
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
■ Focal Changes of the Spleen
Focal changes of the spleen are rare. Ultrasound detects them in approximately 0.2% of cases. They are characterized sonographically in a similar way to hepatic masses, i. e., according to echogenicity, size, shape, delineation, and number. Compared with hepatic masses, the nosological assessment of a focal splenic mass is often difficult and becomes possible
only when taking into account the history and clinical symptoms of the patient as well as the sonographic follow-up. In case of doubt, the diagnosis is confirmed by definite cytology/ histology via ultrasound-guided fine-needle aspiration cytology/biopsy.6
The differential diagnostic considerations in the sections below adhere to the morphologi-
cal typing. This is somewhat fraught with problems since, for instance, splenic cysts may be demonstrated as being anechoic, hypoechoic, or also hyperechoic. Therefore, an additional classification according to the frequency of diagnosis has been introduced.
Anechoic Mass
Spleen |
|
Anechoic Mass |
||
|
|
|
Nonfocal Changes of the Spleen |
|
|
|
|
Focal Changes of the Spleen |
|
|
|
|
|
Hypoechoic Mass |
|
|
|
|
|
|
|
|
|
Hyperechoic Mass |
|
|
|
|
Splenic Calcification |
Dysontogenetic Cysts
Pseudocysts
Infective Cysts
Splenic cysts are rare benign lesions with an |
breaks down into “true” cysts (dysontogenetic, |
incidence of less than 0.1%. They are differen- |
epidermoid, and dermoid cysts) and pseudo- |
tiated by their origin as being infective (hydatid |
cysts (not lined with epithelium). |
disease) or noninfective. The latter group |
|
Dysontogenetic Cysts
















































True splenic cysts are characterized by an epithelial lining and are congenital in origin. On ultrasound, they appear as round, smooth, anechoic lesions, well-defined from the surrounding splenic texture, with signs of distal acoustic enhancement. Sometimes echogenic septation, internal echoes, sedimentation phenomena, sequestered tissue, and calcification of the wall may be observed. The contents of
the cyst may be watery clear or dark brown as a result of hemorrhage. In other cases the lumen of the (pseudo-)cyst is filled with pulpy material. On ultrasound, the fluid within the cysts is at times visualized with a floating, moving hyperechoic mass (“snowstorm”), corresponding to cell conglomerates, sequestered tissue, or crystals ( 5.3).
