
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
Focal Fatty Infiltration
Infiltration
Hyperechoic focal changes are rare. A focal fatty infiltration demonstrates a hyperechoic but regular structure (Fig. 4.54).
Fig. 4.54 Hyperechoic ovoid lesion of the pancreatic head: Focal fatty change.
4
Focal Changes
Irregular (Complex Structured) Lesion
Pancreas
Diffuse Pancreatic Change
Focal Changes
Anechoic Lesion
Hypoechoic Lesion
Isoechoic Lesion
Hyperechoic Lesion
Irregular (Complex Structured) Lesion
Dilatation of the Pancreatic Duct
Chronic Pancreatitis
Focal Chronic Pancreatitis
Pseudocyst/Intracystic Hemorrhage
Cystic Neoplasias (Cystadenoma/Cystadenocarcinoma)
Chronic Pancreatitis

















































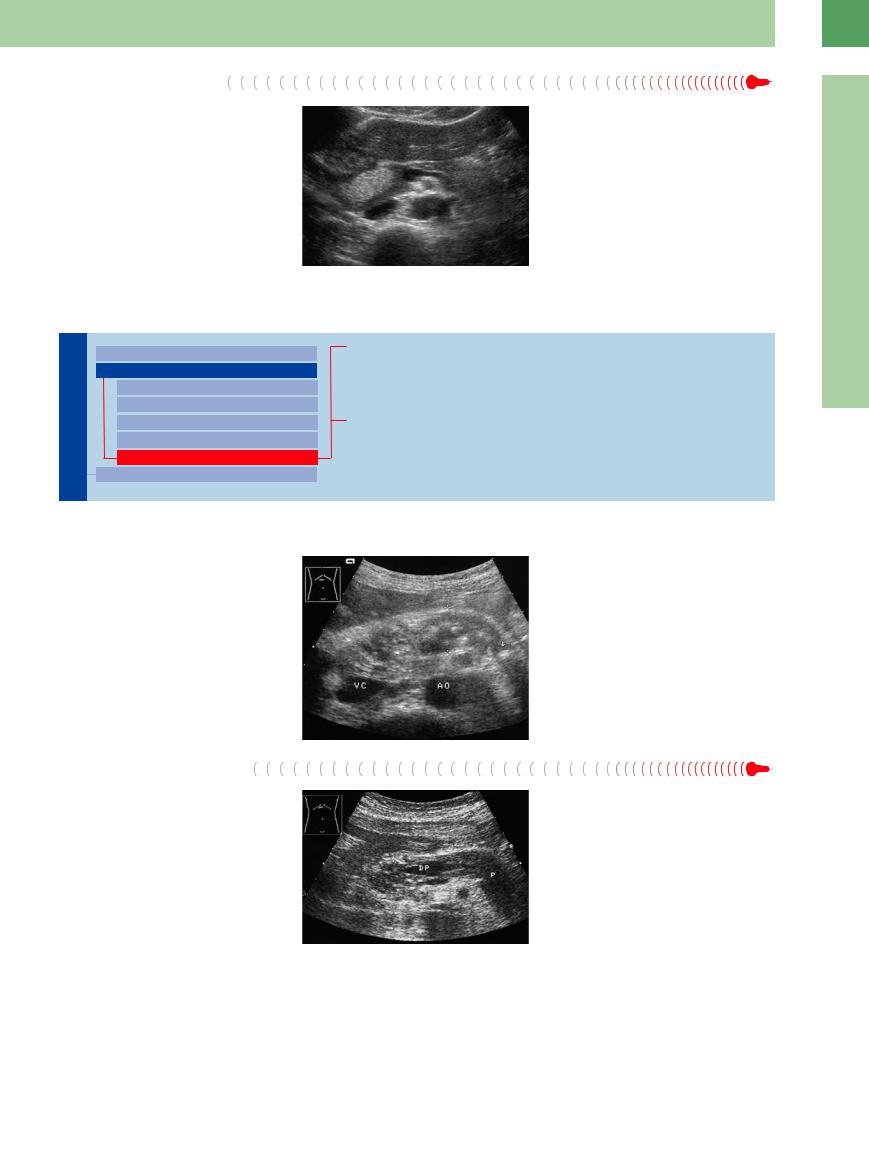
If several or all of the criteria are present, quite often chronic pancreatitis will present a busy picture, where the individual criteria (cyst, fibrosis, calcification, dilated duct) can only be discerned with difficulty or not at all (Fig. 4.55).
