
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index

9
Kidneys
Metastasis






















































It is not uncommon for tumors to metastasize to the kidney by the hematogenous route. Metastases most often originate from the breast, bronchi, or gastrointestinal tract, or from the lymphogenous or hematogenous spread of ipsilateral or contralateral renal cancers.
Renal metastases appear sonographically as round, nodular, relatively homogeneous, hypoechoic masses located in the renal parenchyma or the sinus echo complex (Fig. 9.74).
Fig. 9.74 Patient with non-small cell carcinoma (NSCLC) |
b CEUS: marginal enhancement, suspicious for metasta- |
and mass in the kidney (images courtesy of Professor C. |
sis. Fine-needle biopsy confirmed metastasis of a bron- |
Goerg, University Hospital Giessen and Marburg, Mar- |
chial cancer. |
burg, Germany). |
|
a Hypoechoic mass. |
|
Papillary Adenoma,
Adenoma, Oncocytoma, Inflammatory Tumor
Oncocytoma, Inflammatory Tumor 






Fat-like adenoma. Adenoma (classification in Table 9.7) is a benign, well-circumscribed neoplasm of 1 cm or smaller. It is usually solitary. Papillary adenoma is the most common neoplasm of the epthelium of the renal tubules. It is found in 10% to 40% of specimens.13
Sonographically, adenoma appears hyperechoic. It has smooth margins. No reliable sonographic criteria are available ( 9.4 g,h).
9.4 g,h).
Oncocytoma. Oncocytoma is also a slowgrowing tumor that is always benign. Its echogenicity varies from hyperto hypoechoic, but the presence of cystic parts excludes the diagnosis of an oncocytoma. In CEUS, an oncocytoma displays a slower uptake of contrast medium and an overall decidedly diminished vascularity (Fig. 9.75).
Fig. 9.75 Renal oncocytoma (CT, operation).
a Sharply circumscribed, very hypoechoic mass. Based on ultrasound morphology, the fine echogenic wall (arrows) is suggestive of a hemorrhagic cyst. N = kidney.
b Color Doppler shows subtle but constant vascularity, ruling out a cyst or abscess in favor of a solid mass.
Inflammatory tumor. By contrast, inflamma- |
tory tumors in the sinus echo complex are |
tory tumors (like tumor-simulating vasculitis) |
virtually indistinguishable from masses due to |
are rare lesions that tend to have ill-defined |
bacterial inflammation, although the former |
margins and an inhomogeneous, hypoechoic |
show intense vascularity on color duplex ex- |
structure. In gray-scale ultrasound inflamma- |
amination. |
Complex Structure
Kidneys |
Anomalies, Malformations |
|||
|
|
|
||
|
|
|
Diffuse Changes |
|
|
|
|
||
|
|
|
Circumscribed Changes |
|
|
|
|
||
|
|
|
|
Anechoic Structure |
|
|
|
|
Hypoechoic or |
|
|
|
|
Isoechoic Structure |
|
|
|
|
Complex Structure |
|
|
|
|
|
|
|
|
|
Hyperechoic Structure |
|
|
|
|
Echogenic Structure |
Abscess, Pyonephrosis
Xanthomatous Pyelonephritis
Hematoma, Intracystic Hemorrhage
Renal Cell Carcinoma, Cystic Renal Carcinoma, Malignant Lymphoma
Renal Trauma
Abscess, Pyonephrosis 















































Large abscesses and pyonephrosis present so- |
forming hyperechoic and hypoechoic areas. |
presentation, fine-needle aspiration, CT, or the |
|
nographically as an irregular mass that de- |
They may also contain bizarre, anechoic com- |
histological analysis |
of surgical specimens |
stroys the normal renal architecture while |
ponents. The diagnosis is based on the clinical |
(Fig. 9.61, Fig. 9.76, |
11.3 g–i on p. 394). |
356
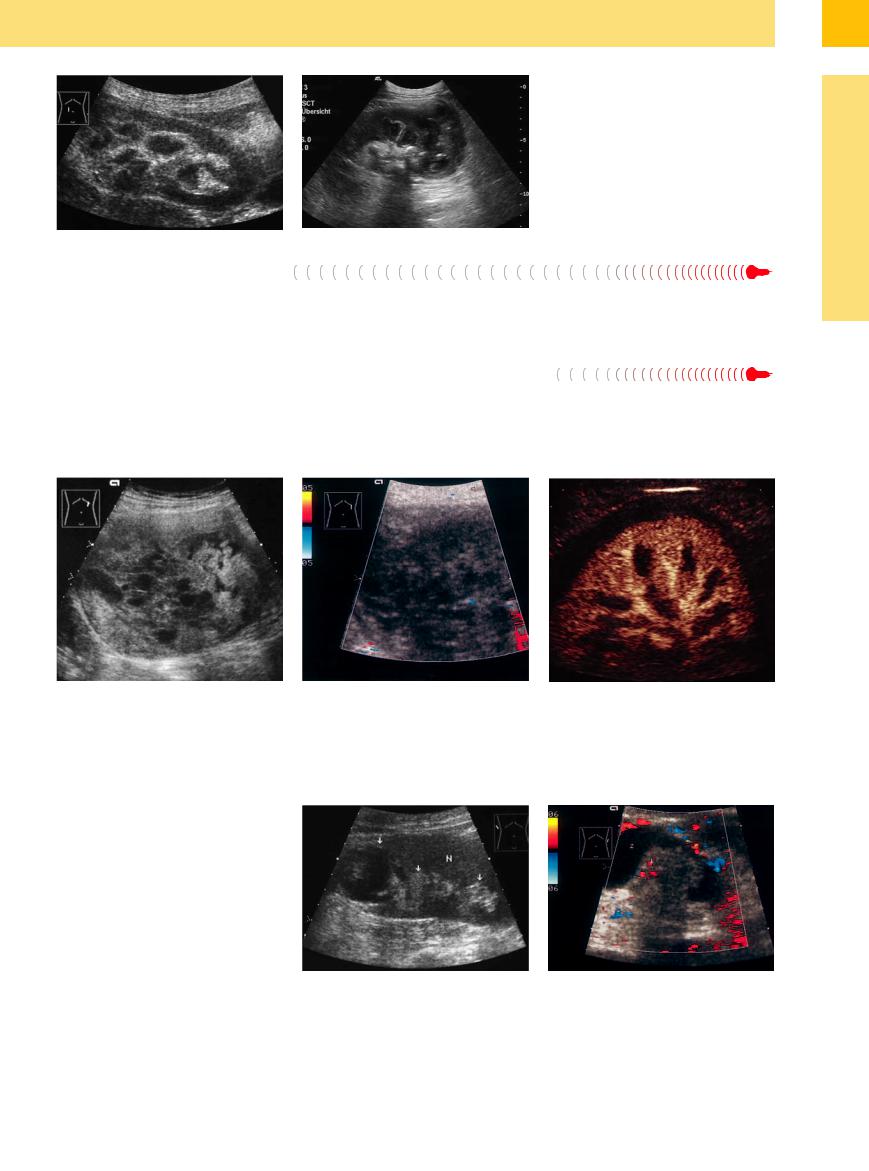
Fig. 9.76
a Pyonephrosis: hypoechoic mass with ill-defined margins, partially filled with echogenic material (debris, inspissation).
b Xanthomatous pyelonephritis: complex mass at the lower renal pole; hyperechoic margins around the medullary pyramids and hyperechoic spots (image courtesy Dr. Katleen Möller, Berlin, Germany).
changes are accompanied by the presence of (sometimes combined with focal urinary tract (Fig. 9.76b).
Hematoma, Intracystic Hemorrhage

















