
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
■ Dilatation of the Pancreatic Duct
Under good conditions ultrasound will demonstrate the normal pancreatic duct in the body of the pancreas as a narrow anechoic ligamentous structure with delicate, smooth, echogenic margins. If it appears marked or dilated, it may be traced all the way to the papilla of Vater.
In dilatation of the pancreatic duct, appropriate views (oblique or sagittal epigastric) will help identify and differentiate the nature of the obstruction. A ductal diameter of 2 mm or less is considered normal (Fig. 4.60). A marginal diameter of 2–3 mm is found in a postprandial state and is much more common after biliary disorders; its assessment can be difficult, in which case additional diagnostic measures are called for to settle the issue. A widely dilated duct is found in chronic pancreatitis and tumors. Table 4.10 lists possible causes.
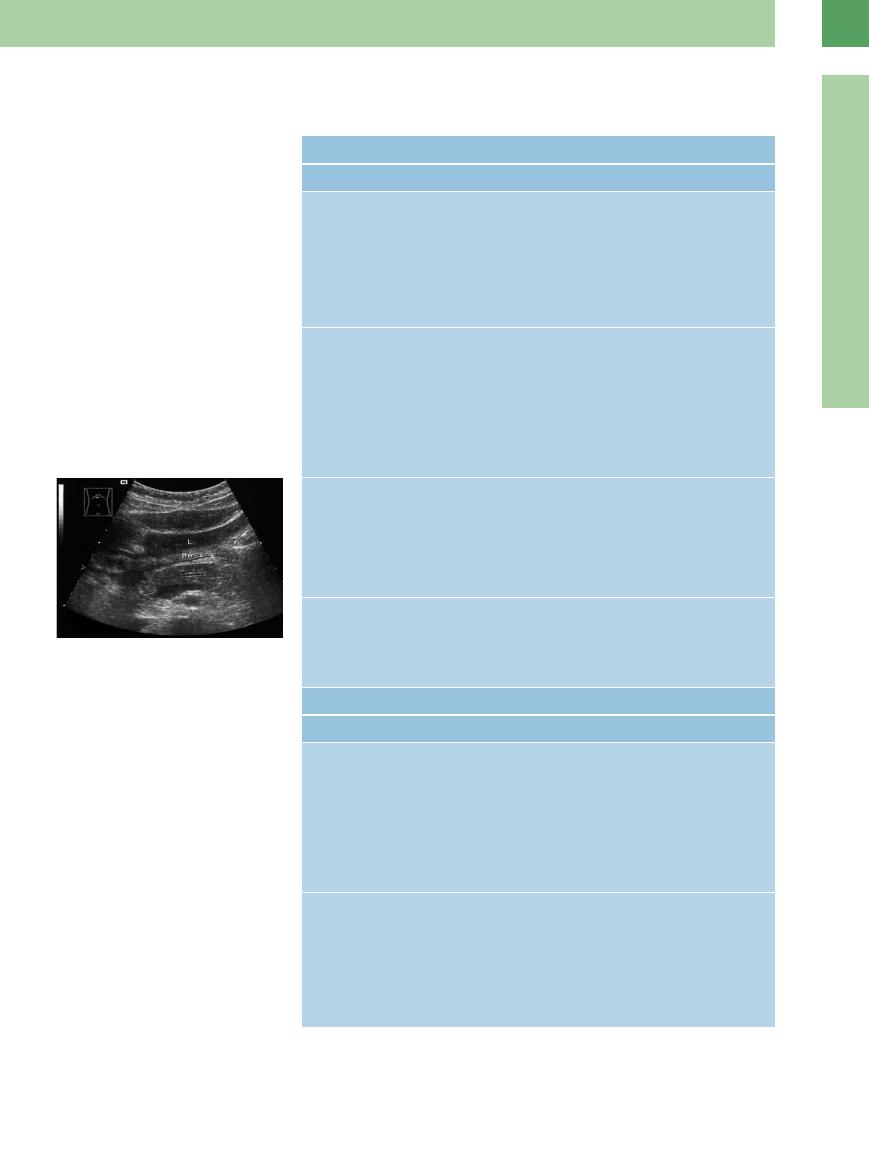
Fig. 4.60 Accentuated pancreatic duct.
Postprandial: marked pancreatic duct (calipers) of normal diameter in postcholecystectomy; echogenic peripancreatic bandlike mass (arrows): fatty necrosis after biliary pancreatitis? MA = stomach; L = liver.
