
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
9
Kidneys
Hyperechoic Structure
Kidneys |
Anomalies, Malformations |
|||
|
|
|
||
|
|
|
Diffuse Changes |
|
|
|
|
||
|
|
|
|
Large Kidneys |
|
|
|
|
Small Kidneys |
|
|
|
|
Hypoechoic Structure |
|
|
|
|
Hyperechoic Structure |
|
|
|
|
|
|
|
|
|
Irregular Structure |
|
|
|
Circumscribed Changes |
|
|
|
|
||
Hypoxemic Renal Shock
Diabetic Nephropathy
Acute and Chronic Glomerulonephritis Chronic Pyelonephritis
Analgesic Nephropathy
Septic–Toxic Kidneys Severe Metabolic Disorders
Light-chain Deposition Disease, Waldenström Macroglobulinemia Amyloidosis
Infiltration by Lymphoma Atrophic Kidneys
An increase in echogenicity is to be found in |
renal function: increased echogenicity is corre- |
many acute and chronic renal diseases. It indi- |
lated with the change in renal function (Table |
cates no special nephropathy, but it is an im- |
9.2). |
portant sonographic sign indicating disturbed |
|
Table 9.2 Hyperechoic transformation of the renal parenchyma in kidney diseases |
|
Acute renal diseases |
Chronic renal diseases |
● Metabolic disease (diabetes, paraproteinemia) |
● Amyloidisis |
● Hypoxemic renal shock |
● Diabetic nephropathy |
● Nephrotic syndrome |
● Chronic glomerulonephritis |
● Acute glomerulonephritis |
● Chronic interstitial nephritis |
● Acute pyelonephritis (focal abscess) |
● Analgesic nephropathy |
● Hydronephrosis |
● Nephrosclerosis: hyperechoic vascular wall thickness, only some |
● Interstitial nephritis |
vascular spots; increased RI |
● After furosemide |
|
● Acute nephritis |
|