Focal Chronic Pancreatitis
Fig. 4.55 Chronic pancreatitis: without the splenic vein as landmark structure (here thrombosed/obliterated), the pancreas is almost impossible to delineate (calipers); the “lively” image is due to ductal ectasia (arrow), here, with calculi, fibrosis, calcification, and microcysts. VC = vena cava; AO = aorta.
Here, too, the texture is heterogeneous, but because of the fibrosis and/or calcification it is micronodular to macronodular. Differential diagnosis has to consider pancreatic cancer (Fig. 4.56).
Fig. 4.56 Mottled hypoechoic/hyperechoic texture of the pancreatic head (P): focal chronic pancreatitis in the head, cut-off of the pancreatic duct (DP) (caution: cancer!).
191
4
Pancreas
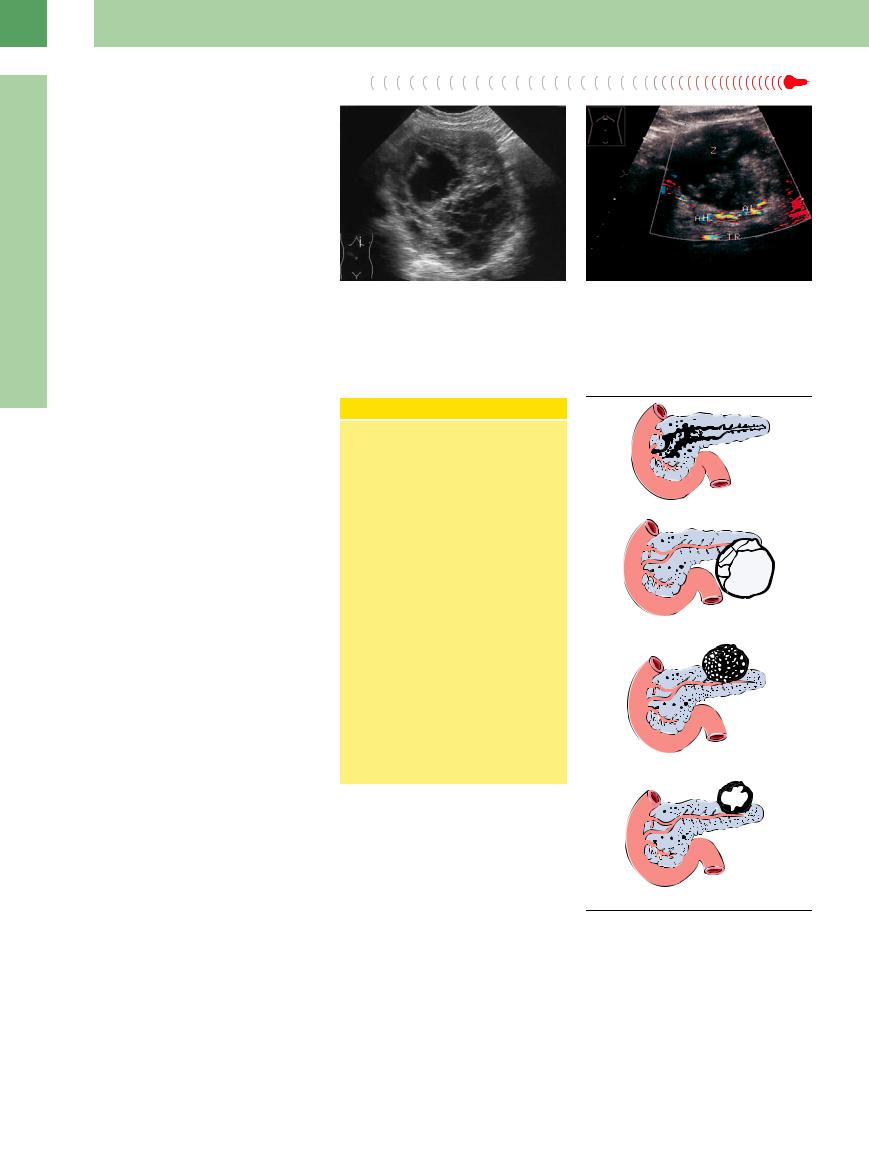
Fig. 4.57
a Large, symptomatic pancreatic pseudocyst: complex masses in the left upper abdomen. Initial symptoms were caused by mass effects from the cyst.