5.3).
This may complicate differentiation from a solid mass. There are no certain sonographic signs ruling out pseudocysts. In 52 patients with noninfective splenic cysts (24 true cysts and 28 pseudocysts) there was no significant difference regarding size, intrinsic echogenicity, or contents of the cyst. Only calcification of the wall appeared to be more frequent in pseudocysts.
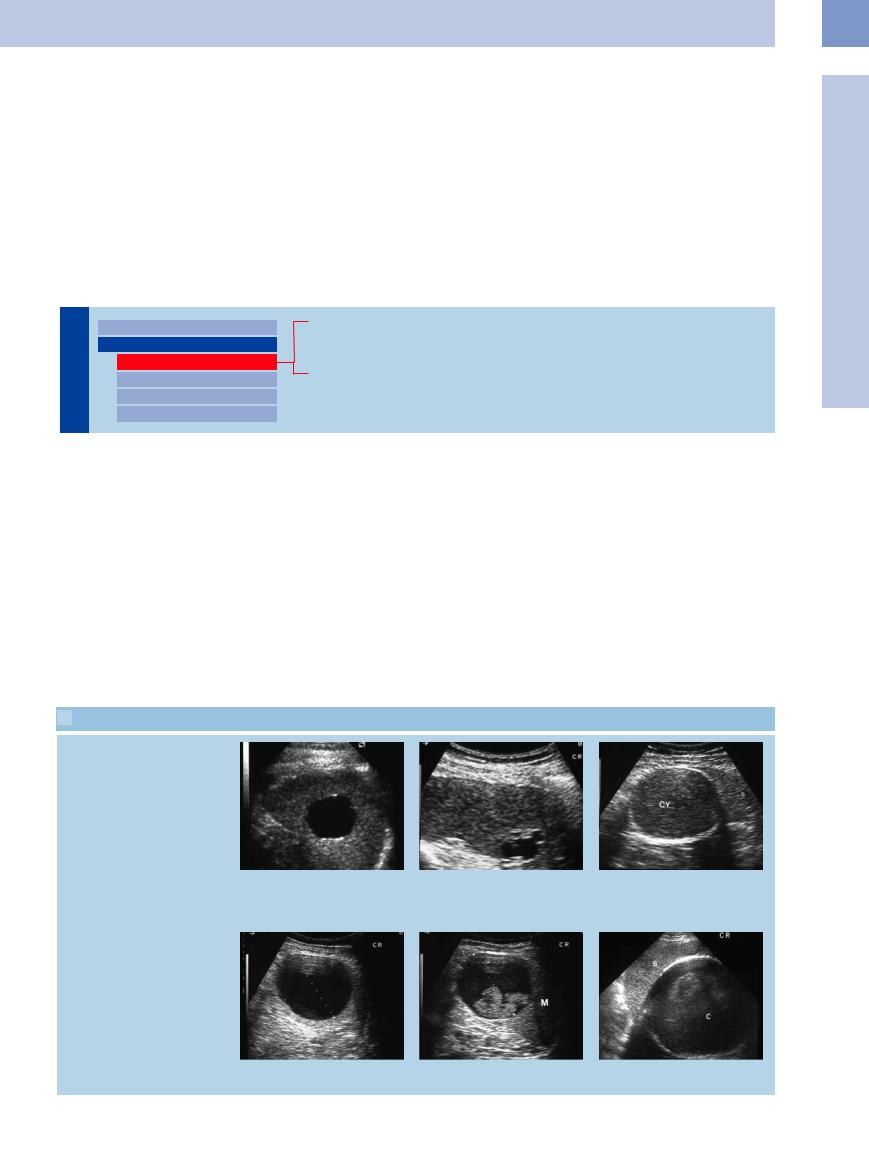
 5.3 Splenic Cysts
5.3 Splenic Cysts
Di erent ultrasound appearance of splenic cysts
5
Focal Changes of the Spleen
a Central anechoic, smoothly delineated round mass within the spleen, with posterior acoustic enhancement, compatible with splenic cyst.
b Anechoic splenic cysts of irregular margin and with small septa.
c Hyperechoic splenic cyst (cy) with signs of intramural calcification.
d and e Anechoic splenic cyst (d). Follow-up demonstrates intrinsic sedimentation phenomena as in old hemorrhage (e). M = spleen.
f Large cyst (C) with signs of intramural calcification. S = spleen.
213
5
Spleen
Pseudocysts





















































By definition, pseudocysts are not lined with epithelium. Almost without exception they are solitary lesions and, as such, sequelae to liquefactive, hemorrhagic, or encapsulating processes after pancreatitis, splenic trauma ( 5.4b,c), infarction (
5.4b,c), infarction ( 5.4a,g,h), and metastasis (see
5.4a,g,h), and metastasis (see  5.10). Since splenic trauma and infarction are somewhat common, the percentage of secondary cysts (ca. 80%) is not only far larger than that of primary cysts (ca. 20%), but also larger than for other parenchymal organs (e. g., the liver).
5.10). Since splenic trauma and infarction are somewhat common, the percentage of secondary cysts (ca. 80%) is not only far larger than that of primary cysts (ca. 20%), but also larger than for other parenchymal organs (e. g., the liver).
General treatment of splenic cysts depends on the clinical symptoms, which in turn are a function of the size of the cyst. Cysts with a diameter of less than 5 cm should be followed up. Large lesions occupying the spleen are mostly treated by surgery, even today when conservative procedures are increasingly preferred. Ultrasound-guided catheter drainage with sclerosing agents is becoming a more viable option. Rupture and carcinoma of splenic cysts have been reported in a few rare cases.
Table 5.3 lists other rare anechoic splenic masses ( 5.4 d–f,i).
5.4 d–f,i).