Depending on its stage, a hematoma may con- |
erogeneous, or hyperechoic structure, raising |
cystic hemorrhage. CDS may be helpful, but |
tain anechoic to hyperechoic components that |
serious sonographic differential diagnostic |
CEUS provides an unequivocal diagnosis of |
create an irregular echo pattern. Intracystic |
problems, especially since renal carcinomas |
hemorrhage when an enhancement is lacking |
hemorrhage leads to a hypoechoic (rare), het- |
also tend to bleed and may be masked by intra- |
(Figs. 9.68, 9.70, 9.77). |
9
Circumscribed Changes
Fig. 9.77 Intracystic hemorrhage.
a Large, sharply circumscribed, irregular structure with cystic and solid components.
b Color duplex shows no vascularity even at a low pulse repetition frequency. This means that a tumor is unlikely.
c Traumatic hematoma; CEUS demonstrates no enhancement in the perirenal hematoma (images courtesy of Professor C. Goerg, University Hospital Giessen and Marburg, Marburg, Germany).
Renal Cell Carcinoma, Cystic Renal
Cystic Renal Carcinoma, Malignant Lymphoma
Carcinoma, Malignant Lymphoma






















Diffuse RCC, cystic RCC, and high-grade lymphoma are additional lesions that can acquire a heterogeneous echo texture as they enlarge.
Predominantly regressive tumor changes in the form of calcifications and cystic tumor liquefaction determine the ultrasound appearance of the affected kidneys. Anechoic elements may also represent displaced blood vessels or focal obstructions of the pyelocaliceal system (Fig. 9.78, Fig. 9.79,  9.4).
9.4).
Fig. 9.78 Cyst-associated renal cell carcinoma (arrows). Histology: chromophobe clear-cell renal cell carcinoma (pseudocystic transformation to cystic RCC occurs only in clear-cell and papillary carcinomas).
a Nonhomogeneous, hyperechoic tumor areas accompanied by partially infiltrated, tumor-compressed cysts with irregular margins. N = kidney.
b Cyst-associated renal cell carcinoma: cystically transformed (necrosis, hemorrhage, Z) clear-cell carcinoma. Color duplex shows scant vascular signals.
357
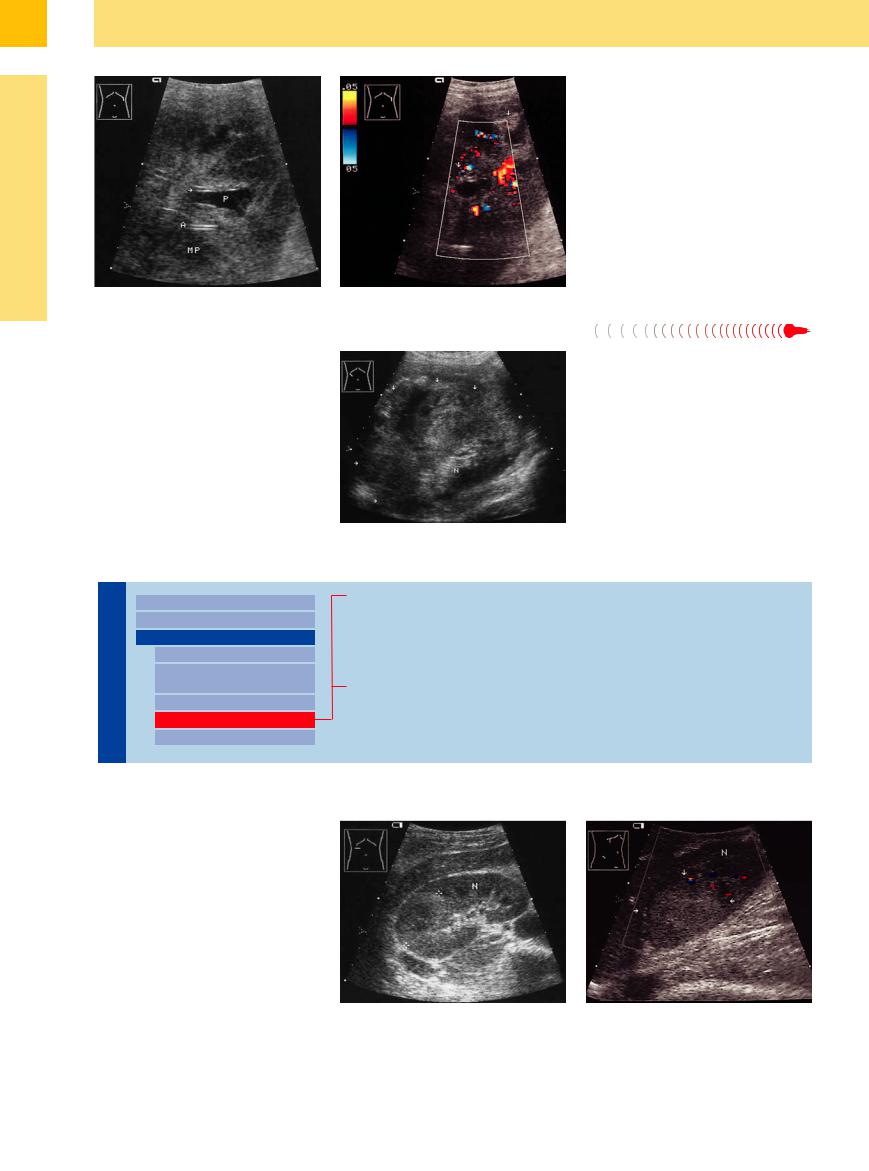
9
Kidneys
Fig. 9.79 High-grade renal lymphoma (enlarged view).
a Irregular mass with echo-free components (obstructive pyelectasis, P) and vascular structures (arrow, A) along with intact, hypoechoic medullary pyramids (MP).
b Color Doppler: echo-free to hyperechoic areas (arrows), irregular tumor vessels.
Renal Trauma 





























In trauma, an irregular (hyperto anechoic) circumscribed transformation of the regular structure and an additional perirenal anechoic mass can be seen.
