
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
14
Thyroid Gland
Tumor
Malignant tumors are difficult to diagnose. Large tumors tend to develop areas of ne-
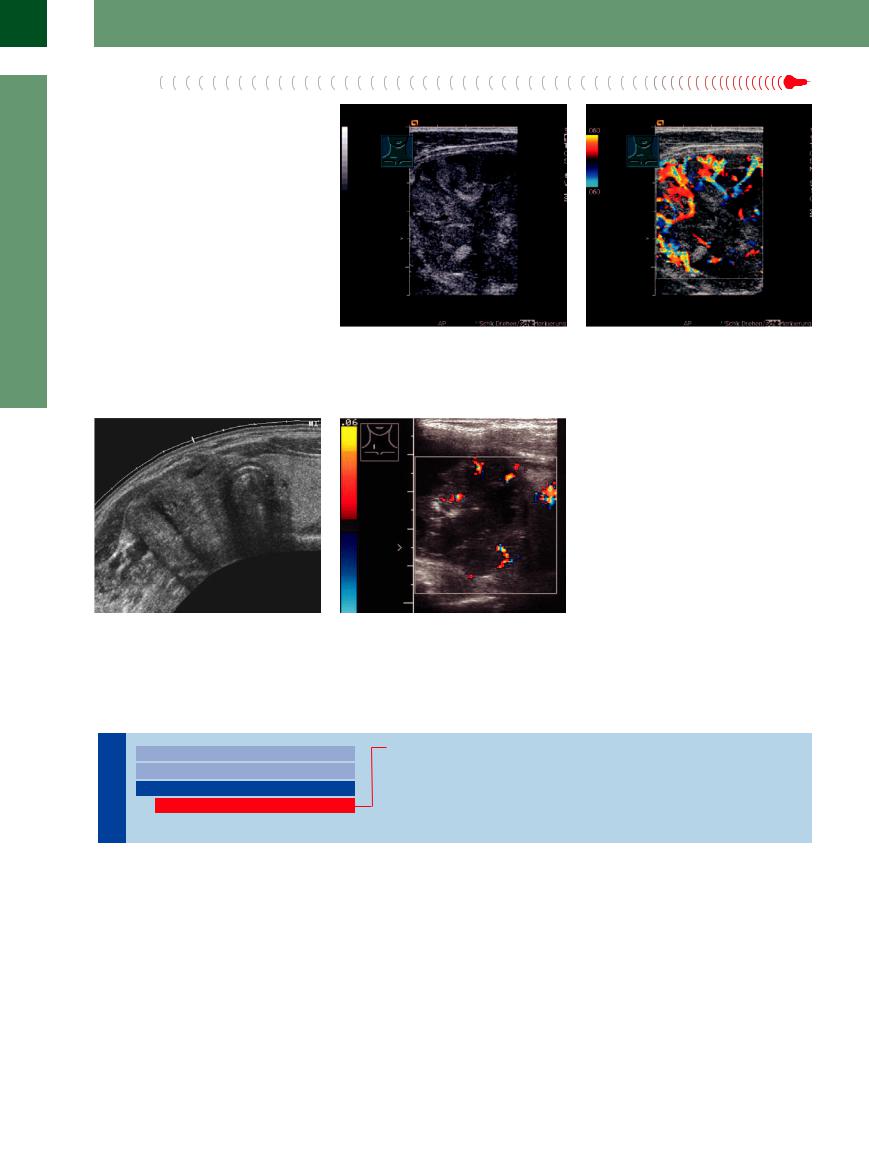
crotic liquefaction. Together with calcifications, they produce a complex echo pattern with an irregular sonomorphologic structure that is difficult to interpret (Figs. 14.62, 14.63, 14.64). The diagnosis relies chiefly on radionuclide findings (cold nodules), clinical manifestations (enlarging goiter), and FNA biopsy.
Fig. 14.62 Follicular carcinoma within a goiter. Fine-needle biopsy (FNB) in the ventral lateral areas: follicular neoplasia. Histology: follicular carcinoma.
a Intensive complex structure.
b Marked vascularization.
Fig. 14.63 Papillary thyroid carcinoma of the right thyroid lobe: diffuse hypoechoic structure, small shadows (image courtesy of Dr. H. Strobel, University of Erlangen, Germany).
f Fig. 14.64 Undifferentiated anaplastic carcinoma shows an irregular structure with a hypoechoic background, internal vascularity, central anechoic foci of liquefaction, and echogenic microcalcifications.