Table 5.3 Differential diagnosis of anechoic splenic masses
Frequent |
Rare |
● Dysontogenetic |
● Abscess |
splenic cyst |
● Hemorrhage |
● Pseudocyst |
● Infarction |
● Infective splenic |
● Metastasis |
cyst |
● Pseudoaneurysm |
|
● Lymphangioma |
|
● Hemangioma |
|
● Hamartoma |
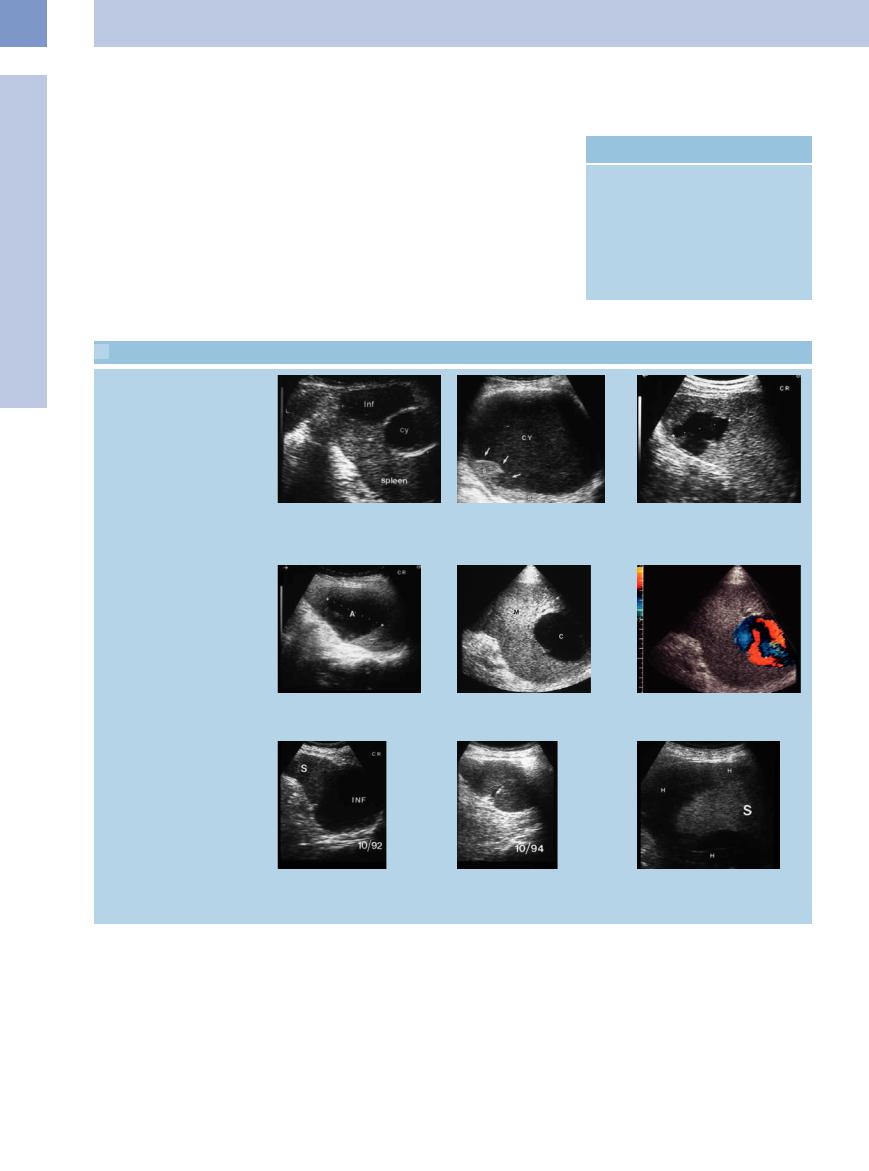
 5.4 Pseudocysts and Other Anechoic Masses
5.4 Pseudocysts and Other Anechoic Masses
Pseudocyst, traumatic cystic lesion
a–d Pseudocysts. |
b Large old post-traumatic splenic cyst |
a Central anechoic cyst (Cy) with calcified |
(CY). The splenic parenchyma is lacerated |
wall. Triangular hypoechoic structure at |
(arrows). S = spleen. |
the margin as in splenic infarction (Inf). |
|
Abscess and pseudoaneurysm
c Central anechoic splenic mass after bicycle accident, compatible with splenic hemorrhage.
d Anechoic splenic abscess (A).
e and f Anechoic intrasplenic mass in hepatic cirrhosis, with demonstration of arterial flow. Intrasplenic pseudoaneurysm; the patient underwent splenectomy. C= cyst-like mass; M = spleen.