The most important information is the extent of viable renal tissue. CEUS adds to the color Doppler assessment with clear delineation of hematomas, and additional evidence for a persistent bleed (Fig. 9.70, Fig. 9.80).
Fig. 9.80 Extensive traumatic haematoma of the right kidney. Heterogeneous mass (arrows); band-shaped nonreflective marginal area dorsally (perirenal fluid collection). The extent of the destruction may be estimated in CEUS as the best diagnostic procedure. N = kidney.20
Hyperechoic Structure
Kidneys |
Anomalies, Malformations |
|||
|
|
|
||
|
|
|
Diffuse Changes |
|
|
|
|
||
|
|
|
Circumscribed Changes |
|
|
|
|
||
|
|
|
|
Anechoic Structure |
|
|
|
|
Hypoechoic or |
|
|
|
|
Isoechoic Structure |
|
|
|
|
Complex Structure |
|
|
|
|
Hyperechoic Structure |
|
|
|
|
|
|
|
|
|
Echogenic Structure |
Renal Abscess, Carbuncles
Hemorrhagic Cyst
Renal Cell Carcinoma
Angiomyolipoma
Scars
Renal Abscess, Carbuncles
Carbuncles 













































Renal abscesses, like hepatic abscesses, often acquire a structure that is hyperechoic to the normal parenchyma. Renal carbuncles are staphylococcal abscesses that arise in the kidney owing to hematogenous spread (Fig. 9.81).
Fig. 9.81 Mass in the renal parenchyma (N) caused by abscess formation.
a Circumscribed mass with an echogenic border (cursors).
b Color Doppler shows an absence of vascularity in the abscess structure (arrows), which is surrounded by displaced normal parenchyma (N). The kidney is generally enlarged with a washed-out appearance. Patient presented clinically with headache and septic fever.
358

9
Circumscribed Changes
geneous structure. The structure alone is therefore not a reliable criterion for the differentia-
the appearance of its peripheral rim and fine internal vascularity or, if necessary, by ultra-
Fig. 9.82 Two renal tumors. The large tumor (T) is a renal cell carcinoma (RCC), appearing as an oval mass isoechoic or slightly hyperechoic to the renal parenchyma (N). It has a faint peripheral rim and creates an outward bulge in the renal sinus. These features are typical of RCC (and could justify surgery without further testing). The second mass is a small, echogenic tumor (arrow), most likely a benign nonepithelial tumor.
Fig. 9.83 Echogenic renal cell carcinoma (T). The differential diagnosis includes angiomyolipoma, and therefore CT should be performed.
a Transverse scan through the right upper quadrant shows a sharply circumscribed, homogeneously echogenic mass with a vascularized rim. N = kidney.
b Flank scan reveals intratumoral vessels. The vascular rim and intratumoral vessels are consistent with renal cell carcinoma, not angiomyolipoma. N = kidney.
Angiomyolipoma 


















