■ Differential Diagnosis of Hyperthyroidism
Types of Autonomy
Gland |
|
|
|
Diffuse Changes |
|
Unifocal Autonomy |
||
|
|
|
||||||
|
|
|
Circumscribed Changes |
|
Bifocal Autonomy |
|||
|
|
|||||||
|
|
|
|
|
Differential Diagnosis of Hyperthyroidism |
|
|
|
Thyroid |
|
|
Multifocal Autonomy |
|||||
|
|
Types of Autonomy |
|
|||||
|
|
|
Disseminated Autonomy |
|||||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Approximately 40% of all hyperthyroidism has an immune etiology. Almost all children and adolescents with hyperthyroidism have Graves disease as an underlying condition. In older patients with hyperthyroidism, functional autonomy (usually multifocal) is present in 70–80% of cases. In iodine-deficient areas, hyperthyroidism occurs predominantly in a latent form (low basal TSH with normal peripheral hormone levels) that is generally missed unless a routine basal TSH determination is performed (just as overt hyperthyroidism is often missed because of nonspecific clinical symptoms). Disseminated autonomies outside of Graves hy-
perthyroidism and a transitory hyperthyroidism in AIT are rare.
Both the sonographic and scintigraphic detection of autonomous foci in nodular goiters present major difficulties, because multiple confluent nodules give the thyroid a generally hypoechoic appearance in which these echopenic (potentially autonomous) nodules cannot be individually identified.
Special problems arise in cases where autonomously functioning nodules coexist with Graves hyperthyroidism (Marine–Lenhart syndrome, 1% incidence;  14.4 p–r) or autonomous foci develop in a setting of autoimmune lymphocytic thyroiditis (Hashimoto disease;
14.4 p–r) or autonomous foci develop in a setting of autoimmune lymphocytic thyroiditis (Hashimoto disease; 
14.4 s–u). Definitive treatment for hyperthyroidism should be withheld in these cases, and replacement therapy should be instituted despite the hyperthyroidism.
Given the important role of ultrasonography in patients who present with hyperthyroid symptoms, the causes of hyperthyroidism are listed in  14.4 and illustrated with ultrasound images and correlative radionuclide scans. The examples do not include hyperthyrosis factitia (hyperthyroidism induced by the exogenous administration of thyroid hormones) or hyperthyroidism as a paraneoplastic syndrome.