Table 4.9 Differential diagnosis of textural and ductal changes
Structural change |
|
|
|
Di use |
Focal |
|
|
|
|
● Anechoic lesion |
|
|
|
– |
pancreatic cysts/pseudocysts |
|
|
– |
renal/splenic cyst |
|
|
– |
splenic artery |
|
|
– |
gastroduodenal artery |
|
|
– |
arterial aneurysm |
|
|
– |
pancreatic duct |
|
|
– |
dilated prepapillary CBD |
● Hypoechoic structure |
● Hypoechoic lesion |
||
– |
juvenile pancreas |
– |
pancreatic cancer |
– |
acute pancreatitis |
– |
necrosis/hemorrhage |
– |
cancer with di use spread |
– |
hemorrhagic pancreatic cyst/pseudocyst |
– |
autoimmune chronic pancreatitis |
– |
abscess |
– |
early chronic pancreatitis |
– |
focal acute pancreatitis of the head/tail |
|
|
– |
metastasis of cancer |
|
|
– |
metastasis of lymphoma |
|
|
– peripancreatic inflammatory lymph node |
|
● Hyperechoic texture |
● Hyperechoic/echodense lesion |
||
– pancreas in the elderly |
– |
intraparenchymal calcification |
|
– |
pancreatic lipomatosis / fibrolipomatosis |
– |
intraductal calculi of the pancreatic duct |
– pancreatic fibrosis in hemochromatosis/ |
– |
stented pancreatic duct |
|
|
cystic fibrosis |
– |
intraductal gas bubbles |
– chronic pancreatitis (with fibrosis/ calci- |
– |
calcified splenic artery |
|
|
fication) |
– |
calcified tumor |
● Irregular/heterogeneous structure |
● Irregular/heterogeneous lesion |
||
– |
pseudocystic transformation of the |
– |
pancreatic pseudocyst |
|
pancreas |
– |
cystadenoma |
– |
acute pancreatitis |
– |
pancreatic cancer |
– |
chronic pancreatitis |
– |
focal chronic pancreatitis |
Size change |
|
|
|
Di use |
Focal |
|
|
● Enlarged |
● Variant shapes |
||
– |
autoimmune pancreatitis |
– |
dumbbell |
– |
acute pancreatitis |
– |
tadpole |
– |
pancreatic edema |
● Pancreatitis |
|
– |
di use cancer |
– |
pancreatitis of the head |
– |
pancreas divisum |
– |
pancreatitis of the tail |
|
|
– |
segmental autoimmune pancreatitis |
|
|
– secondary neoplastic lesions (lymphoma, |
|
|
|
|
metastases) |
●Reduced
–pancreas in elderly people
–atrophic pancreas (terminal stage of chronic pancreatitis)
–status post necrotizing pancreatitis
–status post surgery
–advanced pancreatitis fibrosis (hemochromatosis)
●Pancreatic cancer
●Enlarged head in pancreas divisum and annular pancreas
4
Dilatation of the Pancreatic Duct
195
4
Pancreas
Table 4.10 Causes of pancreatic duct dilatation |
|
|
