
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
■ Focal Changes
Focal changes may display the whole gamut of |
matory or tumorous. Every so often these focal |
most important of these focal changes. It can- |
echogeneity from anechoic to hyperechoic and |
changes are the result of papillotomy or ductal |
not be excluded from diagnostic consideration |
heterogeneous lesions. They may correspond |
procedures, which can be confirmed quite |
unless another origin of the lesion in question |
to anomalies or variants, and may be inflam- |
easily from the patient’s history. Cancer is the |
has been proven. |
Anechoic Lesion
|
|
|
Diffuse Pancreatic Change |
|
Pancreas |
||||
Hypoechoic Lesion |
||||
|
|
|
Focal Changes |
|
|
|
|
Anechoic Lesion |
|
|
|
|
Isoechoic Lesion |
|
|
|
|
Hyperechoic Lesion |
|
|
|
|
Irregular (Complex Structured) Lesion |
|
|
|
|
Dilatation of the Pancreatic Duct |
|
|
|
|
||
Cysts
Pseudocysts
Fluid Collections/Necrosis
Vessels/Duct System
Table 4.4 lists the most important differential diagnoses of the anechoic pancreatic mass.
Table 4.4 Differential diagnosis of the anechoic pancreatic mass |
|
||
Congenital cyst |
Retention cyst |
Necrosis |
Pseudocyst |
● Anechoic |
● Anechoic |
● Anechoic/ |
● Anechoic/ |
● Round |
● Round or polygonal |
hypoechoic |
heterogeneous |
● Enhancement |
● Frequently < 1 cm |
● Polycyclic |
● Almost round |
● Liver/kidney cysts |
together with: |
● Mottled or banded |
● Echogenic wall |
|
● Fibrosis, calcifica- |
● No halo |
|
|
tion, ductal ectasia |
|
|
Cysts
Congenital cyst. Congenital cysts may be solitary or multiple; in the latter case they are more frequently accompanied by polycystic kidney disease. Compared with cysts secondary to inflammation or the frequent cysts in chronic pancreatitis, they are extremely rare and incidental findings. They are characterized by an epithelial lining.
Retention cyst. Retention cysts are small, come in multiples, and are usually found together with other signs of chronic pancreatitis. They are filled with clear pancreatic secretion.
Hydatid cyst. Intrapancreatic hydatid cyst is a true rarity; as is the case with their hepatic counterparts, these cysts will display a hyperechoic inflammatory wall.
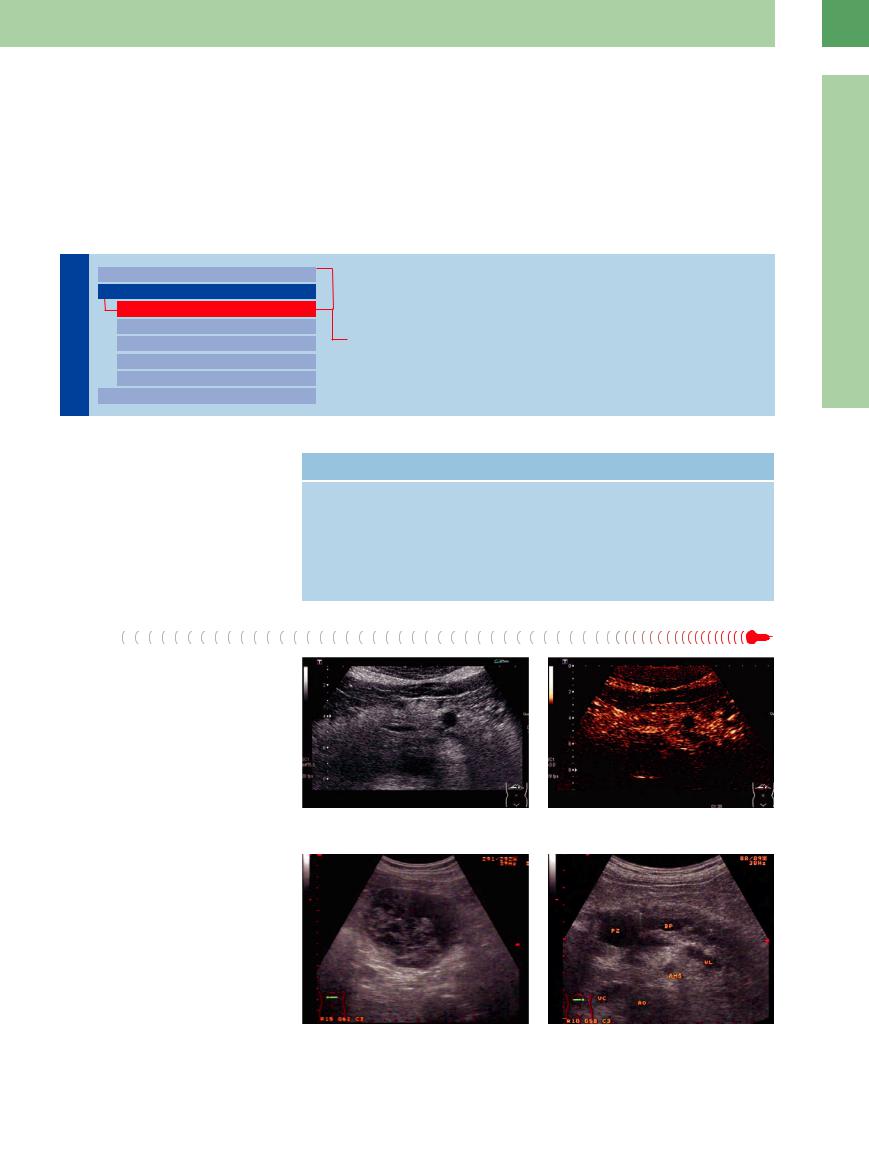