b Incipient pseudocyst (Z) after acute pancreatitis: complex mass displacing the hepatic artery (AH) and splenic artery (AL). Color duplex. TR = celiac axis.
Cystic Neoplasias (Cystadenoma/Cystadenocarcinoma)






























Cystic neoplasias of the pancreas are rare. Ductal carcinomas comprise approximately 80% of all malignant neoplasms of the pancreas, and some 90% of those are ductal adenocarcinomas. Variants of the cystic neoplasms are (Fig. 4.58):14
●Mucinous cystic neoplasm (MCN)
●Serous cystic neoplasm (serous microcystic adenoma, SMA)
●Intraductal papillary mucinous neoplasm (IPMN)
●Solid pseudopapillary neoplasm (SPN)
Cystadenomas have cystic and solid parts. Until the late 1970s, the spectrum of cystic diseases consisted mainly of serous (microcystic, mostly benign) and mucinous (at the time of diagnosis often malignant) neoplasms. The development and widespread use of new imaging techniques led to the delineation of new categories of cystic pancreatic neoplasms; new entities were described.14
Serous microcystic benign adenoma, mostly found in older women, often presents difficulties in differential diagnosis because of a concomitant duct occlusion. Ultrasound depicts a mainly solid formation with numerous small (honeycomb-like) cysts up to 2 cm in size, whereas in macrocystic adenoma, which occurs mostly in middle-aged women, large cystic formations are immediately detectable. With CDS, tumor vessels are detectable in both types, but more so in microcystic adenoma ( 4.3a–f).
4.3a–f).
Cystic lesions of the pancreas are summarized in the following outline:
IPMNs are characterized by intraductal proliferation of mucin-producing cells, which are arranged in papillary patterns. In many IPMNs hypersecretion of mucin leads to a cystic dilatation of the involved ducts. In a few IPMNs, focal or diffuse intraductal papillary growth causes duct dilatation ( 4.3 g–k). IPMNs are frequently localized in the main duct of the head region and should be operated at an early stage; those from secondary ducts seem to have a better prognosis.
4.3 g–k). IPMNs are frequently localized in the main duct of the head region and should be operated at an early stage; those from secondary ducts seem to have a better prognosis.
The most common differential diagnosis of pseudocyst is versus neoplasms with a cystic
Cystic Lesions of the Pancreas
Neoplastic epithelial
Benign
●Serous cystic adenoma
●Mucinous cystic adenoma
Borderline
●IPMN
●Mucinous cystic neoplasm
●Pseudopapillary cystic neoplasm
Malign
●(Serous)/mucinous cystadenocarcinoma
●Intraductal papillary mucinous carcinoma
Nonneoplastic epithelial
●Congenital cyst
●Retention cyst
●Lymphoepithelial cyst
●Duodenal wall cyst
Nonneoplastic nonepithelial
●Pancreatitis-associated pseudocyst
●Parasitic cyst
●Pseudoaneurysm
appearance (cystic ductal carcinoma, MCN and SPN14) ( 4.3 m–o; Table 4.8). In particular, the SPN may be similar to a pseudocyst (
4.3 m–o; Table 4.8). In particular, the SPN may be similar to a pseudocyst ( 4.3 l). Wall thickening in excess of 1 cm, tumor markers in the fine-needle aspirate (CEA, Ca 15.3, Ca 72.4, Ca 19.9) and low amylase and lipase indicate cystic neoplasms. EUS (sensitivity 93%; CT only 36%) and CEUS confirm the diagnosis. In CEUS, necrosis, detritus, and blood clots (see Fig. 4.25) in a pseudocyst can be readily discriminated from solid (vascularized) tumors and septae in cysts (
4.3 l). Wall thickening in excess of 1 cm, tumor markers in the fine-needle aspirate (CEA, Ca 15.3, Ca 72.4, Ca 19.9) and low amylase and lipase indicate cystic neoplasms. EUS (sensitivity 93%; CT only 36%) and CEUS confirm the diagnosis. In CEUS, necrosis, detritus, and blood clots (see Fig. 4.25) in a pseudocyst can be readily discriminated from solid (vascularized) tumors and septae in cysts ( 4.3c,m).