Angiomyolipomas are a common type of benign nonepithelial tumor. They contain thickwalled vessels, smooth muscle, and fatty tissue in varying proportions. In ultrasound they appear round or oval, smoothly defined, and mostly homogeneous and more hyperechoic than the renal sinus; but slightly less echogenic when containing only a small amount of fat or when hemorrhage is present. In harmonic imaging an incomplete shadowing sometimes presents (Fig. 9.84, Fig. 9.85). No intratumorous vessels are detectable in CDS except an entering vessel. In CEUS, a rapid enhancement and wash-out after 19 minutes is detectable.
The sensitivity and specificity of ultrasonography for typical angiomyolipomas can be as high as 85%, but the positive predictive value is
lower because of the small hyperechoic RCC. As result, some examinations require CT (Fig. 9.85b).
The tumor can be confidently diagnosed by the fat attenuation observed on CT, but this line of investigation is almost superfluous when typical ultrasound findings (also in color duplex and CEUS) are present. The accuracy of CT is compromised by an absence of fat in some tumors (< 10%). (Fig. 9.84, Fig. 9.85).
Multiple, bilateral angiomyolipomas occur in the setting of tuberous sclerosis (von Hip- pel–Lindau syndrome). This disease is frequently associated with clear-cell renal carcinomas, however, and so additional tests should be performed (Fig. 9.85a, Table 9.8).
Table 9.8 Sonographic features of angiomyolipoma
●Hyperechoic mass
●Size 1–3 cm (occasionally > 5 cm)
●Globular shape
●Harmonic imaging: incomplete shadowing (high specificity and sensitivity)
●Rarely causes a bulge in the renal outline
●CDS: No detectable intratumoral vessels, power Doppler, eventually intratumoral vessels and feeding artery vascularity
●Multiple in 80% of patients with tuberous sclerosis; only angiomyolipomas associated with this condition may undergo malignant transformation
359

9
Kidneys
Fig. 9.84 Hemangiomyolipoma, showing distinctive sonographic features: echogenic intraparenchymal tumor with a fine, reticular internal structure and smooth contours.
Fig. 9.85 |
b Color duplex shows no detectable internal vascularity |
a Three hemangiomyolipomas (arrows). Multiple angio- |
here. |
myolipomas, often in both kidneys, occur in tuberous |
|
sclerosis (Bourneville–Pringle disease). |
|
Scars
“Scarring” refers to any circumscribed, fibrous area of postinflammatory or postinfarction contraction. The areas may be solitary or multiple and tend to undergo focal calcification, producing high-level entry echoes with acoustic shadowing. Scars may appear as linear or large-area lesions ( 9.6).