Ultrasound findings. Ultrasound studies of congenital cysts will demonstrate the characteristic criteria of cysts and show them to be anechoic. Retention cysts are also anechoic, often with irregular texture, and frequently hard to differentiate from other anechoic structures such as cross-sectional views of the splenic artery, ectatic pancreatic duct with stricture, dilated prepapillary CBD, and intraparenchymal necrosis (Fig. 4.22a–d).
Fig. 4.22
a Pancreatic cyst. Anechoic mass in the pancreatic body.
c Lymphoepithelial cyst. Complex cystic mass.
b CEUS: lack of enhancement within the anechoic mass.
d Chronic pancreatitis with small pseudocyst (PZ) in the head. DP = pancreatic duct, VL = splenic vein, AO = aorta, VC = vena cava, AMS = superior mesenteric artery.
4
Focal Changes
179
4
Pancreas
pseudocysts may resolve and vanish. The rate of regression after acute pancreatitis is 50% within 6 weeks. The classification of pancreatic pseudocysts is shown in Table 4.5.
Ultrasound findings. Some pseudocysts are extremely large, filling most of the abdomen, and ultrasound may not be able to retrace them to the pancreas with certainty. Anechoic pseudocysts are rare; in most cases, they are filled with hyperechoic irregular intrinsic structures that (in infection, incidence 5%) are rooted in pus and detritus or necrosis and blood clots (Figs. 4.23, 4.24, 4.25).
In the differential diagnosis pseudocysts have to be considered separately from cystic tumors; distinctive features are listed in  4.3
4.3
(see Table 4.8).
Pathomorphology of Pancreatic Cysts |
|
|
True cysts |
|
Pseudocysts |
● Congenital cysts. Their wall is made up of |
● Pseudocysts are the result of self-diges- |
|
simple epithelium, as in liver and kidney |
tion of the pancreas in trauma or inflam- |
|
cysts, and they are frequently found in |
mation. |
|
conjunction with them. |
● The walls of pseudocysts arise from the |
|
● Retention cysts. These represent ectatic |
reaction of the surrounding tissue to the |
|
duct and branch segments and are filled |
accumulation of inflammatory fluid. |
|
with clear pancreatic juice. |
● Pseudocysts are most often filled with |
|
● Neoplastic cysts. These originate from |
greenish or hemorrhagic cloudy secre- |
|
cystadenomas or cystadenocarcino- |
tion. |
|
mas; their walls and septae made up |
|
|
of neoplastic epithelium and contain tu- |
|
|
mor vessels. |
|
|
Table 4.5 Classification of pancreatic pseudocysts16 |
|
|
Type I |
Pseudocysts in acute necrotizing pancreatitis: |
|
|
● usually, no communication with pancreatic duct |
|
|
● normal ERCP |