14.4 and illustrated with ultrasound images and correlative radionuclide scans. The examples do not include hyperthyrosis factitia (hyperthyroidism induced by the exogenous administration of thyroid hormones) or hyperthyroidism as a paraneoplastic syndrome.
504
Clinical Classification of Hyperthyroidism
Even isoechoic adenomas, such as hypoechoic microfollicular adenomas, may be autonomous and lead to hyperthyroidism. In nodular goiters as well, the likelihood that adenomatous nodules will develop increases with the age of the goiter. The following types of autonomy can arise:
●Unifocal autonomy (one nodule)
●Bifocal autonomy (two nodules)
●Multifocal autonomy (multiple nodules)
●Disseminated autonomy involving the entire thyroid (rarer than the other types)
Autoimmune thyroid diseases that are associated with hyperthyroidism include the following:
●Immunogenic Graves hyperthyroidism
●Hypertrophic Hashimoto thyroiditis
●de Quervain thyroiditis (including silent thyroiditis)
●Chronic thyroiditis with transient hyperthyroidism or postpartum thyroiditis
●Amiodarone-induced thyroiditis
 14.4 Hyperthyroidism: Ultrasound Findings and Correlative Scintiscans
14.4 Hyperthyroidism: Ultrasound Findings and Correlative Scintiscans
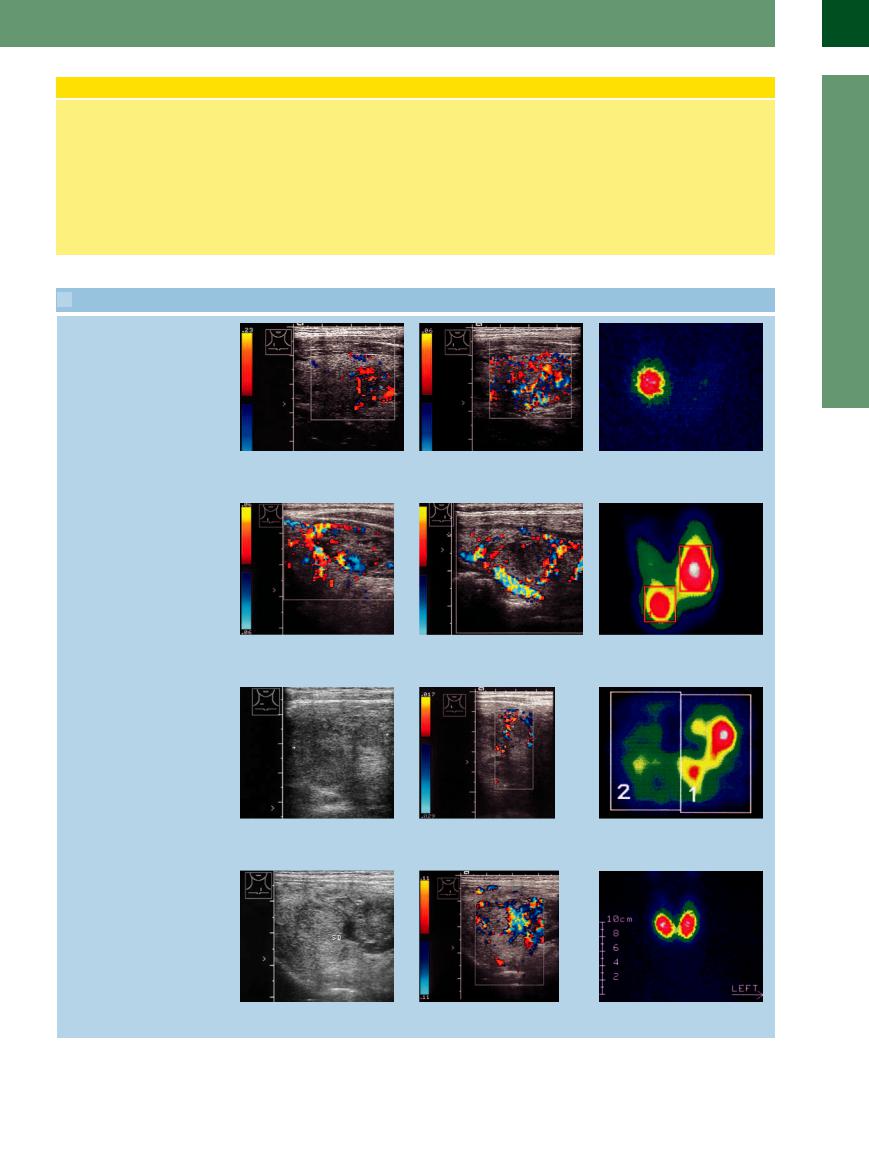
Unifocal autonomy
a Color Doppler scan at a relatively high |
b Scan at a low PRF shows di use |
c Unifocal autonomy on the right side. |
PRF shows circumscribed hypervascularity |
vascularity that is most intense superiorly. |
The other portions of the thyroid are |
only in the area of the adenoma. |
|
suppressed. |
Bifocal autonomy
14
Differential Diagnosis of Hyperthyroidism
d Patchy hypoechoic adenomatous nod- |
e Similar adenomatous nodule in the left |
ule in the lower part of the right lobe (A), |
lobe with peripheral vascularity. |
showing marked peripheral vascularity. |
|
Multifocal autonomy
g Transverse scan on the right side shows |
h Longitudinal scan on the right side |
an irregular, generally hypoechoic back- |
shows a hypoechoic mass with scant |
ground pattern in the right lobe. |
peripheral vascularity. |
Disseminated autonomy
j Nodular goiter (SD). |
k Coarse, patchy vascularity, subtly delin- |
|
eating a nodular lesion. |
f Predominantly left-sided goiter with bifocal autonomy.
i Multifocal autonomy.
l Disseminated autonomy with confirmed hyperthyroidism.