Hypoxemic Renal Shock
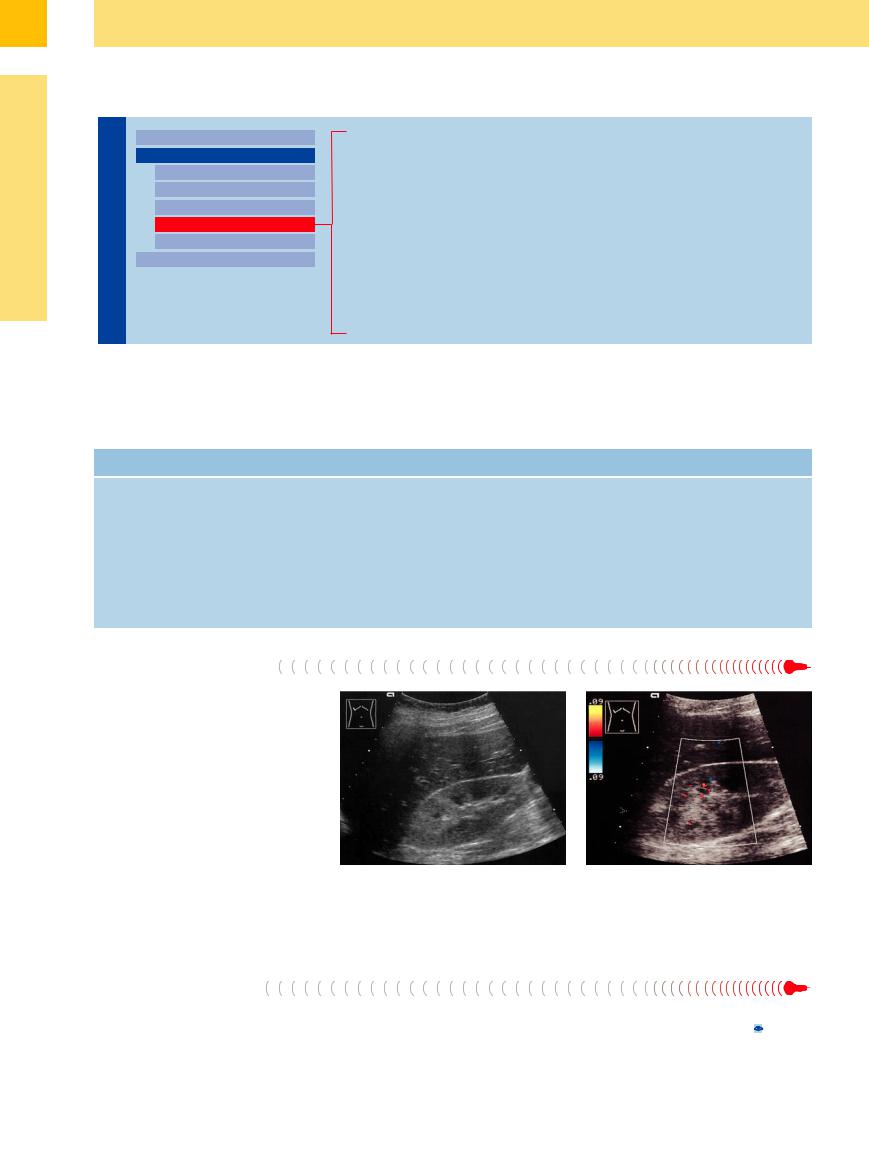
A septic–toxic illness or hypoxemia can cause severe kidney damage with renal failure, leading to subtle but definite changes consisting of a diffuse increase in cortical echogenicity and very echopenic medullary pyramids. These changes are caused by diffuse tubular damage and necrosis, and also by decreased vascularization in cases with severe hypoxemia, so that hypovascular or avascular areas are found in color Doppler scanning (Fig. 9.40).
|
|
|
Fig. 9.40 Hypoxemic renal shock in the setting of acute |
b Color Doppler shows scant color signals, reflecting the |
|
|
|
cardiac and renal failure. The patient was resuscitated for |
marked decrease in vascularity. |
|
|
|
ventricular fibrillation (after sniffing butane gas, with a |
|
|
|
|
fatal outcome). |
|
|
|
|
a The parenchyma is broadened and hyperechoic, result- |
|
|
|
|
ing in a narrow sinus echo complex. Prominent hypo- |
|
|
|
|
echoic medullary pyramids. |
|
Diabetic Nephropathy |
|
|
||
Today, diabetic nephropathy has become the |
drome), shows increased cortical echogenicity |
the medullary pyramids stands in sharp con- |
||
most frequent indication for hemodialysis. Re- |
due to histopathological changes in basement |
trast to the very echogenic cortex ( 9.1; Table |
||
nal ultrasound examination in glomerular dis- |
membrane collagenization, glomerular en- |
9.3, Table 9.4). |
||
eases, |
including |
diabetic glomerulosclerosis |
largement, mesangial cell proliferation, and |
|
(also |
known as |
Kimmelstiel–Wilson syn- |