Infarction, spontaneous hematoma
g and h Somewhat older anechoic splenic infarction (INF) in endocarditis of the aortic valve; follow-up demonstrated spontaneous resolution. S = spleen.
i Anechoic encasement of the spleen (S) in malignant lymphoma, compatible with spontaneous subcapsular hemorrhage
(H).
214

Infective  Cysts
Cysts




















































Infective cysts are rare and are encountered in less than 2% of all patients infected with hydatid disease. In most cases the infective agent is
Echinococcus cysticus or E. granulosus.
Most cysts are visualized as anechoic lesions with a double wall (pericyst and endocyst). In rare cases they may demonstrate daughter cysts. In exceptional cases these secondary cysts may be very small and numerous; and, because of the large number of wall echoes (Fig. 5.26), the sonographic appearance of such a hydatid cyst may resemble that of a hyperechoic lesion. Intracystic membranes, hydatid sand (scolicial sedimentation), and calcification have been reported. In general, the ultrasound appearance of infective splenic cysts is quite characteristic, although sometimes they are hard to differentiate from incidental splenic cysts. When there is doubt, the diagnosis has to be confirmed by laboratory work-up.
Fig. 5.26 Anechoic mass with festoon-like structures corresponding to a hydatid cyst. K = kidney.
Hypoechoic Mass
Spleen |
|
Anechoic Mass |
||
|
|
|
Nonfocal Changes of the Spleen |
|
|
|
|
Focal Changes of the Spleen |
|
|
|
|
|
Hypoechoic Mass |
|
|
|
|
|
|
|
|
|
Hyperechoic Mass |
|
|
|
|
Splenic Calcification |
Invasive Lymphoma
Splenic Infarction
Splenic Abscess
Splenic Trauma
Splenic Metastasis
Table 5.4 lists the most important hypoechoic Table 5.4 Differential diagnosis of hypoechoic splenic masses
splenic masses. |
Rare |
Frequent |
|
● Invasive lymphoma |
● Hypoechoic hypervascularized tumor |
● Infarction |
● Metastasis |
● Abscess |
● Tuberculosis |
● Splenic trauma |
● Sarcoidosis |
|
● Collagen disease |
|
● Amyloidosis |
|
● Histoplasmosis |
|
● Leukemic infiltrate |
|
● Microabscess |
|
● Peliosis |
|
● Cyst |
Invasive Lymphoma

























Splenic involvement may generally be diagnosed sonographically by the presence of splenomegaly (primarily in low-grade lymphoma) or the demonstration of hypoechoic focal or diffuse textural lesions of the splenic parenchyma. However, splenic involvement cannot be ruled out with certainty even if the spleen is of normal size and homogeneous texture.
Ultrasound differentiates between five different patterns of invasion (Fig. 5.27), with micronodular and macronodular invasion as well as the bulky formation corresponding to focal parenchymal lesions.
Diffuse splenic involvement. As in all parenchymal organs, the greatest difficulty ultrasound faces is with diffuse splenic involvement, and this is the main reason for the poor sensitivity (albeit for all imaging modalities) in diagnosing possible invasion of the spleen. Compared with the adjacent liver, a normal spleen will appear to be somewhat more homogeneous. The (subjective or operator-de- pendent) assessment of the splenic texture should be based on the liver as an in-vivo reference ( 5.5a–c).
5.5a–c).
Focal parenchymal lesions. The value of ultrasonography in the detection of focal parenchymal lesions is well known; it has a sensitivity of more than 90% and a specificity of 96%. Since the general incidence of focal splenic destruction is low, hypoechoic splenic masses in patients with lymphoma have to be regarded as neoplastic with a high degree of certainty (
5.5 d–g).
Frequently, large lesions will display an inhomogeneous echo texture ( 5.5h,i) with hyperechoic areas. Calcification is rare and will occur mostly after treatment.
5.5h,i) with hyperechoic areas. Calcification is rare and will occur mostly after treatment.