9.6).
Cause. It can be difficult to establish a cause in any given case (pyelonephritic abscess formation, stone-related, tuberculous, or secondary
to anemia or infarction), although funnelshaped scars most likely result from an old renal infarction ( 9.6a) while pyelonephritic scars tend to have an irregular shape (
9.6a) while pyelonephritic scars tend to have an irregular shape ( 9.6 d).
9.6 d).
Multiple wavy parenchymal notches and rarefactions are characteristic features of peripheral renal arteriosclerosis in a setting of long-standing hypertension ( 9.6a–c). These cases show undulant thinning of the parenchyma with no overall decrease in renal size.
9.6a–c). These cases show undulant thinning of the parenchyma with no overall decrease in renal size.
Rarely, sites of irregular scarring are also seen in panarteritis nodosa.
Scars in the setting of chronic, nondestructive interstitial nephritis are diffuse and therefore produce an irregular renal surface with indistinct outlines. Calcifications may occur.
True scars require differentiation from a parenchymal notch in duplex kidneys, a medially tilted scan plane through the renal hilum, and fetal lobulations ( 9.6f, Fig. 9.66a).
9.6f, Fig. 9.66a).
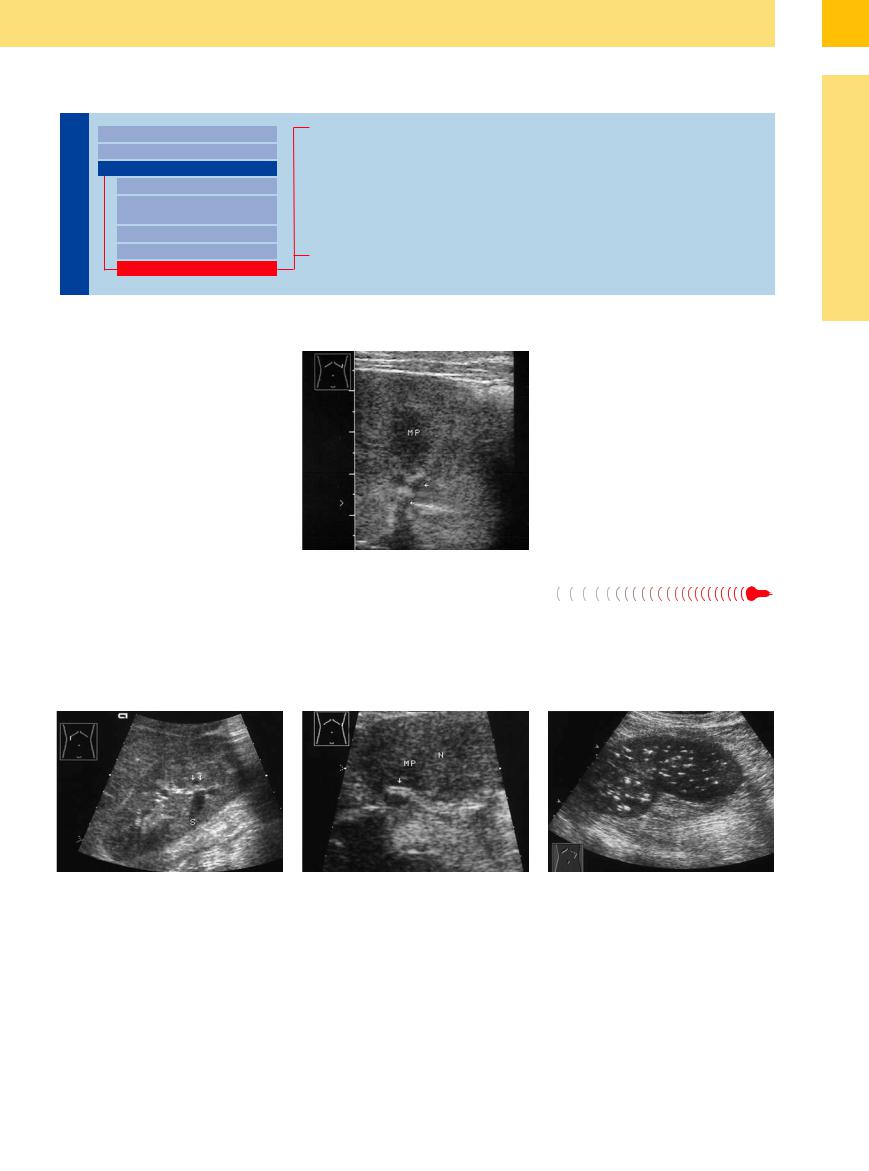
 9.6 Scars and Parenchymal Notching
9.6 Scars and Parenchymal Notching
Vascular scars
a Wedge-shaped infarction scar (arrows) in a patient with absolute arrhythmia in atrial fibrillation. P = renal pelvis.
Postinflammatory scars and differential diagnosis
b Vascular scar (arrows) with focal thinning of the parenchyma. The patient presented clinically with advanced atherosclerosis. N = kidney.
c Broad, echogenic atherosclerotic scar extending focally through the entire parenchyma (arrow) in a patient with chronic hypertension and generalized atherosclerosis. N = kidney.
d Pyelonephritic scar (arrow). Cystic transformation of two medullary pyramids and a calix (arrows), appearing as “caliceal cysts” in the urogram.
e Broad, echogenic scar in the anterior parenchyma following renal tuberculosis.
f Anteroinferior notching of the parenchyma (arrow): scan plane cuts the renal hilum.
360
Kidneys
Anomalies, Malformations Diffuse Changes Circumscribed Changes
Anechoic Structure
Hypoechoic or Isoechoic Structure
Complex Structure Hyperechoic Structure Echogenic Structure
Papillary Calcification
Interlobar and Arcuate Arteries
Bacterial Gas Bubbles
Parenchymal Calcification
Nephrocalcinosis, Medullary Sponge Kidney
Renal Tuberculosis, Putty Kidney
Pyelocaliceal Stone, Staghorn Calculus
Papillary Calcification
Calcification
















