Ductal diameter marginal (2–4 mm) (Smooth) |
Ductal diameter > 4 mm (Smooth) |
Ductal diameter > 4 mm (Convoluted) |
● Postprandial |
● Pancreatic cancer |
● Chronic pancreatitis |
● Cholelithiasis |
● Periampullary cancer |
● Atrophy in chronic pancreatitis |
● Pancreas divisum |
● Chronic pancreatitis |
|
|
● Obstructive chronic pancreatitis |
|
Pancreas |
Diffuse Pancreatic Change |
Postprandial |
|
Focal Changes |
Bile Duct Disorder |
||
Dilatation of the Pancreatic Duct |
Acute/Recurrent Pancreatitis, Pancreas Divisum |
||
Marginal/Mild Dilatation |
|||
Chronic Pancreatitis |
|||
|
Marked Dilatation |
||
|
Periampullary Cancer, Cancer of the Pancreatic Head |
||
|
|
||
Postprandial |
|
||
An accentuated pancreatic duct is a physiologic |
Fig. 4.61 Pancreatic duct of marginal diameter (0.26 cm, |
||
sequel to a rich meal. Diagnosis depends on the |
cursors) postprandially, slightly undulating (differential |
||
medical history and clinical findings (Fig. 4.61). |
diagnosis also early chronic pancreatitis). |
||
|
|||
Bile Duct Disorder
Disorder


















































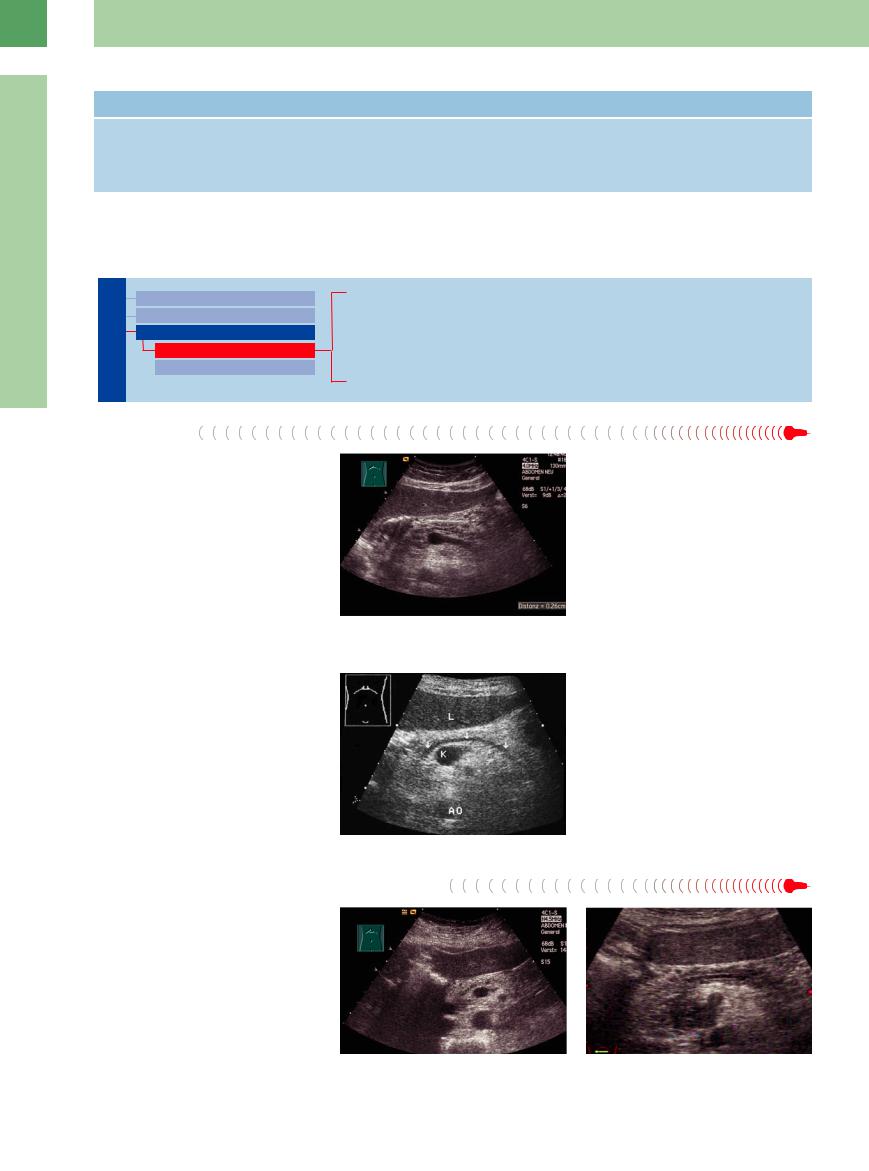
Marginal diameters of 2–3 mm occur as a postprandial functional response, but often they are based on a disease of the biliary system. In the case of somewhat blurred ducts, it is well worth the effort to take a look at the gallbladder and to trace the CBD all the way to the papilla of Vater. In many cases, ductal dilatation is nothing more than postcholecystectomy, often preceded by recurrent colics (with accompanying pancreatitis?). The underlying cause must be increased intraductal pressure (Fig. 4.62).
Fig. 4.62 Dilated pancreatic duct (arrows) in postcholecystectomy. K = venous confluence; AO = aorta; L = liver.
Acute/Recurrent Pancreatitis, Pancreas Divisum
Pancreatitis, Pancreas Divisum
Here, ductal dilatation is infrequent since this is counteracted by the edema. However, if there is a pancreatic duct dilatation detectable the differential diagnosis should include biliary pancreatitis and obstruction of the pancreatic duct caused by tumors (IPMN, ductal carcinoma) or pancreas divisum (Fig. 4.62, Fig. 4.63,  4.1k).
4.1k).
Fig. 4.63
a Pancreatic duct of marginal diameter (0.28 cm, cursors) in histologically proven IPMN.
b Slightly dilated pancreatic duct in carcinoma of the pancreatic head (uncinate process, hypoechoic mass).