5
Focal Changes of the Spleen
215

5
Spleen
 5.5 Invasion by Lymphoma
5.5 Invasion by Lymphoma
Di use invasion
a Di use splenic invasion in malignant |
b and c Di use hypoechoic invasion of the entire spleen in Hodgkin disease. Resolved |
lymphoma. |
under therapy (c). |
Focal (micronodular and macronodular) lesions
d Micronodular splenic invasion in Hodg- |
e Micronodular hypoechoic splenic |
f Macronodular hypoechoic splenic mass |
kin disease. The hypoechoic masses re- |
masses in malignant lymphoma. |
(TU) in malignant lymphoma. |
solved under chemotherapy. |
|
|
g Hypoechoic mass within the spleen; splenectomy confirmed primary lymphoma of the spleen.
h and i Large complex masses within the spleen, with hyperechoic as well as hypoechoic areas, in Hodgkin disease. Resolved under therapy (i).
Perisplenic invasion
j Perisplenic invasion (L) originating at the hilum in malignant lymphoma.
k and l Extensive invasion (TU) of the spleen in malignant lymphoma; subcapsular hemorrhage (H). Under therapy, demarcation of the intrasplenic lesion (arrows) with resolution.
m and n Perivascular invasion (TU) at the splenic hilum in Hodgkin disease (arrowheads); resolved under therapy (n). S = spleen.
o Perivascular invasion in malignant lymphoma.
216

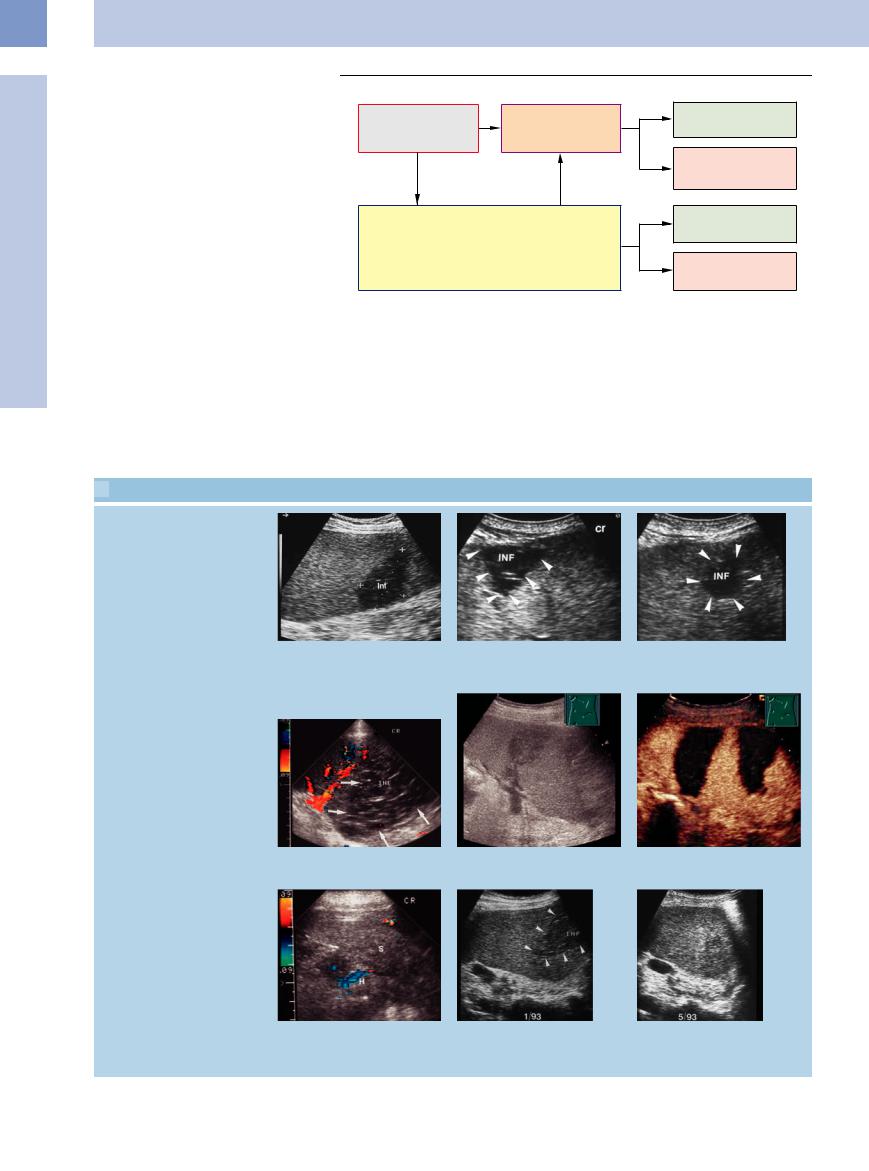
Corresponding with the macroscopic findings, low-grade non-Hodgkin lymphoma as well as Hodgkin lymphoma tend toward diffuse or micronodular invasion, whereas highgrade non-Hodgkin lymphoma will lead to larger masses.