subsequent glomerulosclerosis. The lucency of |
|
338
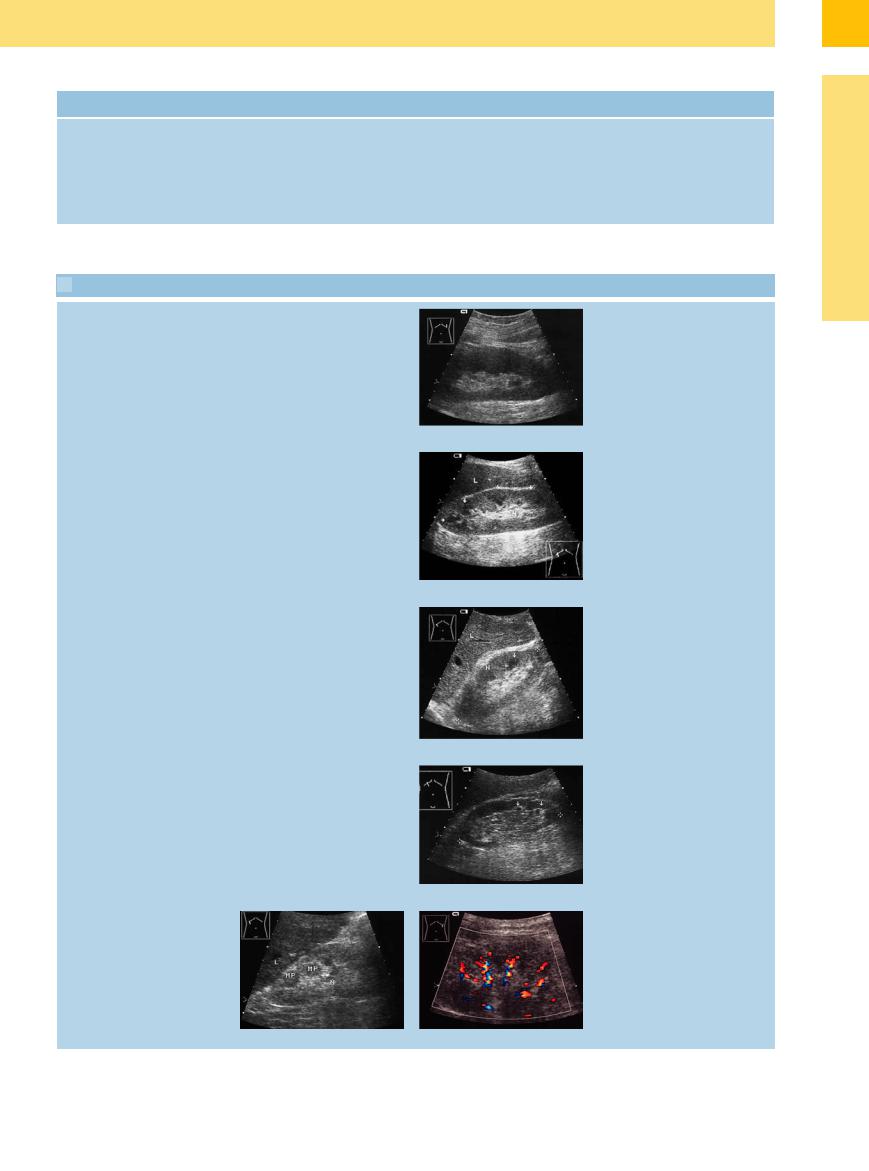
Table 9.3 Sonographic changes seen in different stages of diabetic nephropathy
Early stage |
Advanced stage |
● Renal enlargement (volume increase) |
● Normal renal size with rarefied parenchyma |
● Increased cortical echogenicity |
● Increased cortical echogenicity |
● Markedly hypoechoic medullary pyramids |
● Cystic transformation or disappearance of |
|
medullary pyramids |
|
● Wavy contours |
 9.1 Diabetic Nephropathy3
9.1 Diabetic Nephropathy3
Stages I and II |
Hyperperfusion with a volume increase |
Stage III |
Incipient nephropathy with microalbumi- |
|
nuria (hyperperfusion with a volume |
|
increase) |
Stage IV |
Overt nephropathy (proteinuria) |
Stage V |
Renal failure (nephrotic syndrome) |
Stage V with dialysis
End stage
●Small kidneys
●Intensely hyperechoic parenchyma
●Disappearance of medullary pyramids
●Secondary cysts, calcifications
a Large kidneys, normal structure.
b Enlarged kidneys (N), normal width, incipient hyperechoic cortex, prominent hypoechoic medullary pyramids (arrows). L = liver.
c Normal-size kidneys (N), parenchymal thickness still normal, increased cortical echogenicity; broadened, hypoechoic medullary pyramids.
d Renal size still normal, rarefied cortex, markedly hypoechoic “cystic” pyramids with echogenic demarcation.
e and f Small kidneys (N) (8.7 cm), rarefied cortex, degeneration or disappearance of the medullary pyramids (MP), parenchymal destruction (possible secondary cysts, calcifications). Spotty, deformed vascular segments. L = liver.