196
Chronic Pancreatitis

















































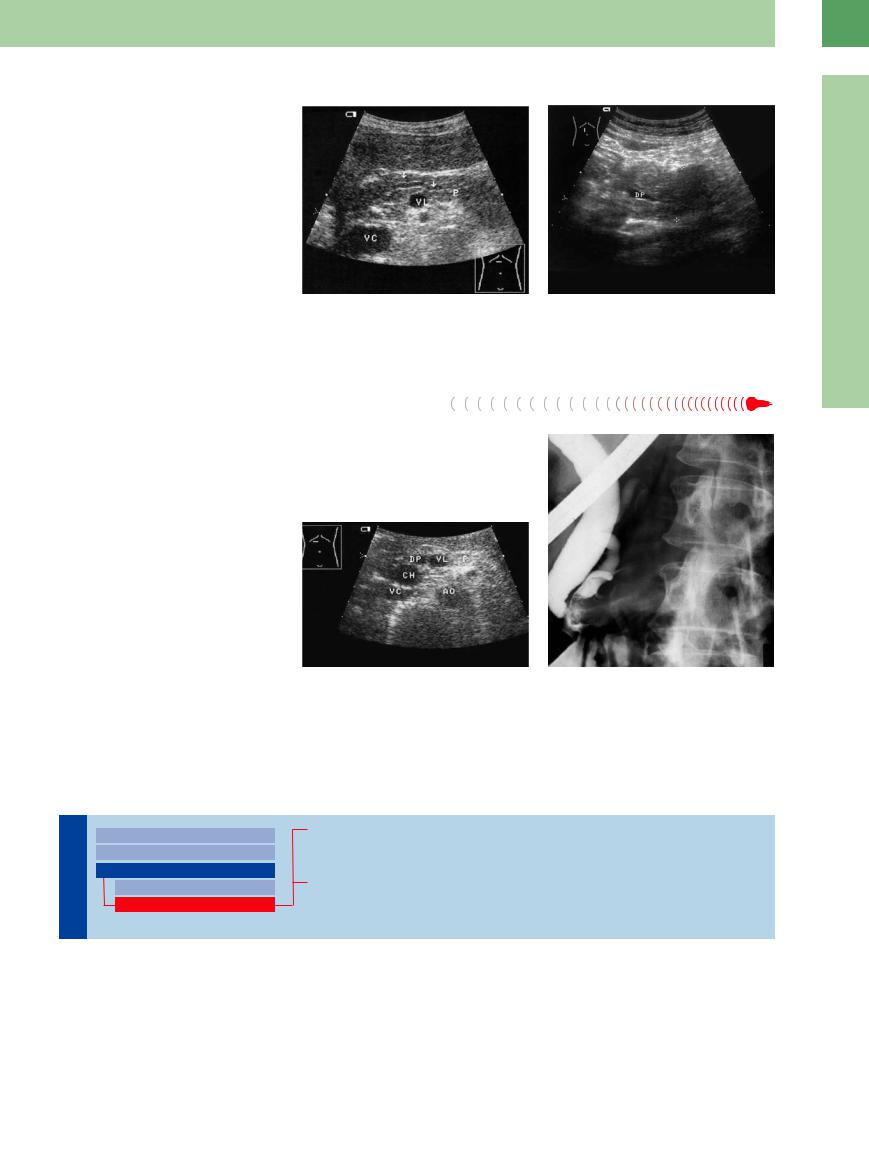
Dilatation of the pancreatic duct in chronic pancreatitis tends to be quite marked rather than marginal. The obstruction may be caused by intraductal calculi, prepapillary gallstones, protein plugs, and in a few rare cases a slowgrowing malignancy. Fibrosis of the adjacent tissue can result in a tortuous duct with varying diameter, beaded irregularities, and twisting, mostly in an advanced state (Fig. 4.64).
Fig. 4.64 |
b Early chronic pancreatitis, paramedian longitudinal |
a Dilated undulating pancreatic duct (arrows) in chronic |
scan direction. Slightly dilated duct (DP), tapering off as |
pancreatitis (P). This appearance of the duct is character- |
a result of focal pancreatitis of the head. |
istic of chronic pancreatitis and not ductal obstruction. VL |
|
= splenic vein; VC = vena cava. |
|
Periampullary  Cancer,
Cancer, Cancer of
Cancer of  the
the Pancreatic
Pancreatic Head
Head
Here, dilatation of the pancreatic duct is normal and in the advanced stage quite marked. Cancer of the tail of the pancreas will not result in ductal dilatation, while cancer of the uncinate process will sometimes do so. Cancer in the head of the pancreas is usually accompanied by dilatation of the CBD (Fig. 4.65).
4
Dilatation of the Pancreatic Duct
Fig. 4.65 Dilated prepapillary ducts.
a Transverse epigastric view: cross-sectional view of the pancreatic duct (DP) and CBD (CH) (double duct sign); periampullary ductal cancer of the pancreas. The carcinoma could not be demonstrated on ultrasound or CT. AO = aorta; VC = vena cava; VL = splenic vein; P = pancreas.
b ERCP film with tumorous void in the pancreatic duct.
Marked Dilatation
|
|
|
|
|
Diffuse Pancreatic Change |
|
|
|
|
|
|
||
|
|
|
|
|
Focal Changes |
|
|
|
|
|
|
||
|
|
|
|
|
Dilatation of the Pancreatic Duct |
|
Pancreas |
||||||
Marginal/Mild Dilatation |
||||||
|
|
|
|
|
Marked Dilatation |
|
Chronic Pancreatitis
Intraductal Mass
Pancreatic Cancer
Chronic Pancreatitis

















































Acute relapsing pancreatitis or persistent pain in chronic pancreatitis are often caused by stones obstructing the pancreatic duct. The calculi will be visualized when following the duct sonographically to the papilla of Vater. One
treatment option is ultrasound-guided lithotripsy following papillotomy (Fig. 4.66a,b).