Perisplenic invasion. Perisplenic invasion by lymphoma is extremely rare; it may originate at the hilum and spread in caplike fashion ( 5.5j). Perisplenic invasion is also possible by the lymphoma spreading from the thorax through the diaphragm into the capsule of the spleen. In individual cases this may result in complications such as hemorrhage, rupture (
5.5j). Perisplenic invasion is also possible by the lymphoma spreading from the thorax through the diaphragm into the capsule of the spleen. In individual cases this may result in complications such as hemorrhage, rupture ( 5.5k,l), and infarction of the spleen.
5.5k,l), and infarction of the spleen.
Hypoechoic perivascular structural transformation. Hypoechoic perivascular structural transformation of the hilar vessels of the spleen is a rare entity in patients with malignant lymphoma. The etiology is unknown and it resolves under chemotherapy. Similar changes have been reported in the liver as hypoechoic structural lesions encompassing the portal vein (“periportal cuffing”). They have also been ob-
1. Diffuse invasion |
2. Micronodular invasion |
|
(diameter of largest |
|
mass < 3 cm) |
|
3. Macronodular invasion |
|
(diameter of largest |
|
mass ≥ 3 cm) |
4. “Bulky” type |
5. Perisplenic invasion |
served in systemic hematological malignancy as well as in benign disorders. Apart from invasion by the lymphoma, perivascular edema
Fig. 5.27 Schematic illustration of the different sonographic patterns of splenic invasion in malignant lymphoma.
by lymphatic obstruction has also been suggested as one possible explanation (
5.5 m–o).
Splenic Infarction 


















































Splenic infarction is relatively common. It is the result of embolic or thrombotic obstruction of branches of the splenic artery.
Clinical symptoms. The clinical picture is unremarkable and is characterized by:
●Lack of any symptoms
●Diffuse abdominal pain, or
●Left upper quadrant pain
Because of the textural difference with regular parenchyma, splenic infarction and its complications may be visualized by ultrasound (Fig. 5.28).
Acute splenic infarction. Ultrasound shows acute splenic infarction as more or less hypoechoic lesions of the parenchymal texture, differing in size and extending to the splenic margin ( 5.6a). If the ultrasound beam parallels the longitudinal axis of the infarct, it may be possible to visualize the typical pyramid or wedge-shaped defect, with its base pointing toward the surface and the apex toward the hilum (
5.6a). If the ultrasound beam parallels the longitudinal axis of the infarct, it may be possible to visualize the typical pyramid or wedge-shaped defect, with its base pointing toward the surface and the apex toward the hilum ( 5.6b,c). In these cases, the characteristic shape alone is sufficient to differentiate between splenic infarction and the similar sonographic morphology of splenic abscess or neoplastic splenic invasion.
5.6b,c). In these cases, the characteristic shape alone is sufficient to differentiate between splenic infarction and the similar sonographic morphology of splenic abscess or neoplastic splenic invasion.
Color-flow Doppler imaging will delineate infarction from regular splenic parenchyma by the lack of flow signals in the former (
5.6 d).
On CEUS, splenic infarctions demarcate themselves distinctly from the surrounding tissue ( 5.6e,f).
5.6e,f).
The wide spectrum of sonographic visualization in splenic infarction reported in the literature is partly due to the lack of methods
Causes of Splenic Infarction
Thromboembolism. Thromboembolism is the cause of splenic infarction in up to 70% of cases, depending on the patients studied. It complicates various cardiovascular diseases (arteriosclerosis, myocardial infarction with intramural thrombus, atrial fibrillation, bacterial endocarditis, cardiac catheterization, but also in microangiopathy syndromes).
There have been reports of septic emboli in AIDS patients without any underlying cardiac disease.