9
Diffuse Changes
339

9
Kidneys
Acute and Chronic
and Chronic Glomerulonephritis
Glomerulonephritis


































Both acute and chronic (bilateral) glomerulo- |
glomerulonephritis, normal-sized in chronic |
The usually pronounced increase in echoge- |
nephritis are associated with increased cortical |
glomerulonephritis, and smaller in later stages. |
nicity is caused by histological changes. It has |
echogenicity, which is more distinct than in |
The medullary pyramids are prominent, |
been shown that cortical echogenicity corre- |
diabetic nephropathy.7 Again, the medullary |
whereas in later stages the surface becomes |
lates with histological criteria such as sclerosis, |
pyramids appear markedly hypoechoic relative |
undulated. In the dialysis stage, secondary |
tubular atrophy, hyaline casts, and focal leuko- |
to the cortex. The kidney is enlarged in acute |
cysts are frequent (Fig. 9.41, Fig. 9.42). |
cytic infiltration (Table 9.4).8 |
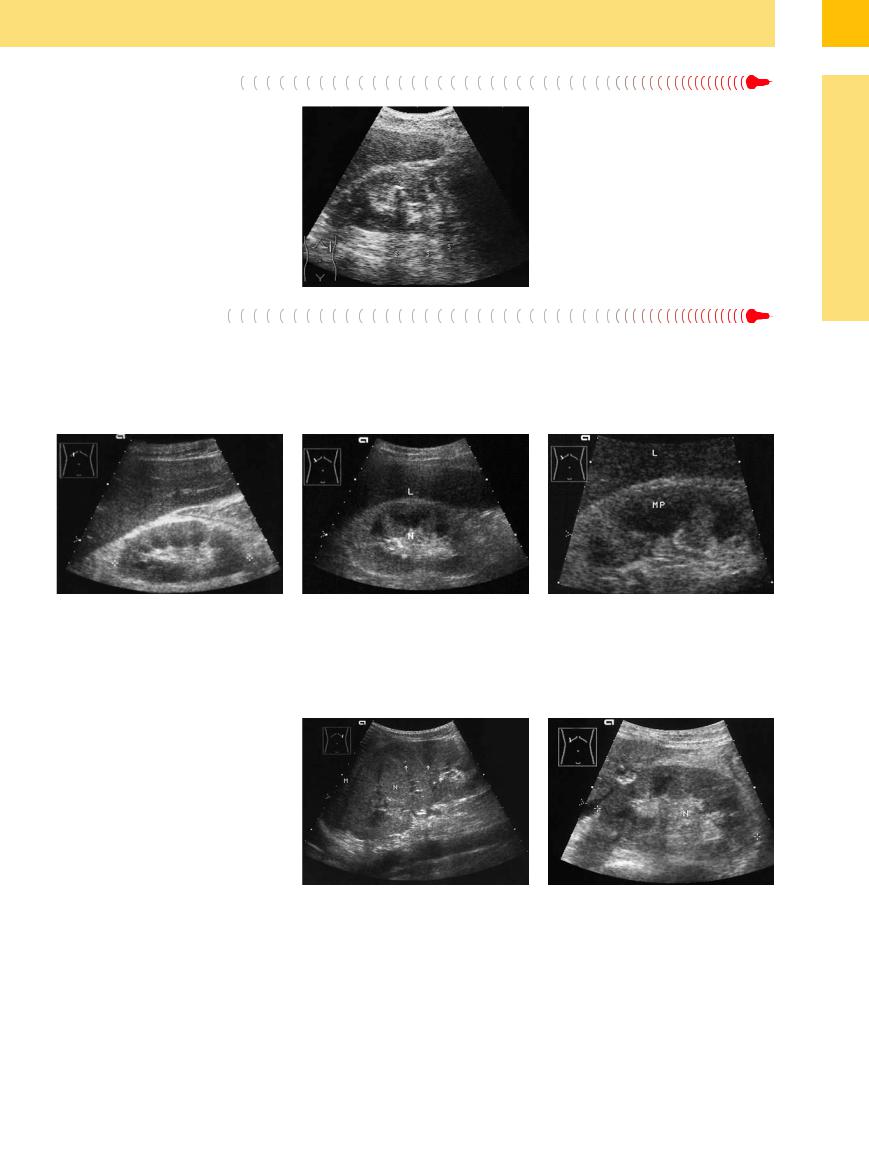
Fig. 9.41 Acute glomerulonephritis: enlarged kidneys (N) |
Fig. 9.42 |
with markedly increased cortical echogenicity and very |
a Chronic glomerulonephritis (prior history of rheumatic |
prominent, hypoechoic medullary pyramids (arrows). |
fever) and chronic renal failure: small kidney (N), hyper- |
|
echoic cortex, disappearance of the medullary pyramids, |
|
shallow notches due to scarring (arrows). L = liver. |
Chronic Pyelonephritis 
























Chronic pyelonephritis is also associated with |
tory conditions listed above, and scarring leads |
increased echogenicity. The kidney is generally |
to areas of parenchymal thinning (Fig. 9.43, |
small, however, compared with the inflamma- |
Table 9.4). |
b Membranous glomerulonephritis due to chronic hepatitis C. Markedly increased echogenicity of the renal cortex compared to the liver structure, normal size (cursors). LE = liver; N = kidney.
Table 9.4 Histopathology of chronic diabetic, glomerular and interstitial nephropathy
Diabetic nephropathy |
Early stage |
|
● Glomerular enlargement, mesangial cell proliferation |
|
● Basement membrane collagenization |
|
Late stage |
|