Prominent dilatation of the pancreatic duct with no obstruction seen on ultrasound or endoscopy is also found in the rare patient with
chronic autoimmune pancreatitis. In these cases, it is of vital importance that possible neoplasms (look out for IPMN) and malignancy be ruled out for certain (Fig. 4.66c,d, Fig. 4.67).
197
4
Pancreas
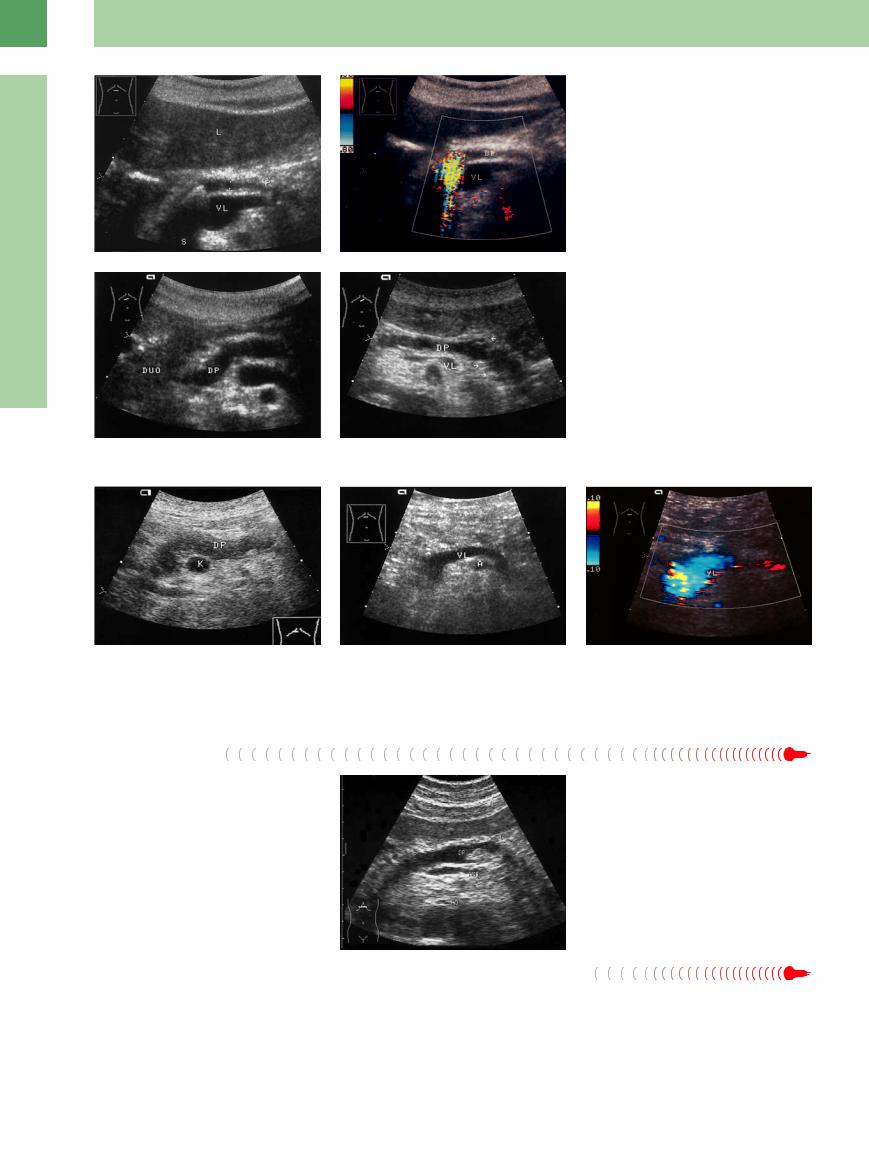
Fig. 4.67 Chronic pancreatitis. Clinical symptoms: diabetes and weight loss. VL = splenic vein; K = venous confluence.
a Prominent undulating dilated duct (DP). ERCP: no obstruction, no branches whatsoever.
b Same patient after 1 year: additional loss of the pancreatic duct. Without knowledge of the previous findings and the clinical symptoms, the diagnosis of chronic pancreatitis would have been missed. VL = splenic vein.
Fig. 4.66
a Dilated pancreatic duct (cursors) in chronic obstructive
(?) pancreatitis with atrophy. Site of the obstruction (stone; acoustic shadow S) at the junction of the head and body. L = liver; VL = splenic vein. Patient presented clinically with diabetes mellitus.
b Color duplex: the “twinkling artifact” aids in stone detection (as with renal stones). VL = splenic vein; DP = pancreatic duct.
c Chronic pancreatitis, extended dilatation of the duct (DP) up to the duodenum (DUO).
d Clublike dilated branch ducts (arrows) are visible in the pancreatic tail. DP = pancreatic main duct. VL = splenic vein.
c CDS: no dilatation of the duct evident, atrophy of the pancreas, no history of pancreatitis, probably primary autoimmune chronic pancreatitis.