Myeloproliferative and lymphoproliferative disorders. The pathogenetic mechanism of splenic infarction in myeloproliferative and lymphoproliferative disease is certainly multifactorial. Apart from congestion due to invasion by malignancy and/or extramedullary hematopoiesis (primarily in myeloproliferative disorders), the mismatch between the increased oxygen consumption incurred by the enlarged organ and the decreased oxygen supply in anemia plays a certain role.
for determining the age of the infarction in retrospect, primarily because of the unspecific clinical picture.
Chronic recurrent infarction. It is chronic (recurrent) infarction ( 5.6 g) in particular, typical in homozygous sickle-cell anemia, for example, that displays calcification and focal hypoechoic as well as hyperechoic masses with
5.6 g) in particular, typical in homozygous sickle-cell anemia, for example, that displays calcification and focal hypoechoic as well as hyperechoic masses with
Sickle-cell anemia. In homozygous sicklecell anemia, oxygen deficiency and/or acidosis will lead to abnormal crystallization of hemoglobin with subsequent erythrocyte adhesion and manifestation of splenic infarction. This complication is less common, but still possible, in carriers (heterozygous sickle-cell anemia).
Thrombophilia. Congenital or acquired thrombophilia (e. g., congenital protein C or S deficiency, lupus anticoagulant, erythropoietin therapy) is an infrequent cause of splenic infarction.
Partial splenic dearterialization. Some children with thalassemia major undergo therapeutic partial dearterialization of the splenic artery with subsequent splenic infarction. In myeloproliferative disorder, hypersplenism may be palliated by embolization of branches of the splenic artery.
increasing atrophy of the organ, corresponding to autosplenectomy ( 5.2). In color-flow Doppler scanning of the scarred spleen, circulation in the organ is markedly reduced.
5.2). In color-flow Doppler scanning of the scarred spleen, circulation in the organ is markedly reduced.
Total splenic infarction. Infarction of the entire spleen may be the result of acute splenic vein thrombosis in the wandering spleen, torsion of the vascular pedicle, acute sequestration in
5
Focal Changes of the Spleen
217
5
Spleen
homozygous childhood sickle-cell anemia, septic disorders, disseminated intravascular coagulation (DIC), or as part of pancreatitis ( 5.6j–l). The sonographic image is characterized by hypoechoic inhomogeneous transformation of the entire splenic texture.
5.6j–l). The sonographic image is characterized by hypoechoic inhomogeneous transformation of the entire splenic texture.