● Glomerulosclerosis |
|
● Broadened mesangium with deposits |
|
● Arteriosclerosis |
|
● Glycogen storage in tubules |
|
● Papillary tip necrosis |
Chronic glomerulonephritis |
● Mesangial cell proliferation |
|
● Glomerular enlargement |
|
● Basement membrane thickening |
|
● Mesangial, capillary, tubular deposits |
|
● Cellular infiltrates |
Chronic pyelonephritis |
● Interstitial scarring |
|
● Lymphoplasmocytic infiltrates |
|
● Tubular atrophy |
|
● Tubular protein deposits |
|
● Scarring of the pyelocaliceal system |
Analgesic nephropathy |
● Papillary necrosis |
|
● Broadening of the capillary basement membrane in the |
|
renal medulla (capillary sclerosis) |
|
● Lipofuscin deposits in tubules |
|
● Lymphoplasmohistiocytic infiltrate with fibrosis, sclerosis |
Fig. 9.43 Old chronic pyelonephritis: small kidney with multiple echogenic sites of parenchymal retraction due to scarring.
340
Severe septic–toxic disease states are characterized by a markedly hyperechoic, swollen renal cortex. These changes are usually associated with renal enlargement. Direct septic– toxic tissue effects such as necrosis, lympho-
leukocytic infiltration, or ischemic vascular changes can occur. Similar changes are found in acute tubular obstruction by urates in the chemotherapy of malignant lymphoma, also in acute renal failure, lupus erythematosus, he-
Fig. 9.44 Analgesic nephropathy: nonhomogeneous, hyperechoic parenchyma with ill-defined contours. Note the hyperechoic areas at the tips of the medullary pyramids (calcifying papillary necrosis, acoustic shadows S).
molytic–uremic syndrome, and acute glomerular renal diseases. After the systemic manifestations have subsided, the diffuse structural changes also regress (Fig. 9.45, Fig. 9.46).
9
Diffuse Changes
Fig. 9.45 Septic–toxic abdomen: normal-size kidney with increased cortical echogenicity and prominent hypoechoic medullary pyramids.
Fig. 9.46 Septic–toxic renal failure.
a The kidney (N) shows greatly increased echogenicity relative to the liver (L). Note the prominent, hypoechoic medullary pyramids.
b Enlarged view shows massive swelling and decreased echogenicity of the medullary pyramids (MP).