|
Type II |
Pseudocysts after an acute episode of chronic pancreatitis: |
|
|
● often, communication with pancreatic duct |
|
|
● ERCP: irregular duct, no stenosis |
|
Type III |
Retention cysts in chronic pancreatitis: |
|
|
● internal communication with pancreatic duct |
|
|
● ERCP: significant stenoses |
|
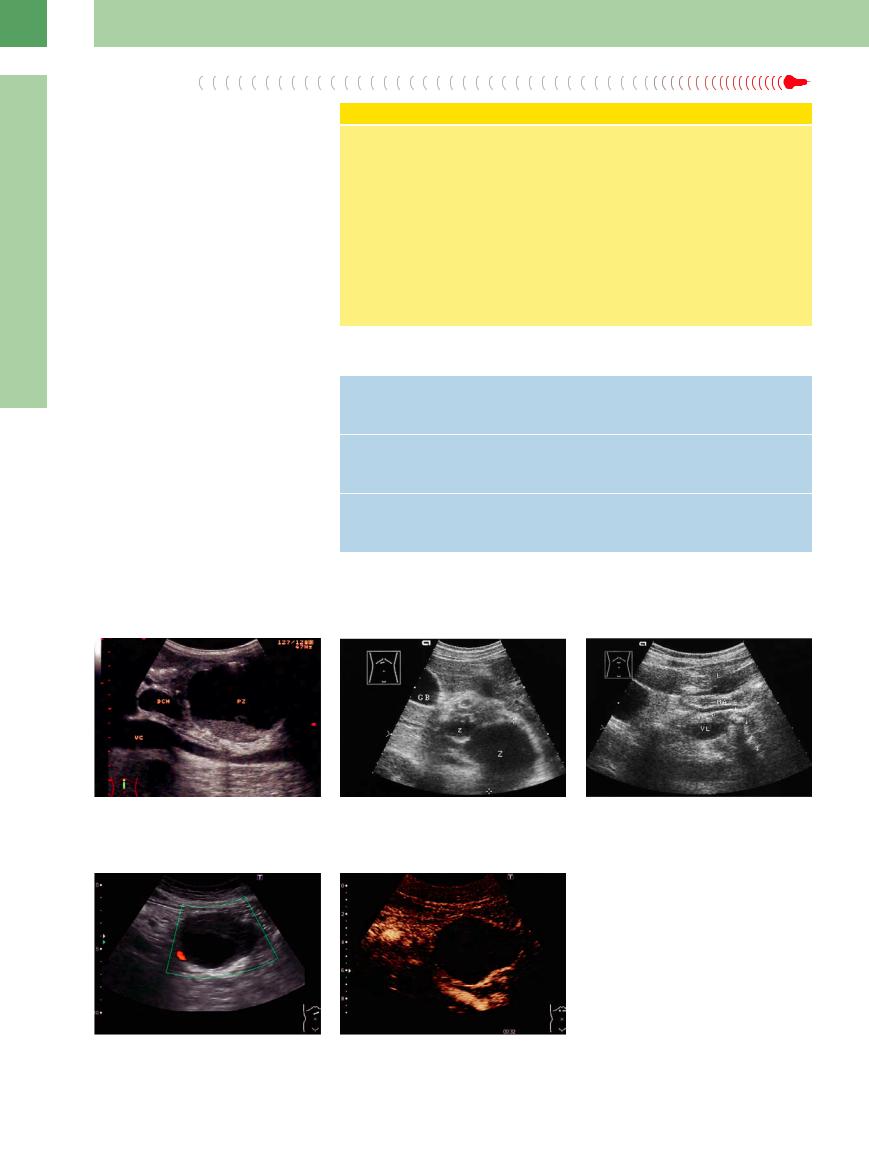
Fig. 4.23 Symptomatic macro-pseudocyst (PZ) with sediment, compressing the main bile duct (DHC). VC = vena cava.
Fig. 4.24
a Microcyst (z) and macrocyst (Z) type II after an acute episode of chronic pancreatitis: anechoic mass in the body and tail of the pancreas. GB = gallbladder.
b Same patient as in a. After 5 months, spontaneous resolution of the pseudocysts; hyperechoic calcification, shadowing (arrows). L = liver; MA = stomach; P = pancreas; VL = splenic vein.
Fig. 4.25 Differential diagnosis: pseudocyst or cystic tumor?
a Power Doppler sonography: no vessels inside.
b CEUS: no enhancement, so a neoplasm can be ruled out (cf. Fig. 4.59).
180

Fluid Collections/Necrosis
As the term implies, the severe course of acute (“hemorrhagic necrotizing”) pancreatitis will produce necrosis within a few days of the onset of the disease. Frequently, it is accompanied by collections of peripancreatic fluid, especially in the lesser sac as circumscribed anechoic masses; peripancreatic hyperechoic masses correspond most likely to fatty necroses, ascites (around spleen, liver, kidney, and in the lower abdomen/rectouterine pouch), and pleural effusion of the left lung (Figs. 4.26, 4.27, 4.28).
The Atlanta classification proposes another classification of cystic lesions in acute and
acute relapsing pancreatitis with regard to the complications:
1.Acute fluid collections (intrapancreatic or peripancreatic)
2.Pancreatic necroses
3.Infected necroses
4.Acute pseudocyst (after 4 weeks)
5.Chronic pseudocyst (in chronic pancreatitis; high enzyme level)
6.Pancreatic or peripancreatic abscess
The differentiation between acute edematous and hemorrhagic necrotizing pancreatitis is based on histopathological criteria. However, native ultrasound or, better, CEUS (as also in
CT angiography) can differentiate the structures that indicate whether tissue is vital or nonvital. In the early stages CT studies may therefore be useful as a second imaging procedure for staging and in obtaining the baseline status when an intervention or surgical operation may be an option.