Intraductal Mass
Mass
By far the most common causes of intraductal mass are echogenic calculi with posterior shadowing, followed by sediments and pus. Protein plugs or IPMN (see  4.3 g–k) are infrequent findings (Fig. 4.68).
4.3 g–k) are infrequent findings (Fig. 4.68).
Pancreatic  Cancer
Cancer



























Dilatation of the pancreatic duct without a dis- |
to the tumor. In selective enlargement, the |
cernible cause of obstruction has to raise a high |
ductal cut-off will be either ovoid, convex, or |
degree of suspicion of malignancy unless an- |
tapering. |
other cause can be confirmed (calculi, fibrosis, |
Concurrent dilatation of the CBD is indica- |
pseudocyst, twisted duct, ductal stricture). Ul- |
tive of a malignancy in the vicinity of the pap- |
trasound will demonstrate the duct all the way |
illa (periampullary cancer) and is termed “dou- |
Fig. 4.68 Ectatic duct (DP) with probably protein plug (DP, arrow) of unknown origin; ERCP: wide open papilla of Vater; biopsy and cytology negative three years later carcinoma of the pancreatic head. Differential diagnosis: IPMN. AMS = superior mesenteric artery; AO = aorta.
ble duct sign.” ERCP with brush cytology and ductal biopsy or CT study will help with the final diagnosis; fine-needle aspiration biopsy should be reserved for uncertain findings and inoperable cases because of the risk of implantation of tumor cells along the needle track.
198
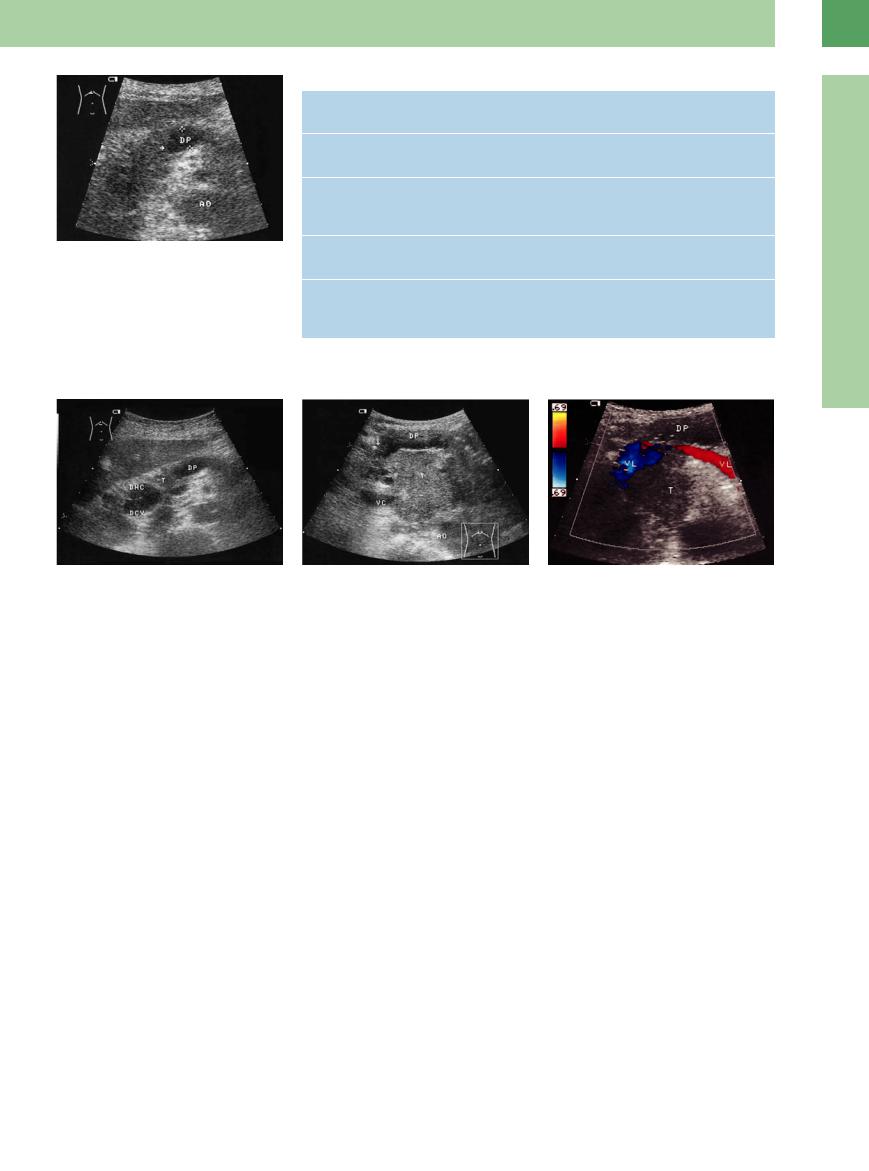
Fig. 4.69 Pancreatic duct (DP) with prestenotic dilatation and ductal cut-off without direct visualization of the tumor; but, together with the cut-off (arrow), the diffuse ill-defined enlarged head of the pancreas suggests the malignancy. AO = aorta.