Severe Metabolic Disorders













































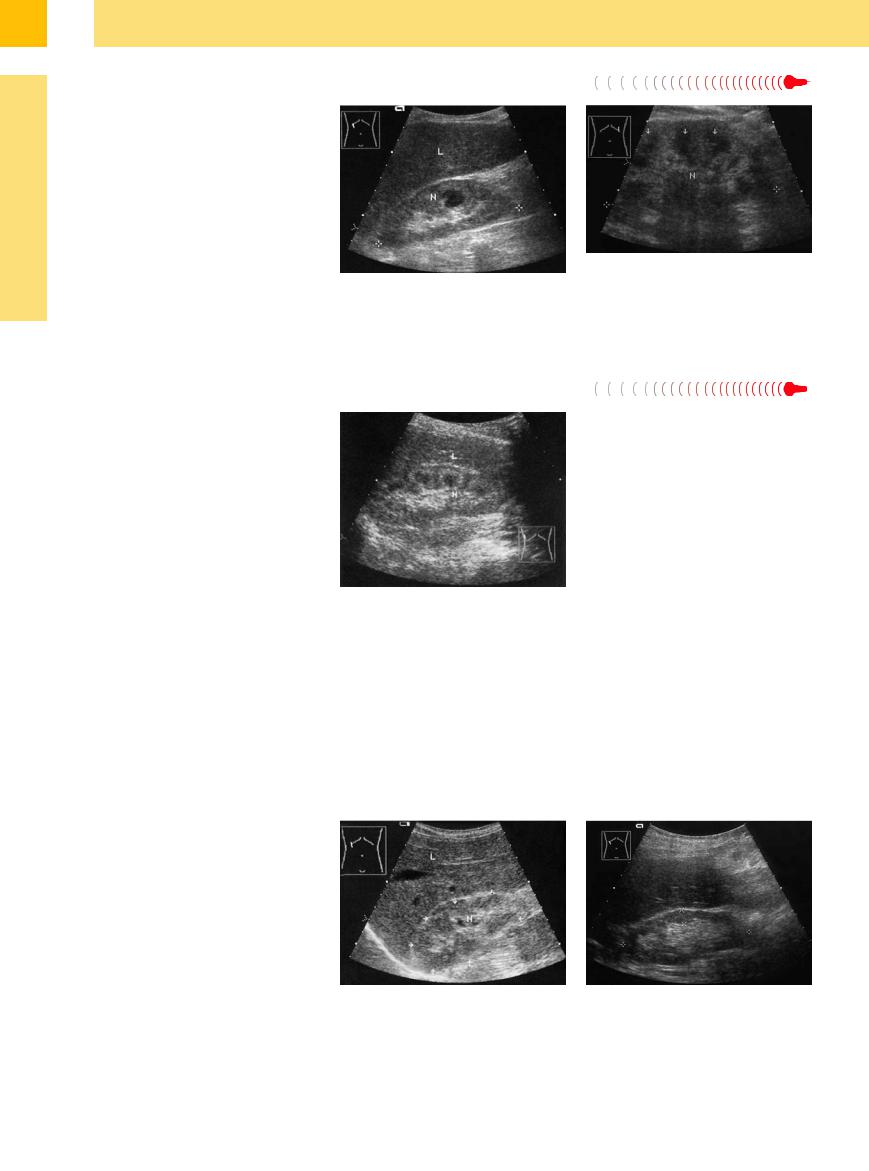
Severe metabolic disorders such as untreated diabetes mellitus, with or without diabetic coma, are occasionally associated with pronounced renal structural changes. Possible causes are severe dehydration, tubular glycogen storage or other storage disorders, as well as glomerular/tubular changes that do not always have a discernible cause, especially if the case cannot be evaluated histologically (Fig. 9.47, Fig. 9.48).