Abscess. Most abscesses arise from infected necrosis; early fine-needle aspiration cytology and culturing of the necroses are necessary in order to decide on possible interventions or surgery. Fever and septic temperature course are indicative of abscess formation (Fig. 4.29).
4
Focal Changes
Fig. 4.26 Severe pancreatitis, necrosis in the body and tail. |
Fig. 4.27 Necrotizing severe pancreatitis. |
Ventrally displaced stomach. WS = vertebral column, VC |
a Gray-scale US: macropseudocyst (PZ), supposed |
= vena cava, AO = aorta. |
thrombosis of the splenic vein (VL). |
Vessels/Duct System
System 
















































Vessels. The splenic and gastroduodenal arteries might be mistaken for microcysts, and the splenic vein for the pancreatic duct (Figs. 4.30, 4.31). Aneurysms of the splenic artery are rare and can be reliably differentiated from cysts by CDS (differentiation of pseudoaneurysms from pseudocysts).
Ductal cysts. The pancreatic duct has numerous variants. In most patients it is found offcenter at the junction between the middle and posterior thirds of the pancreas. It turns caudad in the pancreatic head, and in stenosis of the papilla of Vater it can be traced as a dilated tube running in parallel with the CBD. The cross-sectional view of both ductal systems
b CDS: thrombosis of the splenic vein (VL); pseudocyst (PZ) without color coding. CF = venous confluence, AO = aorta, VC = vena cava, WS = vertebral column.
Fig. 4.28 CDS: severe necrotizing pancreatitis, peripancreatic extended, fluid collection (bursa omentalis), incipient pseudocyst.
Fig. 4.29 Peripancreatic abscess after pancreatitis (patient hospitalized because of septic temperatures, no clinical sign of previous pancreatitis): irregularly defined hypoechoic abscess formation (A) below the liver with adjacent inflammatory lymphadenopathy (LK); paracaval (VC) view high in the epigastrium; evacuated by ultrasoundguided fine-needle aspiration.
will visualize them as round cystic structures. CDS identifies them as ducts and not blood vessels.
Dilated segments of the pancreatic duct due to ductal obstruction in chronic pancreatitis may mimic cysts and be easily misdiagnosed ( 4.1h, Fig. 4.32).
4.1h, Fig. 4.32).
181
4
Pancreas
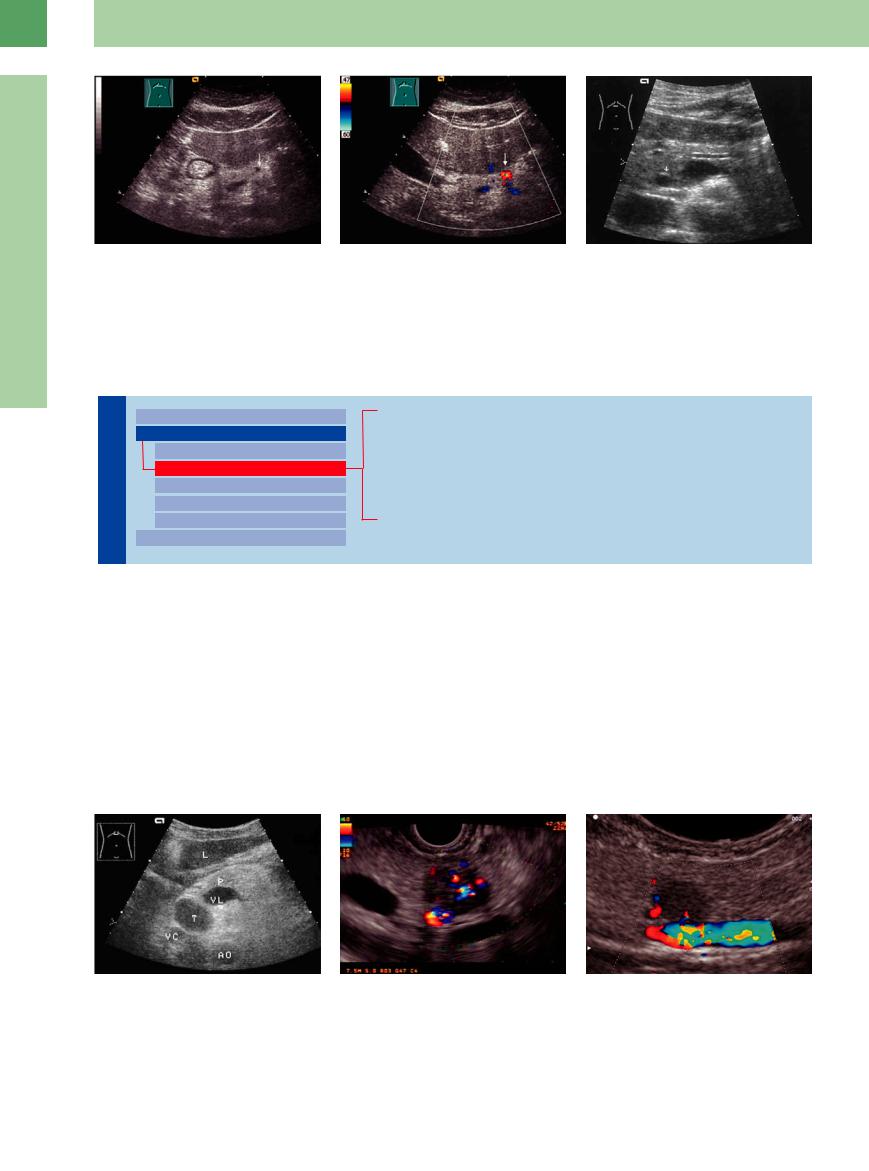
Fig. 4.30 Anechoic cystic structures in the body of the pancreas (arrow).