4.3c,m).
Mucinous cystadenomas and cystadenocarcinomas are not really distinguishable in US; CDS and CEUS do not give reliable enough values for differentiation, but an invasion into vessels signifies malignancy.
IPM carcinomas demonstrate mural nodulation within the dilated duct wall and parietal
Fig. 4.58 Cystic pancreatic tumors (reproduced from Klöppel and Kosmahl14 with permission from Elsevier).
masses with flow signals. Symptomatic IPMN with mural nodulation greater than 10 mm are mostly malignant. CEUS depicts vascularized septae and hyperenhanced masses (Fig. 4.59).
Table 4.8 provides a summary of cystic lesions of the pancreas, Table 4.9 of the differential diagnoses of diffuse and focal changes in the texture and size of the pancreas.
192

Table 4.8 Differential diagnosis of cystic pancreatic lesions (after Klöppel and Kosmahl14)
Cystic tumor lesion |
Clinic findings |
Localization and morphology |
Sonography |
Pseudocyst |
● Mostly male, age range |
● Grossly visible well-demarcated |
● Complex well-defined mass with hyper- |
|
35–60 years |
cystic lesion, containing necrotic- |
echoic wall |
|
● Previous pancreatitis of alco- |
hemorrhagic material |
● No septae, no vascularization |
|
holic, biliary or traumatic ori- |
● Enclosed by a wall of inflammatory |
|
|
gin |
and fibrous tissue |
|
Intraductal papillary |
● Approximately 30% may even- |
mucinous neoplasm |
tually become invasive meta- |
(IPMN) |
stases |
|
● Symptoms of acute and/or |
|
chronic pancreatitis are com- |
|
mon, often incidentally |
|
● Slightly more frequent in men |
|
than in women |
●Most frequently localized in the main duct of the head region, those from secondary ducts seem to have a better prognosis
●Markedly and cystically dilated lumen of the main duct
●“Main duct“ type: 75%
●“Branch duct“ type: 25%
●Within the dilated duct, wall thickening with mural nodulation. Papillary parietal masses with flow signals
●Cystic duct dilatation with hyperechoic material
●Symptomatic IPMN with mural nodulation > 10 mm mostly malignant
●In CEUS, vascularized septae and nodules leaving the space of the dilated duct
Mucinous cystic neo- |
● Almost exclusively women, age |
● Predominantly in the body or tail |
● Polycystic mass > 5 cm irregular cystic |
plasm (MCN) |
usually < 50 years, mean 49 |
● No communication with the ductal |
lesion with solid parts and septae |
|
● Often found incidentally |