505
14
Thyroid Gland
 14.4 Hyperthyroidism: Ultrasound Findings and Correlative Scintiscans (Continued)
14.4 Hyperthyroidism: Ultrasound Findings and Correlative Scintiscans (Continued)
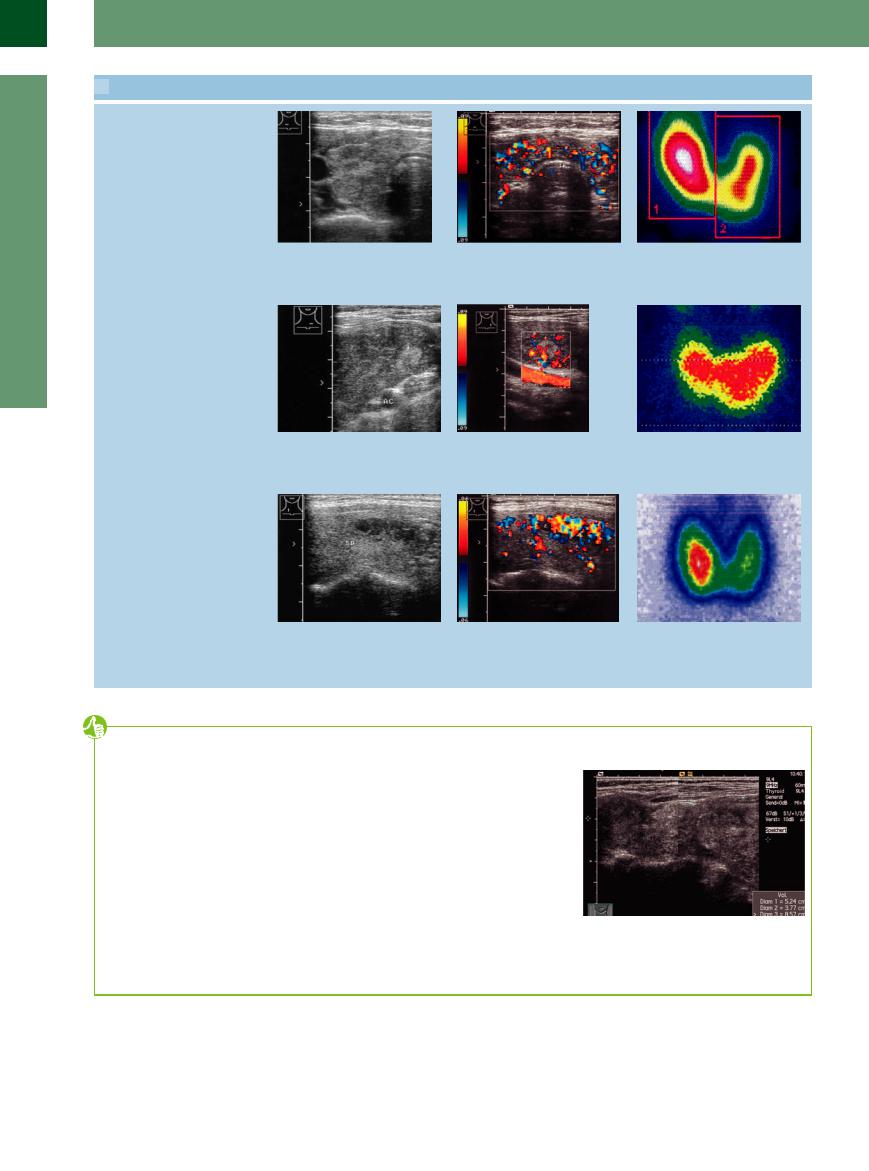
Disseminated autonomy in AIT (PAS type II)
m Right thyroid lobe: coarse, patchy |
n Swelling of the isthmus. Scan at 0.09 m/ |
o Di use goiter predominantly a ecting |
hypoechoic structure. |
s still shows definite vascularity (similar to |
the right lobe. |
|
Graves disease). Maximally high TPO |
|
|
levels, no anti-TSH receptor antibodies. |
|
Graves disease with focal autonomy (Marine–Lenhart syndrome)
p Graves disease: marked hypoechoicity and swelling of the left lobe, also nodules (one marked with an arrow). AC = carotid artery.
q Hypervascularity in one of the nodules, which here appears more reflective than the otherwise hypoechoic thyroid gland. AC = carotid artery.
r Graves thyroid shows increased radiotracer uptake predominantly in the left lobe.
AIT with additional autonomous adenomas
s Hypoechoic thyroid gland (TH) with a circumscribed, anechoic bandlike area on the right side; incipient thyrotoxicosis. Anti-TPO > 1000, anti-TSH receptor antibodies slightly elevated.
t Pronounced hypervascularity in the patchy echo-free lesion in s. The vesselspared area is a cyst (C). The rest of the thyroid also shows increased vascularity.
u Scintiscan shows intense uptake in the right lobe and decreased uptake in the left lobe.
Tips, tricks and pitfalls
●Thyroid gland ultrasonography with high-res- olution probes may yield the appearance of an “inhomogeneous” structure with fine cystic lesions like small vessels or lymph cysts although this is actually a normal finding.
●Small adenomas (< 1 cm) are not visible in radioiodine thyroid scan, therefore this method should not be used; follow-ups with ultrasound are recommended when no hyperthyroidism is present.