Fig. 4.31 Color-flow Doppler scan demonstrates the splenic artery.
Fig. 4.32 Dilated prepapillary CBD (arrow) within the head of the pancreas in chronic pancreatitis. Borderline pancreatic duct (3.2 mm).
Hypoechoic Lesion
|
|
|
Diffuse Pancreatic Change |
|
Pancreas |
||||
Hypoechoic Lesion |
||||
|
|
|
Focal Changes |
|
|
|
|
Anechoic Lesion |
|
|
|
|
Isoechoic Lesion |
|
|
|
|
Hyperechoic Lesion |
|
|
|
|
Irregular (Complex Structured) Lesion |
|
|
|
|
Dilatation of the Pancreatic Duct |
|
|
|
|
||
Neuroendocrine Tumors
Pancreatic Cancer
Metastasis, Malignant Lymphoma, Inflammatory Lymph Node
Abscess
Hemorrhagic/Infected Cyst/Pseudocyst
Focal (Segmental) Pancreatitis/Ventral Anlage
Neuroendocrine Tumors
Tumors 














































Neuroendocrine tumors are rare intrapancreatic tumors, stemming from the neural crest, with possible endocrine activity. Because the pancreas houses a large number of endocrine cells, endocrine tumors may arise here, particularly insulinoma, a mostly benign tumor, while gastrinoma as a rule can be detected only after it has transformed into malignancy. Even rarer entities are glucagonomas, VIPomas, somatostatinomas, and carcinoid.
Because of their small size (< 2 cm), insulinomas are hardly ever visualized on transcutaneous ultrasound; the clinical diagnosis in the fasting state can be confirmed by direct visualization in EUS.
In general, endocrine tumors are detectable in transcutaneous ultrasound studies only with an accuracy of about 60% or less, when tumors are less than 1 cm in size. Ultrasound studies show endocrine tumors to be hypoechoic,
round, and smoothly delineated, and in contrast to metastases and other tumors they demonstrate marked hypervascularity in CDS and hyperenhancement in CEUS.9 The pancreatic duct is not obstructed (Fig. 4.33b,c).10 EUS is the most sensitive imaging modality in the diagnosis of neuroendocrine tumors, but further differentiation by ultrasonography is not possible.