Calcifications at the tips of the renal papillae are characteristic findings in analgesic nephropathy and diabetes mellitus. They appear only as echogenic flecks on ultrasound survey scans, where they often go undetected. They are easily detected and identified in strongly magnified views (Fig. 9.86).
Interlobar and Arcuate Arteries
Arcuate Arteries 



















Fig. 9.86 Medullary (papillary) (MP) calcification (arrows) with an acoustic shadow. Clinical presentation: neglected type 1 diabetes.
The renal artery itself, its branches in the renal |
straight and interlobular arterioles arising |
Ultrasound |
will occasionally |
demonstrate |
sinus, and the interlobar and arcuate arteries |
from the arcuate artery along with the more |
these thickened, echogenic vessels, some of |
||
tend to undergo atherosclerosis and calcifica- |
distal afferent vessels and the postglomerular |
which have |
shadowing wall |
calcifications |
tion in patients with long-standing hyperten- |
efferent vessels). |
(Figs. 9.87, 9.88, 9.89). |
|
|
sion (arteriolosclerosis would affect the |
|
|
|
|
9
Circumscribed Changes
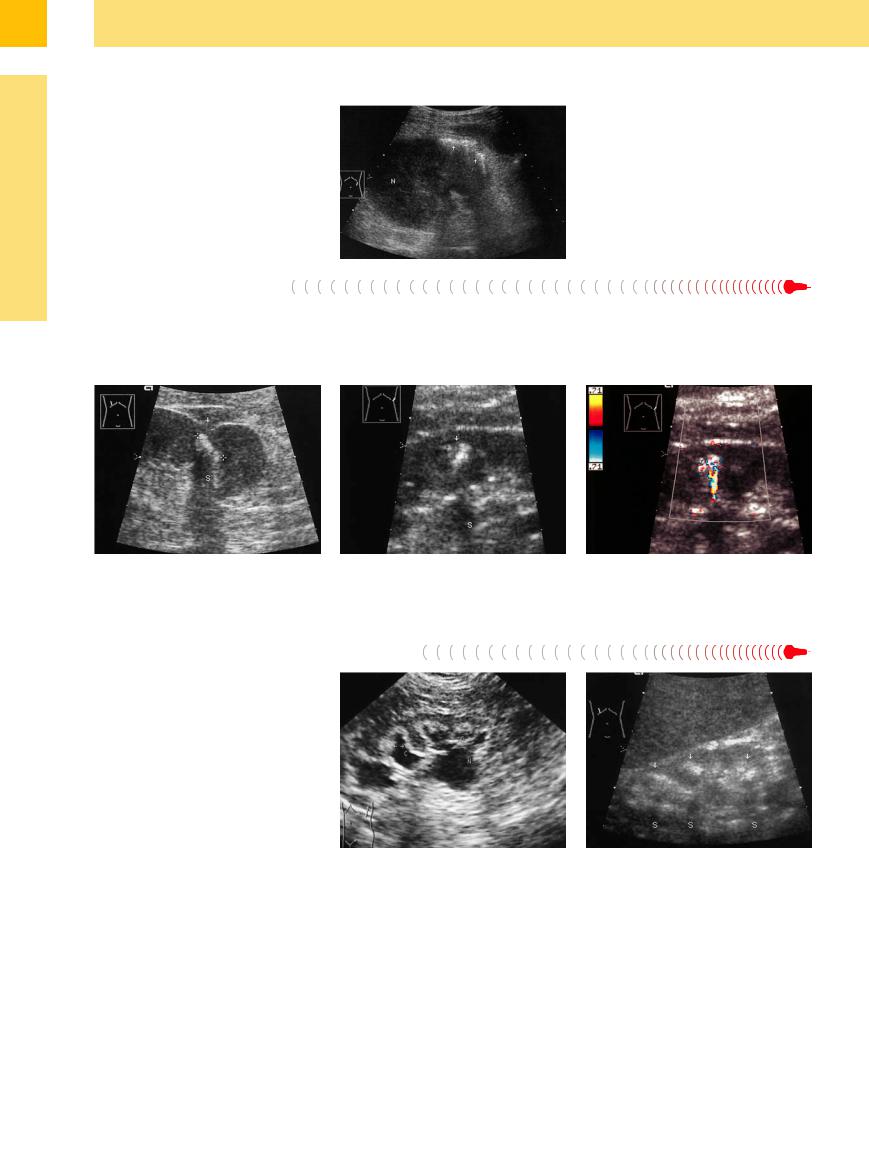
Fig. 9.87 Vascular sclerosis: echogenic renal artery in the renal sinus (arrows), shadowing (S); 60-year-old patient, intensive nicotine abuse, general arteriosclerosis.
Fig. 9.88 Bright double echoes with a central, narrow echo-free band (arrow). Atherosclerosis of the arcuate artery at the base of the medullary pyramid (MP). N = kidney.
Fig. 9.89 Extensive atherosclerosis of the small renal arteries, close-up tangential parenchymal view in pseudoxanthoma elasticum.
361
9
Kidneys
Bacterial Gas
Gas Bubbles
Bubbles
















