●Volume estimation should be done in every thyroid examination; it appears to be accurate enough to be useful and the isthmus is not
measured. The depth may be measured in transverse scan direction in the deepest diameter. Measuring procedures are difficult in large goiters because of the limited probe diameter (4–5 cm), in which case convex probes can be used; alternatively, divided images may be helpful (Fig. 14.65). A volume of over 50 mL results in a less accurate measurement.
●Ultrasonography can demonstrate an impression of the trachea in case of stridor.
Fig. 14.65 Nodular goiter, atypical longitudinal scan left lobe; compound double image: the left thyroid lobe exceeds the image limits, so the length has to be estimated (volume of the left lobe 45 mL, of the whole thyroid ca. 88 mL).
506

References
[1]Braun B, Günther R, Schwerk WB. Ultraschalldiagnostik. Lehrbuch und Atlas. Landshut: Ecomed, 1992
[2]Scherbaum WA. Kongress of Internists Wiesbaden, 1999
[3]Ralls PW, Mayekawa DS, Lee KP, et al. Colorflow Doppler sonography in Graves disease: “thyroid inferno”. AJR Am J Roentgenol 1988; 150(4):781–784
[4]Mann K. Vorsymposium 33. Jahrestagung Deutsche Diabetesgesellschaft Frankfurt, 1999
[5]Baldini M, Castagnone D, Rivolta R, Meroni L, Pappalettera M, Cantalamessa L. Thyroid vascularization by color doppler ultrasonography in Graves’ disease. Changes related to different phases and to the long-term outcome of the disease. Thyroid 1997;7(6): 823–828
[6]Dralle H, Scheumann GFW, Proye C. The value of lymph node dissection in hereditary medullar thyroid carcinoma: a retrospective, European, multicentre study. J Intern Med 1995;238:357–361
[7]Guggenberger D. Ultraschalldiagnostik 94. Dreiländertreffen Basel, 1994
[8]Junik R, Sawicka J, Kozak W, Gembicki M. Thyroid volume and function in patients with acromegaly living in iodine deficient areas. J Endocrinol Invest 1997;20(3): 134–137
[9]Meller J, Zappel H, Konrad M, Roth C, Emmrich D, Becker W. 123 J-Szintigraphie und Per- chlorat-Depletionstest bei der Diagnostik der kongenitalen Hypothyreose. Nuklearmedizin 1998;37(1):7–11
[10]Loviselli A, Bocchetta A, Mossa P, et al. Value of thyroid echography in the long-term fol- low-up of lithium-treated patients. Neuropsychobiology 1997;36(1):37–41
[11]Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 1997;126(3): 226–231
[12]Becker D, Lohner W, Martus P, Hahn EG. Farbdopplersonographische Detektion von fokalen Schilddrüsenautonomien [Color Doppler ultrasonographic detection of focal thyroid nodules]. Ultraschall Med 1999;20(2):41–46
[13]Becker D, Bair HJ, Becker W, et al. Thyroid autonomy with color-coded image-directed Doppler sonography: internal hypervascularization for the recognition of autonomous adenomas. J Clin Ultrasound 1997;25(2): 63–69
[14]Clark KJ, Cronan JJ, Scola FH. Color Doppler sonography: anatomic and physiologic assessment of the thyroid. J Clin Ultrasound 1995;23(4):215–223
[15]Cooper DS, Doherty GM, Haugen BR, et al; American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006;16(2): 109–142
[16]Frates MC, Benson CB, Charboneau JW, et al; Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005;237(3):794–800
[17]Mandel SJ. Thyroid nodules. Endocrine Society 90th Annual Meeting. Meet-The-Profes- sor Sessions. June 15, 2008
[18]Cappelli C, Pirola I, Cumetti D et al. Is the anteroposterior and transverse diameter ratio of nonpalpable thyroid nodules a sonographic criteria for recommending fine-nee- dle aspiration cytology? Clin Endocrinol 2005;63:689–693
[19]Rago T, Vitti P, Chiovato L, et al. Role of conventional ultrasonography and color flowdoppler sonography in predicting malignancy in ‘cold’ thyroid nodules. Eur J Endocrinol 1998;138(1):41–46
[20]Moon WJ, Baek JH, Jung SL, et al; Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011;12(1): 1–14
[21]Schneider AB, Bekerman C, Leland J, et al. Thyroid nodules in the follow-up of irradiated individuals: comparison of thyroid ultrasound with scanning and palpation. J Clin Endocrinol Metab 1997;82(12):4020–4027
14
Differential Diagnosis of Hyperthyroidism
507