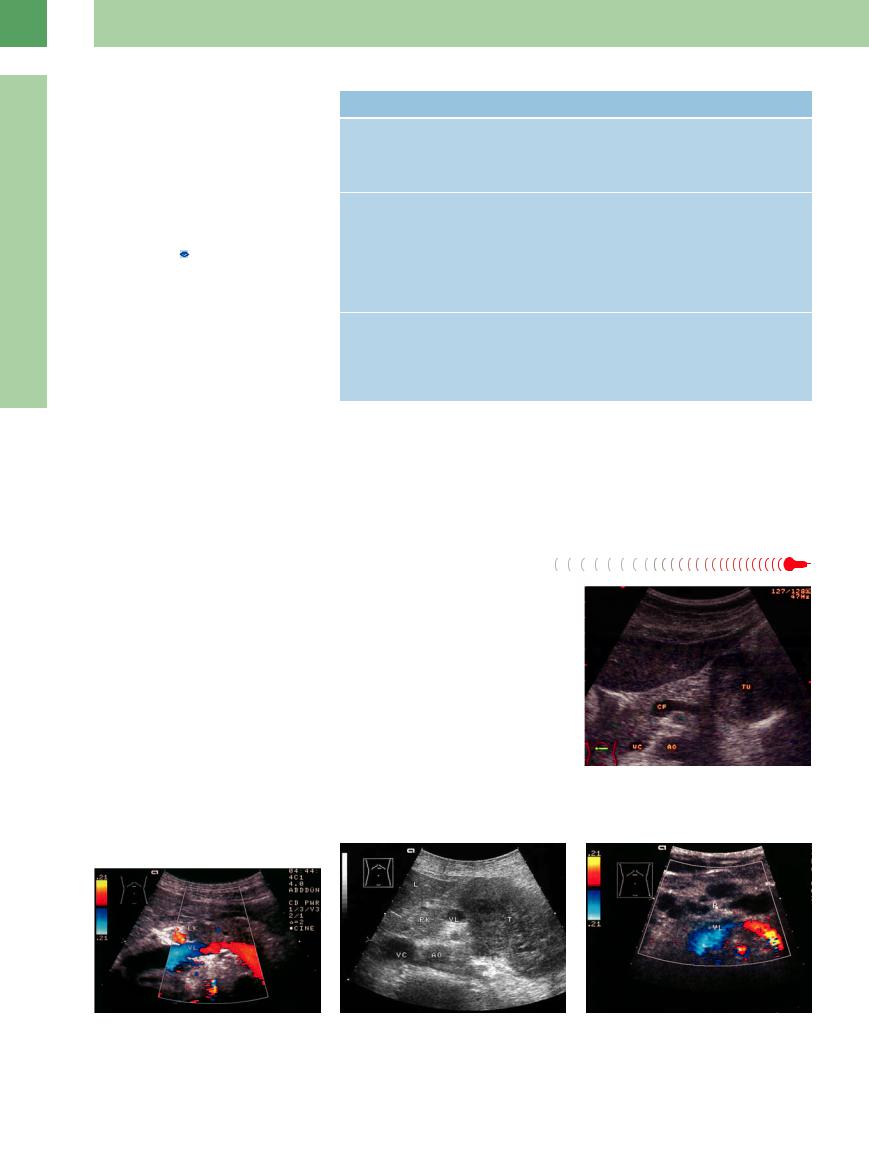
Fig. 4.33
a Metastasizing neuroendocrine tumor (T) of the head of the pancreas. AO = aorta; L = liver; P = pancreas; VC = vena cava; VL = splenic vein.
b Insulinoma in the tail of the pancreas, 8 mm in diame- |
c Gastrinoma, 5 mm body of the pancreas (CDS; EUS).13 |
ter. Also visualized is the splenic vein. CDS; EUS.13 |
|
182

Pancreatic  Cancer
Cancer


















































Pancreatic cancer is a focal, defined, solid lesion. Sonographically it appears hypoechoic with irregular margins, and in CDS no detectable vessels. In CEUS, ductal carcinoma is hypoenhanced compared to the adjacent pancreatic tissue in all phases. Indeed, some rare undifferentiated carcinomas (ca.10%) demonstrate good vascularization and therefore are distinct from endocrine tumors and metastases (e. g., renal cell carcinoma). Indications for the use of CEUS in pancreatic tumors are also to improve the depiction of the dimensions and margins, including relationship of the tumor to adjacent vessels, although not to give a definite answer to the question of resectability ( 4.2).5
4.2).5
The most important criterion of malignancy is an irregular margin, in addition to the bulging contour; other diagnostic characteristics are tumor spread, cut-off of the pancreatic duct, displacement, invasion, and locoregional as well as distant metastasis. Ductal pancreatic cancer arises from the epithelium of the
Histological Classification of Pancreatic Cancer
Benign Pancreatic Tumors
●Adenomas, which are quite rare and arise from the acinar or centroacinar cells
●Cystadenomas with cystic and solid parts: cystadenomas are classified into mucinous, macrocystic adenoma (at the time of establishing the diagnosis mostly benign or borderline lesion with tendency to malignancy) and the rather rare microcystic benign cystadenoma
●Mesenchymal tumors (hemangioma, lipoma)
●Endocrine tumors (insulinoma, gastrinoma, VIPoma, and glucagonoma). Insulinoma and gastrinoma are the most common endocrine tumors; they rarely
exceed 1–2 cm and 2–4 cm in diameter, respectively.