Table 4.11 Differential diagnosis of the pancreatic duct with prestenotic dilatation
Intraductal calculi |
● Hard echo due to calculi, posterior shadowing |
|
● Frequently in the head/body of the pancreas |
Pancreatic cancer |
● Hypoechoic (isoechoic) tumor mass downstream of the stenosis |
|
● Tapered or convex cut-o of the duct (on selective enlargement) |
Metastasis/ |
● Impossible to di erentiate from pancreatic cancer by ultrasound |
lymphoma |
morphology alone (ultrasound-guided fine-needle aspiration biopsy |
|
indicated) |
Periampullary cancer |
● Concurrent dilatation of the pancreatic duct and CBD |
(pancreas/CBD) |
● Usually, no direct visualization of the tumor possible |
Pancreas divisum ● Ductal cut-o in the head/body without definite cause
●Conspicuously enlarged head of the pancreas
●Demonstration of two ductal systems in the pancreatic head
4
Dilatation of the Pancreatic Duct
Fig. 4.70 Small cancer (T) of the pancreatic head; duct (DP) with prestenotic dilatation; dilated CBD (DHC) and cystic duct (DCY). “Double duct sign”.
Fine-needle biopsy and cytohistology is of no benefit in the case of suspected carcinoma in chronic pancreatitis because of the high rate of false-negative results. However, if pain is persistent in chronic pancreatitis, the possibility of carcinoma must be a primary consideration.
Fig. 4.71 Cancer (T) of the uncinate process. AO = aorta; VC = venous confluens; VL = splenic vein.
a With invasion of the pancreatic duct (arrow, DP).
The cumulative risk of pancreatic cancer in chronic pancreatitis is 1.8% after 10 years, and 4% after 20 years.15 Therefore, chronic pancreatitis is considered a precancer. When the symptoms persist, cancer cannot be ruled out and the issue has to be settled by surgery (Figs. 4.69, 4.70, 4.71).
b CDS: invasion of the splenic vein. The invasive spread confirms malignancy and rules out surgery as an option.
Table 4.11 summarizes the differential diagnosis of the pancreatic duct with prestenotic dilatation.
199
4
Pancreas
Tips, tricks, and pitfalls
General
●The pancreas is more easily detectable in abdominal ultrasound than is generally assumed; it is of great value to have a “second look” when intestinal gas interferes with the detection of the organ. Peristalsis, compression, palpation, decubitus position, inspiration, or expiration favor a better visual situation.
●The normal visual point in a transverse scan direction is through the deep inspirated left liver lobe, and for the tail a scan direction through the spleen.
References
[1]Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 2001; 120(3):682–707
[2]Wermke W. 32. Dreiländertreffen Davos 2008
[3]Dellinger EP, Forsmark CE, Layer P, et al. De- terminant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 2012;256: 875-880
[4]Sarner M, Cotton PB. Classification of pancreatitis. Gut 1984;25(7):756–759
[5]D’Onofrio M, Zamboni G, Tognolini A, et al. Mass-forming pancreatitis: value of contrastenhanced ultrasonography. World J Gastroenterol 2006;12(26):4181–4184
[6]Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune
●Fluid collection in the stomach improves the visualization of the pancreas, so a breakfast that includes a drink may lead to better conditions.
●Dynamic criteria: displacement of the organ is detectable on inspiration.
●Consistency: normally, compression of the soft pancreatic head by the caval pulsation can be observed.
Special
●In diminished parenchyma (aging pancreas, cachexia) a lot of vessels are close together; in this case the gastroduodenal artery becomes a leading structure.