Small, vesicular, hood-like echogenic masses may be observed in the setting of emphysematous (bacterial gas-forming) pyelonephritis; the shadowed region usually obscures the abscess itself. These findings indicate an urgent need for treatment (immediate broad-spec- trum antibiotics or nephrectomy) due to existing or impending bacterial sepsis (Fig. 9.67,
Fig. 9.90).
Fig. 9.90 Hood-like bacterial gas collection (arrows) with associated reverberations. An actual abscess cannot be identified. Sepsis, emergency surgery, nephrectomy.
N = kidney.
Parenchymal Calcification
Calcification
Parenchymal calcifications can have a variety of |
athy or analgesic nephropathy. Some calcifica- |
develop as a sequel to old renal trauma, and |
causes. They may reflect an old focal pyelo- |
tions suggest a cystic etiology because of their |
others remain unexplained (Fig. 9.91). |
nephritis, possibly in a setting of renal lithiasis, |
shape. Calcifications also occur in the setting of |
|
as well as calcifications in diabetic nephrop- |
tuberculosis (see below). Other calcifications |
|
Fig. 9.91
a Broad scar with calcification (arrows) of undetermined cause. Acoustic shadow (S).
b Echogenic lesion (arrow), incomplete shadowing (S).
c CDS with high pulse repetition frequency (PRF): “twinkling artifact,” which favors a calcification and not an angiomyolipoma.
Nephrocalcinosis, Medullary Sponge Kidney
Kidney
Nephrocalcinosis and medullary sponge kidney are different renal diseases that are associated with calcifications and have similar or identical ultrasound appearances.
Medullary nephrocalcinosis (medullary sponge kidney) is based on a dysontogenic disorder that can be broadly classified among the cystic diseases. It is characterized by a cystic dilatation of the collecting tubules in the renal papillae, leading to stone formation (a cauliflower or rosette pattern is seen in the intravenous pyelogram). Ultrasonography shows echogenic, rosette-like calcifications with acoustic shadows in the medullary pyramids.
Cortical nephrocalcinosis is marked by calcifications of the parenchyma and medullary pyramids and the formation of kidney stones. Calcifications of the medullary pyramids are the dominant feature. Nephrocalcinosis develops in a setting of hypercalcemia or tubular acidosis. A calcium excess is common to both disorders (increased supply or decreased tubular reabsorption).
If caliceal stones and renal pelvic/ureteral stones are also present, scans will show more or less intense, anechoic areas of caliceal and pelvic ectasia (Fig. 9.92).
Fig. 9.92 |
b Medullary nephrocalcinosis: annular calcifications in |
a Medullary nephrocalcinosis (renal tubular acidosis): |
medullary pyramid projection (arrows). S = acoustic |
echogenic medullary pyramids (arrows) and obstructive |
shadow. |
pyelocaliectasis (C) with multiple renal pelvic and ureteral |
|
stones. N = kidney. |
|
362
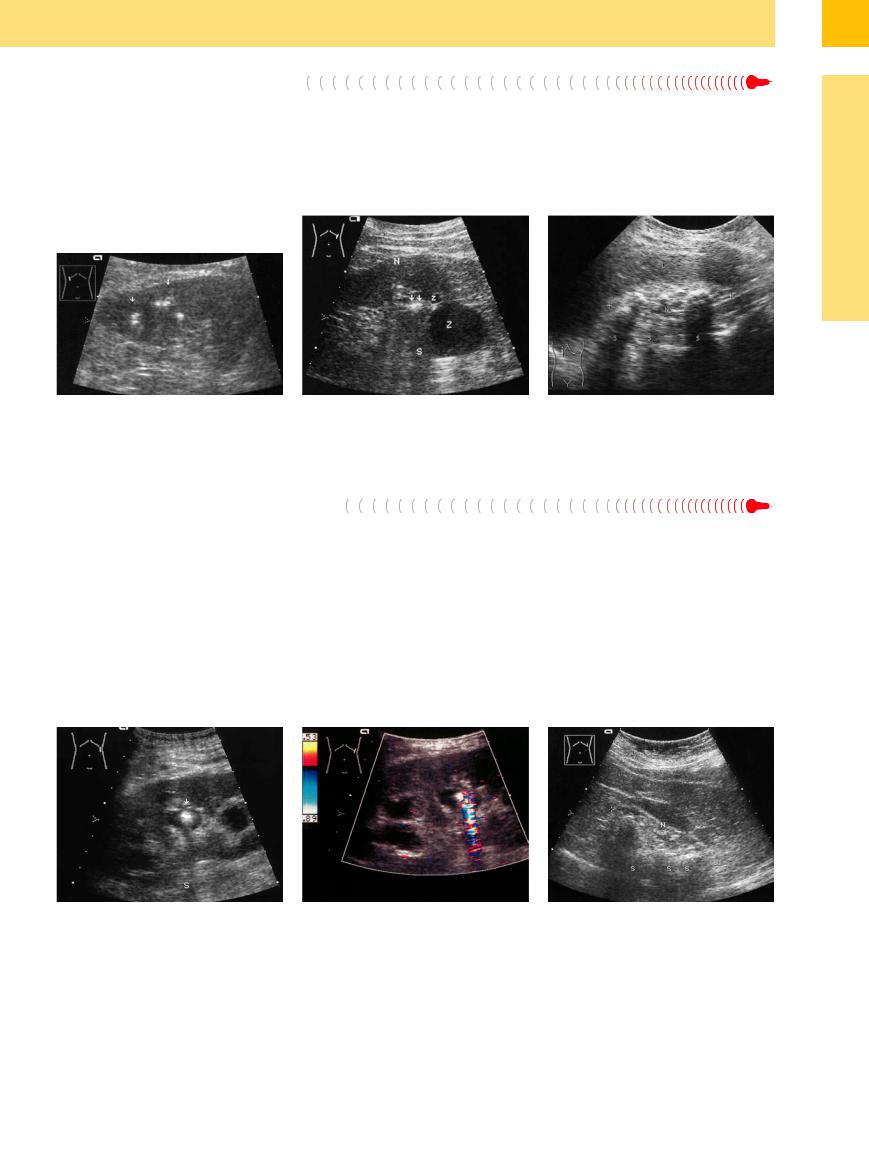
9
Circumscribed Changes
Fig. 9.93 Renal tuberculosis: focal parenchymal calcifica- |
Fig. 9.94 Renal tuberculosis: calcifications (arrows, acous- |
tions (arrows) around a presumably destroyed medullary |
tic shadow S), cavernous cysts (Z). N = kidney. |
pyramid (see also Fig. 9.60). |
|
Pyelocaliceal Stone,
Stone, Staghorn Calculus
Staghorn Calculus
Pyelocaliceal stones or staghorn calculi are easily diagnosed on ultrasound, which demonstrates the bright stone-surface echoes and associated shadows that are typical of stones and calcifications. Occasionally the stones are so small that their presence is revealed only by an acoustic shadow. This introduces a degree of uncertainty, as the shadowing focus may represent a vascular calcification.
Staghorn calculi in the renal pelvis may exhibit various shapes, depending on the orientation of the scan plane. If the scan is directed
through the greatest width of the renal pelvis, it will display a broad, hard linear echo with an equally broad acoustic shadow. But if the plane cuts portions of the stone in the calices, multiple echoes and acoustic shadows will be seen (Fig. 9.96, Fig. 9.97).
Some small stones may be missed within the bright reflections of the sinus echo complex. A helpful guide in these cases is the “twinkling artifact” seen in color duplex scanning. It consists of red and blue color artifacts appearing within the shadow cast by calcified
Fig. 9.96 |
b CDS, high PRF: “Twinkling artifact” (red–blue artifact in |
a Small calculus of the left kidney (arrow); incomplete |
the region of the shadowing). |
shadow (S). |
|
Fig. 9.95 Putty kidney (following tuberculosis): diffuse calcifications, acoustic shadow (S). An actual renal structure is not defined (N, cursors). L = liver.
renal stones (or calcium stones in general:
Fig. 9.91; see also Figs. 4.47, 11.43, 11.44,
11.46). Reportedly, this phenomenon is seen even with very small stones.5 Explanations for that may be changes in velocity, phase or Doppler shifting, or mechanical forces on stones leading to minimal movements. Changing the scan direction leads to a variant twinkling.
Fig. 9.97 Small atrophic kidney (N, cursors) due to renal pelvic stones or (unlikely here) multiple calcifications. Acoustic shadows (S). Excretory urogram showed a silent kidney with no focal opacities (urate stones?).
363