Malignant Tumors
●Ductal carcinomas; accounting for 80% of pancreatic malignancies; they arise from the epithelium of the duct and originate primarily in the head of the pancreas. There are several types:
–Tubular adenocarcinoma
–Mucinous adenocarcinoma
–Adenosquamous carcinoma
–Squamous cell carcinoma
–Pleomorphic giant cell type carcinoma
●Endocrine tumors
●Acinar cell carcinoma
 4.2 Pancreatic Cancer
4.2 Pancreatic Cancer
Tumor localization and structure
4
Focal Changes
Pancreatic carcinoma of the body: tumor attitude in CDS and CEUS
Infiltrative growth, metastases
a Pancreatic carcinoma in the head (uncinate process,T), with obstruction of the pancreatic duct (DP). VL = splenic vein.
d hypoechoic structure, in CDS no vascularization.
g Pancreatic carcinoma of the tail, infiltration of the splenic artery with prestenotic dilatation and partly chromatic spots (blue).
b Pancreatic carcinoma in the head with duct dilatation, CDS: blurred defined polycyclic tumor (T), no intratumoral vessels detectable. KO = venous confluence, P = pancreatic body.
e CEUS, early phase, hypoenhancement in contrast to the rest of the organ.
h Pancreatic carcinoma of the head (T; see  4.2b), infiltration into the CBD (DC) and obstruction of the cystic duct (clinically Courvoisier’s sign) infiltrated celiac trunk; consequently inoperable.
4.2b), infiltration into the CBD (DC) and obstruction of the cystic duct (clinically Courvoisier’s sign) infiltrated celiac trunk; consequently inoperable.
c Pancreatic carcinoma of the tail, polycyclic contours, hypoechoic structure. Infiltration into the splenic vein (CDS).
f In the late phase, accentuated hypoenhancement.
i Pancreatic carcinoma, EUS: multiple hypoechoic lymph nodes the largest with cursors. EUS is the best procedure for the detection of small tumors as well as the evaluation of operability.
183
4
Pancreas
and liver metastases as well as a peritoneal metastasis, are considered to indicate inoperability ( 4.2h). To differentiate other pancreatic tumors see Table 4.6.
4.2h). To differentiate other pancreatic tumors see Table 4.6.
Acinar cell carcinomas have the same appearance as ductal carcinomas. Periampullary cancer is a separate and quite different entity because it is an adenocarcinoma of the mucosa of the papilla of Vater. It leads to a “double duct” sign (dilated CBD and pancreatic duct; see Fig. 4.65).
Ultrasound findings. As in most other malignancies, the texture of pancreatic cancer is hy-
Table 4.6 Sonographic differentiation of the diverse solid tumors of the pancreas including CEUS2
|
B-mode |
CDS/vascularization |
Signal enhancement |
|
Ductal |
● Hypoechoic |
No detectable vessels |
Hypovascular, size/mar- |
|
carcinoma |
● Polycyclic |
|
|
gins better visualized, |
|
● Dilated pancreatic duct |
|
|
chaotic tumor architec- |
|
● Vascular invasion |
|
|
ture |
Metastases |
● Hypoechoic |
No detectable tumor |
Di erent vasculariza- |
|
|
● Round, rarely polycyclic |
vessels (except hyper- |
tion; mostly hypoen- |
|
|
● Well defined |
nephroma, endocrine |
hanced; metastases of |
|
|
● Pancreatic duct rarely |
tumors) |
renal cell carcinoma |
|
|
dilated |
|
|
(NCC) hyperenhanced |
|
● Rarely necrosis or |
|
|
|
|
vascular invasion |
|
|
|
Endocrine |
● Hypoechoic |
Rare vessels detectable |
Extremely vascularized, |
|
tumor |
● Well defined |
|
|
therefore hyperen- |
|
● Pancreatic duct not |
|
|
hancement |
|
dilated |
|
|
|
|
● Rare vascular invasion |
|
|
|
poechoic, although assessment of the texture |
hyperechoic pancreas but also as an isoechoic |
|||
depends on the echogenicity of the pancreas. |
(quite rarely hyperechoic) lesion within a hy- |
|||
Since the gland may present as hypoechoic or |
poechoic pancreas of normal texture. |
|||
hyperechoic, depending on the age of the pa- |
|
|
||
tient, pancreatic cancer will be demonstrated not only as a hypoechoic structure within a
Metastasis, Malignant Lymphoma, Inflammatory
Inflammatory Lymph
Lymph Node
Node
Differential diagnosis of suspected pancreatic cancer has to consider endocrine tumors, metastasis of, e. g., hypernephromas, lung cancer or malignant melanoma, and malignant lymphoma.
Metastases. These appear as smooth round or polygonal structures and are almost impossible to differentiate from primary pancreatic cancer. The same is true for other rare tumors like gastrointestinal stromal tumors (GIST) (Fig. 4.34, Fig. 4.35).