chronic pancreatitis revisited. World J Gastroenterol 2006;12(16):2487–2496
[7]Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaulation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin testin in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998;48:11–17
[8]Jenssen C, Dietrich CF. Endosonographie bei chronischer Pankreatitis [Endoscopic ultrasound in chronic pancreatitis]. Z Gastroenterol 2005;43(8):737–749
[9]Claudon M, Cosgrove D, Albrecht T, et al; EFSUMB Study Group et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—up- date 2008. Ultraschall Med 2008;29(1): 28–44
[10]Will U. in: Schmidt G, Görg C. Kursbuch Ultraschall. 5. ed Stuttgart, New York: Thieme; 2008
[11]DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography
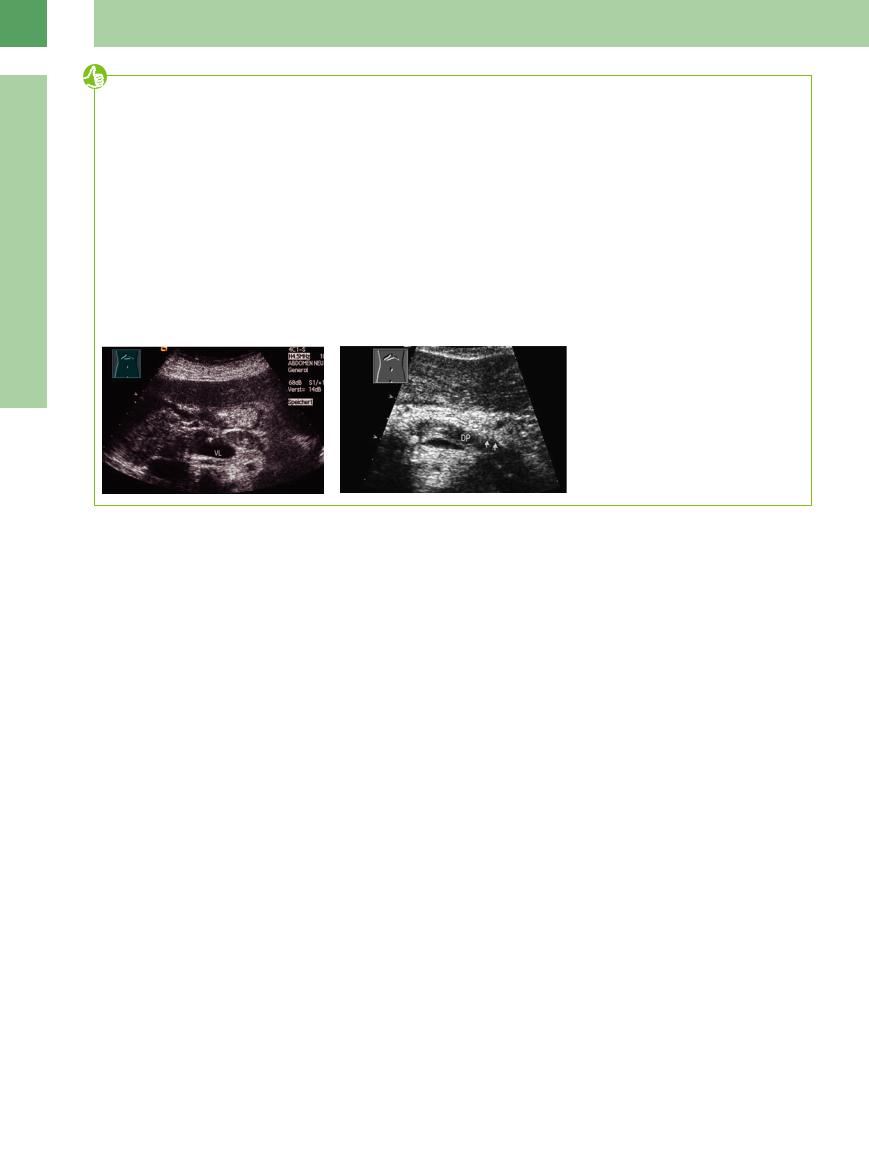
●Hyperechoic fluid or stones in the pancreatic duct conceal a duct dilatation; branches of the main duct are only exceptionally demonstrable (Fig. 4.72; see also Fig. 4.66 d).
The duodenum enclosing the pancreas is, like the jejunum, a small and poorly visible part of the intestine. This situation changes in case of inflammation; in a functional state such as diabetic vegetative neuropathy, intramural fluid collection alters the situation. In this case, the duodenum is demonstrable around the head of the pancreas, because the descending duodenum runs not only strictly caudally (as seen in anatomy) but also in a dorsal direction.
Fig. 4.72
a Chronic atrophic pancreatitis. Echogenic pancreatic duct with stenosis between the stomach (MA) and the splenic vein (VL).
b Atrophic chronic pancreatitis. Dilated hyoechoic duct (DP) with a calculus in the head and visible branches of the main duct (arrows).
and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004;141(10):753–763
[12]Taniguchi T, Seko S, Azuma K, et al. Autoimmune pancreatitis detected as a mass in the tail of the pancreas. Am J Gastroenterol 1995; 90:1834–1837
[13]Riede UN, Werner M. Color atlas of pathology. Stuttgart: Thieme, 2004
[14]Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. Pancreatology 2001;1:648–655
[15]Lowenfels AB, Maisonneuve P, Cavallini G, et al; International Pancreatitis Study Group. Pancreatitis and the risk of pancreatic cancer. N Engl J Med 1993;328(20):1433–1437
[16]D’Egidio A, Schein M; D’Egidio A. Pancreatic pseudocysts: a proposed classification and its management implications. Br J Surg 1991;78 (8):981–984
200