9
Kidneys
Tips, tricks, and pitfalls
Since most renal tumors found today are diagnosed in localities where ultrasound is commonly used, they are most often at a local operable stage. Therefore:
●The kidneys should be always investigated in case of abdominal scanning; what is the renal structure and the vascular architecture? Examination with magnification is called for.
●Renal tumors, renal calculi, infarction scars, cysts may be hidden: careful lamelliform examination of all kidney regions in two sectional planes including lateral decubitus position is indicated.
●Normal kidneys are movable.
–Pay attention to dynamic criteria (movement by respiration, normal movement by the iliopsoas muscles regarding also liver and spleen).
–Examine the patient in a standing position when nephroptosis is suspected.
●Parenchymal thickness correlates with age:
–In youth, the renal parenchyma is robust.
–In older people it is reduced, but to no less than 1.2 cm; values below this level indicate pathological changes through arteriosclerosis, scars, or renal artery stenosis (Fig. 9.98).
●Fusional, positional, and form variants (e. g., horseshoe or pelvic kidneys, lateral humpback, nodular formation) are not uncommon.
–In such findings a second ultrasound exam including CDS (in the case of regular vascularity) should be performed; otherwise, CEUS should be performed.
●Transplanted kidneys are denerved.
–A slight pyelectasis is a nearly normal finding. Pyelectasis also results from an ampullary renal pelvis, from fluid diuresis, and pathologically from highly inflamed pyelitis.
●Renal calculi are frequently not enveloped by fluid and therefore difficult to differentiate:
Look closely: does CDS show a “twinkling artifact”)?
Fig. 9.98 Small kidney with diminished parenchyma. Accidental finding. Clinically, general arteriosclerosis.
References
[1]Banholzer P, Haslbeck M, Mehnert H. Sonographische Größenveränderungen der Nieren bei Typ-I-Diabetes als Früherkennungsmethode der diabetischen Nephropathie. Ultraschall Med 1988;9:255–259
[2]Mogensen CE. Diabetes mellitus and the kidney. Kidney Int 1982;21(5):673–675
[3]Jinzaki M, Ohkuma K, Tanimoto A, et al. Small solid renal lesions: usefulness of power Doppler US. Radiology 1998;209(2):543–550
[4]Bönhof JA, Meairs SP, Wetzler H. Duplexund Farbdoppler-sonographische Kritrien von Nierenarterien-Stenosen. Ultraschall Klin Prax 1990;5:187
[5]Klauser A, Pallwein L, Frauscher F, Helweg G, Peschel R, Debus J. Die Wertigkeit des farbdopplersonographischen „Twinkling-Arte- fakts“ in der Diagnostik der Nephrolithiasis. 24th Dreiländertreffen der ÖGUM, DEGUM, SGUM. Vienna 2000
[6]Farrelly C, Delaney H, McDermott R, Malone D. Do all non-calcified echogenic renal le-
sions found on ultrasound need further evaluation with CT? Abdom Imaging 2008;33(1):44–47
[7]Mostbeck G, Derfler K, Walter R, Herold Ch, Mallek R, Tscholakoff D. Sonographie bei terminaler Niereninsuffizienz—ätiologische Rückschlüsse? Ultraschall Med 1988;9(6): 250–254
[8]Hricak H, Cruz C, Romanski R, et al. Renal parenchymal disease: sonographic-histologic correlation. Radiology 1982;144(1):141–147
[9]Deeg KH, Gerdemann C, Weingärtner K, Seitz
G. Sonographic diagnosis of an unusual case of multilocular cystic nephroma mimicking polycystic kidney disease. Ultraschall Med 2008;29(Suppl 5):264–267
[10]Schmidt G, Ed. Ultrasound Thieme Clinical Companions. New York: Thieme, 2007
[11]Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hep- atic applications. Ultraschall Med 2012; 33(1):33–59
[12]Takebayashi S, Aida N, Matsui K. Arteriovenous malformations of the kidneys: diagnosis and follow-up with color Doppler sonography in six patients. AJR Am J Roentgenol 1991;157(5):991–995
[13]Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol 2006; 49(5):798–805
[14]Mostofi FK, Davis CJ. Histological Typing of Kidney Tumours. 2nd ed. Berlin: Springer, 1998
[15]Ascenti G, Zimbaro G, Mazziotti S, Gaeta M, Settineri N, Scribano E. Usefulness of power Doppler and contrast-enhanced sonography in the differentiation of hyperechoic renal masses. Abdom Imaging 2001;26(6): 654–660
[16]Hofmann R, Schütz R, Leyh H, Braun J. Sonographischer Nachweis von Nierenvenenund Vena-cava-Thrombosen beim Adenokarzinom der Niere. Ultraschall Med 1985;6: 312–315
[17]Snyder ME, Bach A, Kattan MW, Raj GV, Reuter VE, Russo P. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: influence of sex. J Urol 2006;176(6 Pt 1):2391–2395, discussion 2395–2396
[18]Remzi M, Memarsadeghi M. Der kleine, zufällig entdeckte Nierentumour. Aggressionspotential und Abklärung [Small incidental renal tumors. Evaluation and biological parameters]. Urologe 2007;46:478–484
[19]Lau DM, Siegel MJ. Pediatric abdominal masses. In: Bluth EI, Arger PH, Benson CB, Ralls PW, Siegel MJ, eds. Ultrasound. 1. ed. New York: Thieme, 2000: 425
[20]Schmidt G, Görg C. Kursbuch Ultraschall . 5. ed Stuttgart: Thieme, 2008
[21]Smith AD, Remer EM, Cox KL, et al. Bosniak category IIF and III cystic renal lesions: outcomes and associations. Radiology 2012; 262(1):152–160
[22]Schmidt G. Differential Diagnosis in Ultrasound Imaging. Stuttgart: Thieme, 2006
364