Lymphoma. The pancreas is permeated by an extensive intrapancreatic and extrapancreatic lymph system, and therefore malignant lymphoma will manifest itself as intrapancreatic as well as extrapancreatic neoplasm. It is seen as a flat or bulky, primarily hypoechoic, lesion (Fig. 4.36).
Inflammatory lymph node. Sometimes there are inflammatory lymph nodes along the pancreatic margin that may be difficult to separate from the gland. In most cases, confirmation of suspected malignancy has to await fine-needle aspiration biopsy.
Fig. 4.34 Smoothly margined tumor (TU), differential diagnosis: pancreatic cancer. Gastrointestinal stromal tumor of the stomach, infiltration into the pancreatic tail. CF = venous confluence, AO = aorta, VC = vena cava.
Fig. 4.35 Metastasis ((LK) of non-small-cell bronchial car- |
Fig. 4.36 |
b Pancreatic metastases, partly peripancreatic, of a low- |
cinoma in the pancreatic body (CDS). VL = splenic vein. |
a High-grade non-Hodgkin lymphoma (T) in the body/tail |
grade non-Hodgkin lymphoma; diagnosed 6 years previ- |
|
of the pancreas, sole manifestation. Diagnosis confirmed |
ously, slow growth (CDS). VL = splenic vein. |
|
by ultrasound-guided fine-needle aspiration biopsy. PK = |
|
|
head of pancreas; AO = aorta; VC = vena cava; VL = |
|
|
splenic vein. |
|
184

Abscess
Abscess occurs as an anechoic or, more commonly, hypoechoic mass; frequently, it demonstrates intrinsic structures and may then appear as a hypoechoic inhomogeneous lesion (Fig. 4.29). In CEUS, abcesses can be well differentiated by peripheral hypervascularization and absence of central enhancement.
Hemorrhagic/Infected Cyst/Pseudocyst
Cyst/Pseudocyst 






































Pancreatic pseudocysts may display a variety of textures, but normally they are anechoic. Bleeding or calcification in the wall will result in a rather hypoechoic mass, even resembling a tumor or abscess (Fig. 4.37).
4
Focal Changes
Fig. 4.37
a Intracystic hemorrhage of a pancreatic pseudocyst, smooth margin, hypoechoic to complex structured mass.
b Infection of a pseudocyst. Fine echoes caused by bacterial gas with gas-related reverberations.
Focal (Segmental) Pancreatitis/Ventral
Pancreatitis/Ventral Anlage
Anlage 


































Focal pancreatitis of the head/tail of the pancreas resembles a tumor, making differentiation from true malignancy difficult. The suspected diagnosis of focal pancreatitis is based on the patient’s history and clinical symptoms, but it should be remembered that pancreatic cancer may also trigger acute pancreatitis. A special form is “groove pancreatitis”, a focal pancreatitis affecting the head of the pancreas around the intrapancreatic CBD. In this case, any increased tumor marker will subside after resolution of the acute symptoms, while in pancreatic cancer the marker will increase steadily. Fine-needle aspiration biopsy is of differential diagnostic value only if it is positive.
Compared to standard US, CEUS seems to increase diagnostic confidence in the differential diagnosis between mass-forming pancreatitis and pancreatic carcinoma.5
Undulating pancreatic duct, calcification, and fibrosis in the residual pancreas support the diagnosis of focal chronic pancreatitis; ductal cut-off and well-defined dilated duct support pancreatic carcinoma (see section “Dilatation of the Pancreatic Duct”). Further diagnostics are endoscopic ultrasound fine-needle aspiration (EUS-FNP), and, if indicated, exploratory laparotomy; elastography may be helpful in the differential diagnosis. In general, the older the patient, the more often a cancer is to be found.
A ventral anlage often presents as a hypoechoic mass in the caudal–dorsal parts of the pancreatic head, including the uncinate process, and may be mistaken as focal pancreatitis (Figs. 4.17b, 4.38, 4.39, 4.40, 4.41, Table 4.7).
Fig. 4.38
a Focal pancreatitis in the head of the pancreas (P): hypoechoic, swollen, well-defined head with calcifications, probably intraductal calculi (arrow): the swelling in the pancreatic head is almost impossible to differentiate from cancer. L = liver; AO = aorta; VL = splenic vein.
c Focal pancreatitis in the pancreatic head, “groove pancreatitis” with a duct stone, extracted endoscopically. Hypoechoic area in the uncinate process around the common bile duct (DC). VL = splenic vein.
b Left-sided pancreatitis in the tail. Hypoechoic mass, undisturbed course of the splenic vein (CDS).
d Stenosis of the intrapancreatic common bile duct (arrow); inflammatory wall thickening (calipers).
185