system |
● Hypervascularized tumor; arteries with |
|
|
● Unilocular or multilocular smooth |
flow signals (enhancement in CEUS) |
|
|
surface and diameters between 2 |
● No dilated pancreatic duct |
|
|
and 35 cm |
● Invasion into vessels signifies malignancy |
|
|
● Excellent prognosis when com- |
|
|
|
pletely resected, but potentially |
|
|
|
malignant |
|
Serous cystic neo- |
● Localization in the body/tail |
● Single well-circumscribed, slightly |
plasm (SCN); serous |
● Predominantly female; age |
bosselated round tumor, with diam- |
microcystic adenoma, |
range 35–90 years, mean 65 |
eters ranging from 1 to 25 cm |
(SMN), up to 60%, se- |
● Clinically asymptomatic, usu- |
● Numerous small cysts |
rous oligocystic and ill- |
ally found incidentally |
● Central stellate scar, calcifications |
demarcated adenoma, |
|
(except SOIA) |
(SOIA), 30% of all SCN |
|
● No invasion |
Solid pseudopapillary |
● In any region of the pancreas |
● Diameters ranging from 3 to 18 cm |
neoplasm |
or attached to it |
● Hemorrhagic cystic degeneration |
|
● Predominantly women at age |
forming irregular blood formations |
|
20–30 years, mean 26 |
● Demarcated by pseudocapsule, |
|
● Usually detected incidentally, |
possibly calcifications |
|
in case of bleeding also pain |
● Di erential diagnosis: pseudocysts, |
|
● Excellent prognosis in 85–90%, |
cystic forms of endocrine tumors |
|
but potentially malignant |
|
Ductal adenocarcino- |
● Elderly people |
● Cysts usually no larger than 0.5 cm |
ma with cystic fea- |
● Localization in any region, pre- |
by ectatic duct-like structures or |
tures |
dominantly in the head |
nonneoplastic retention cysts |
|
● Mostly indicated by symptoms |
● Tumor necroses; otherwise produce |
|
of pain, loss of weight or |
large cystic cavities especially in |
|
appetite |
poorly di erentiated tumors |
●Solid tumor
●Numerous small cysts up to 2 mm, some larger cysts are possible
●Intensively vascularized
●Often preocclusive duct dilatation
●Hyperenhanced on CEUS with small areas of nonenhancement
●Well-defined round tumor
●Confluence of lesions of pseudocystic degeneration
●Not distingishable from pseudocysts in B- mode sonography; probably only by CDS or CEUS
●Small cysts with large pseudocyst-like lesions
●Solid parts and nodules with flow signals in CDS, enhancement in CEUS
●Main duct obstruction
Fig. 4.59 Intraductal papillary mucinous carcinoma, confirmed by operation.