Fig. 9.47 Neglected case of type I diabetes mellitus: massive parenchymal swelling, increased echogenicity (relative to the spleen, M), prominent hypoechoic pyramids (arrows). Lateral flank scan displays the aortic band at the bottom of the image. N = kidney.
Fig. 9.48 Toxic renal failure secondary to extensive thrombosis of the portal venous system: large, echogenic kidney (N), prominent hypoechoic pyramids, perirenal ascites.
341
9
Kidneys
Light-chain Deposition Disease, Waldenström
Deposition Disease, Waldenström Macroglobulinemia
Macroglobulinemia
Light-chain deposition disease is a nonspecific condition that leads to renal failure based on the precipitation of light-chain paraproteins in the distal tubules, a hypercalcemic tubulopathy in hypercalcemia syndrome, and/or an accompanying deposition of amyloid. Similar changes are encountered in Waldenström macroglobulinemia and amyloid kidney.
Ultrasound may demonstrate renal enlargement and increased cortical echogenicity. Often there is only mild enlargement with an indistinct parenchymal texture (Fig. 9.49).
Fig. 9.49 Light-chain deposition disease, hypercalcemic crisis, renal failure.
a Right kidney (N): parenchyma has a somewhat indistinct structure and appears slightly hyperechoic to the liver (L). Prominent medullary pyramids.
b Acute renal failure requiring dialysis in a patient with paraproteinemia (and initial hypercalcemia) and a plasmacytoma. The kidneys are still of normal size (N, cursors). The parenchyma shows markedly increased echogenicity with enlarged, hypoechoic to anechoic medullary pyramids (arrows).
Amyloidosis 






























Amyloidosis is also associated with an increase in cortical echogenicity and prominent hypoechoic pyramids. We see, then, that these diffuse structural changes occur in a variety of diseases and cannot be considered specific sonographic features; indeed, they are very nonspecific. Only if the underlying disease is known can the echogenicity changes be referred to a specific renal complication. In the beginning the kidneys are normal in size and markedly hyperechoic. In later stages they become smaller and even diminutive at the dialysis stage (Fig. 9.50).
Fig. 9.50 Presumed renal amyloidosis: echogenic renal cortex with increased parenchymal volume causing slight narrowing of the sinus echo complex. Hypoechoic, illdefined medullary pyramids (rheumatoid arthritis, renal failure with nephrotic syndrome). L = liver; N = kidney.
Infiltration by Lymphoma
by Lymphoma














































The infiltrating lymphomatous tissue leads to renal enlargement (see above). Increased echogenicity may be seen, but the bulges in the renal outline (frequently heterogeneous in structure) and irregular tumor vascularity serve to distinguish this disease from those listed above (Fig. 9.32, Fig. 9.79).
Atrophic  Kidneys
Kidneys 


















































Generally the detection of renal atrophy does not point to a specific cause (Fig. 9.51), although bilateral atrophic kidneys are more likely to have a diabetic etiology (only in the late dialysis stage) or a glomerular cause, or may result from analgesic nephropathy.
Unilateral renal atrophy, or bilateral atrophy that differs in severity between the sides, usually has a different cause such as pyelonephritis or vasopathy (Fig. 9.52).
As the name implies, the kidneys appear small in ultrasound. The parenchyma is thinned, and the renal outlines are smooth (glomerular, diabetic) or wavy (pyelonephritis
Fig. 9.51 Small, hyperechoic right kidney (N, arrows) due to an unknown cause. Slight structural irregularity. L = liver.
Fig. 9.52 Renal atrophy (85 mm) due to arteriosclerosis and arteriolosclerosis: irregular, rarefied, slightly hyperechoic parenchyma (cursors) with a correspondingly broadened sinus echo.
342