a Cystic mass with thickening of the wall and dorsal hyperechoic parts.
b CEUS: vascularized wall and dorsal solid parts indicate a malignoma (histology: intraductal papillary mucinous carcinoma).
4
Focal Changes
193
4
Pancreas
 4.3 Cystic Neoplasms of the Pancreas
4.3 Cystic Neoplasms of the Pancreas
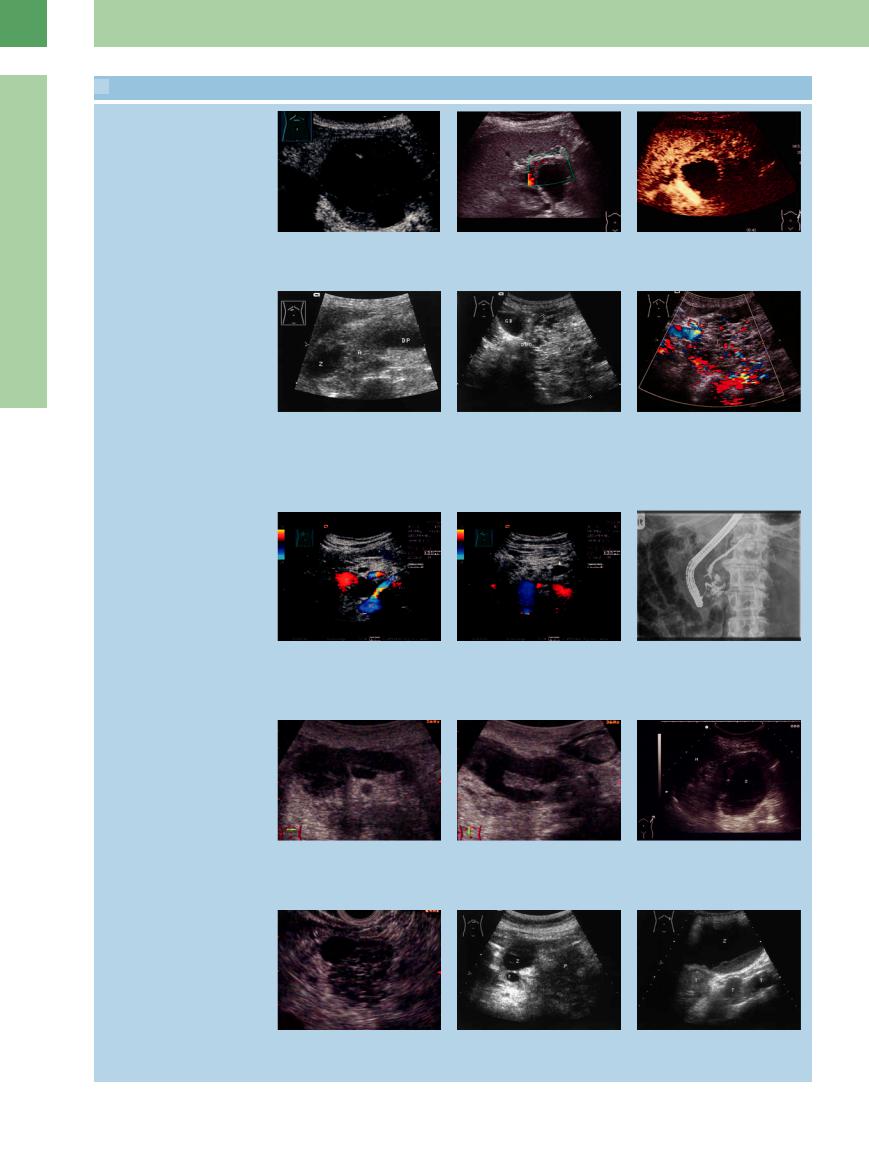
Mucinous cystic neoplasm (macrocystic)
a Cystadenoma of the pancreas: tumor |
b Power Doppler in the mural node de- |
c CEUS: the solid parts show a hyperen- |
with cystic (CEUS: completely nonenhanc- |
tection of vessels. |
hancement, the cystic parts remain |
ing), and solid parts (CEUS: markedly en- |
|
without signals. |
hanced). |
|
|
Serous cystic neoplasm (microcystic)
Intraductal papillary mucinous and solid pseudopapillary neoplasm
Cancers: intraductal papillary mucinous carcinoma, ductal carcinoma with cystic feature
d Microcystic cystadenoma of the pancreas (calipers), confirmed by histology: hypoechoic microcystic tumor with a larger cyst (Z); cut-off of the pancreatic duct (DP). After 2 years follow-up there has so far been no change of this finding in an elderly female patient. A = splenic artery.
g–i Intraductal papillary mucinous neoplasm (IPMN).
g Septated cystic mass in the pancreatic head. C = venous confluence, VL = splenic vein, VC = caval vein, AO = aorta. VR = left renal vein.
j IPMN with pancreas divisum. Extremely dilated pancreatic duct with ballooning in the head and cystic-nodular structure.
m Mucinous cystic carcinoma (EUS); complex structured tumor.
e Microcystic cystadenoma: large tumor (T; cursors) of the head of the pancreas with microcysts. DUO = duodenum, GB = gallbladder.
h Second cystic mass in the pancreatic body (arrow).
k IPMN, longitudinal scan.
n Ductal adenocarcinoma with cystic transformation (Z); the tumor in the tail (T) ill-defined with infiltration growth.
f CDS: Spot-like vascularization. VP = portal vein.
i ERP: the two cystic formations (arrows) are derived from the pancreatic duct. Histological examination (transpapillar biopsies): main duct IPMN (body), branch duct IPMN (head).
l Supposed solid pseudopapillary neoplasm, incidental finding in a 25-year-old woman; complex structured cystic tumor
(Z) of the pancreatic tail with thick walls. M = spleen.
o Cystic pancreatic carcinoma of the tail: Large nodal masses (T), inoperable. Increasing number of cysts under observation.
194