Healing phase. Increasing echogenicity and shrinking size of the infarct characterize the healing phase. Sometimes local textural inhomogeneity or calcification will remain as a scar. In 80% of cases the infarction will heal without any complication ( 5.6h,i).
5.6h,i).
Complications. Twenty percent of patients will suffer complications, including:
●Increasing liquefaction of the infarct (
5.7a–c)
●Development of subcapsular hemorrhage ( 5.7 d–f)
5.7 d–f)
●Flow phenomena identifiable on color-flow Doppler scanning and also by CEUS as indication of pseudoaneurysm or arteriovenous fistula ( 5.7 g–l)
5.7 g–l)
●Demonstration of free intraperitoneal fluid (blood) ( 5.7 m,n)
5.7 m,n)
Fig. 5.28 Sonographic visualization and complications of splenic infarction. |
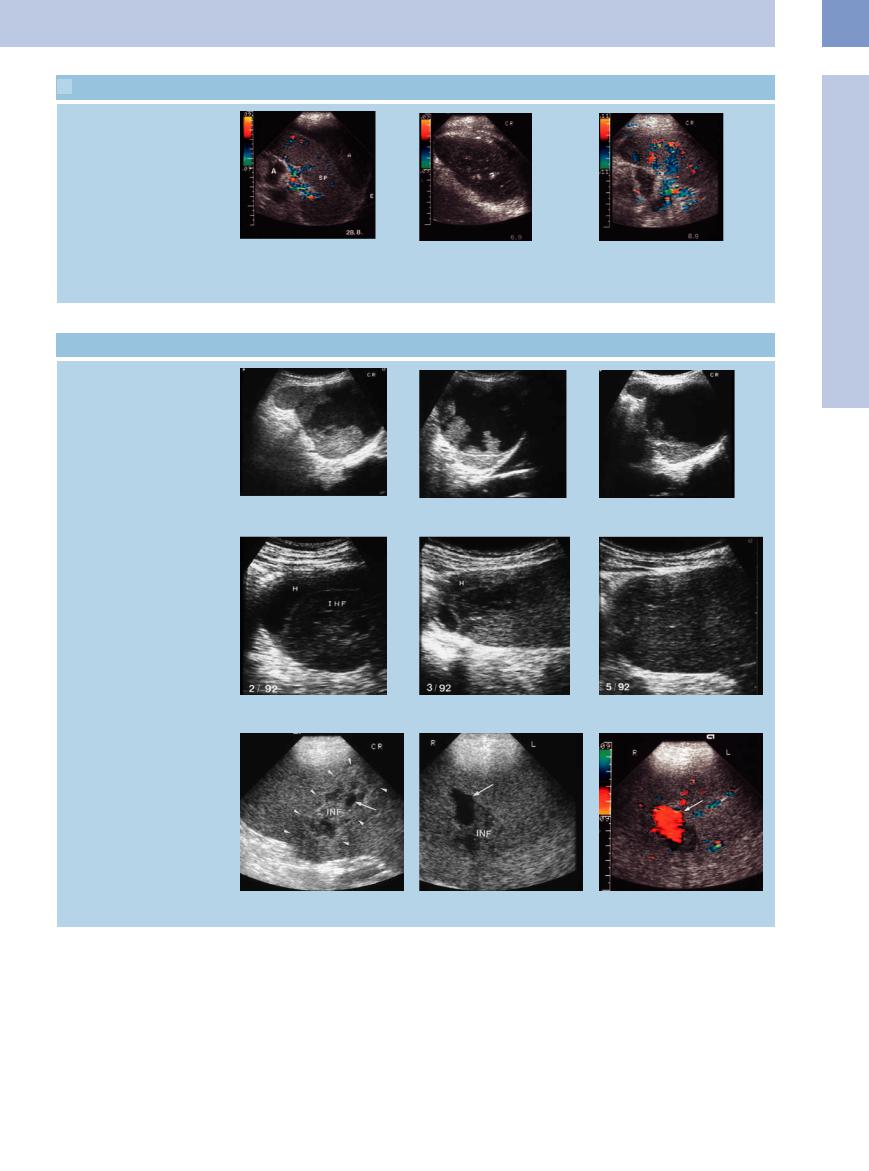
 5.6 Acute and Chronic Recurrent Splenic Infarction
5.6 Acute and Chronic Recurrent Splenic Infarction
Splenic infarctions
a Hypoechoic wedge-shaped splenic le- |
b and c Wedge-shaped splenic infarct (INF); in a slightly di erent view mimicking a |
sion in myelodysplasia, corresponding to |
round mass (c). |
an infarction. |
|
d Large hypoechoic splenic infarction. No e and f Hypoechoic splenic infarctions are easily recognizable in gray-scale image; flow signals within the infarction. CEUS demonstrates the distinct demarcation of the infarction.
g Small echogenic spleen lacking any vascular flow signals; chronic recurrent infarction in homozygous sickle-cell anemia. H = hematoma; S = spleen.
h and i Course of splenic infarction (INF).
h Hypoechoic appearance during the acute stage.
i Later, increasing echogenicity of the infarct.
218
 5.6 Acute and Chronic Recurrent Splenic Infarction (Continued)
5.6 Acute and Chronic Recurrent Splenic Infarction (Continued)
Perisplenic abscess
j–l Acute pancreatitis. |
k During the course, transient hypochoic |
l Two days later, revascularization is |
j Formation of a hypoechoic perisplenic |
transformation of the spleen, lacking flow |
evident. |
signals because of splenic artery obstruc- |
|
|
abscess (A). SP = spleen; E = pleural |
|
|
tion. |
|
|
exudation. |
|
|
|
|
 5.7 Complications of Splenic Infarction
5.7 Complications of Splenic Infarction
Liquefaction of the infarct
a–c Large liquefying splenic infarct with pseudocyst formation in endocarditis.
Subcapsular hemorrhage
d–f Apart from a splenic infarct (INF) there is a subcapsular hematoma (H) with subsequent resolution.
Pseudoaneurysm and arteriovenous (AV) fistula
g–i Complex transformation of splenic tissue with small anechoic area (arrow). There are arterial flow signals compatible with intrasplenic pseudoaneurysm (arrow). INF = infarct.
5
Focal Changes of the Spleen
219
5
Spleen
 5.7 Complications of Splenic Infarction (Continued)
5.7 Complications of Splenic Infarction (Continued)
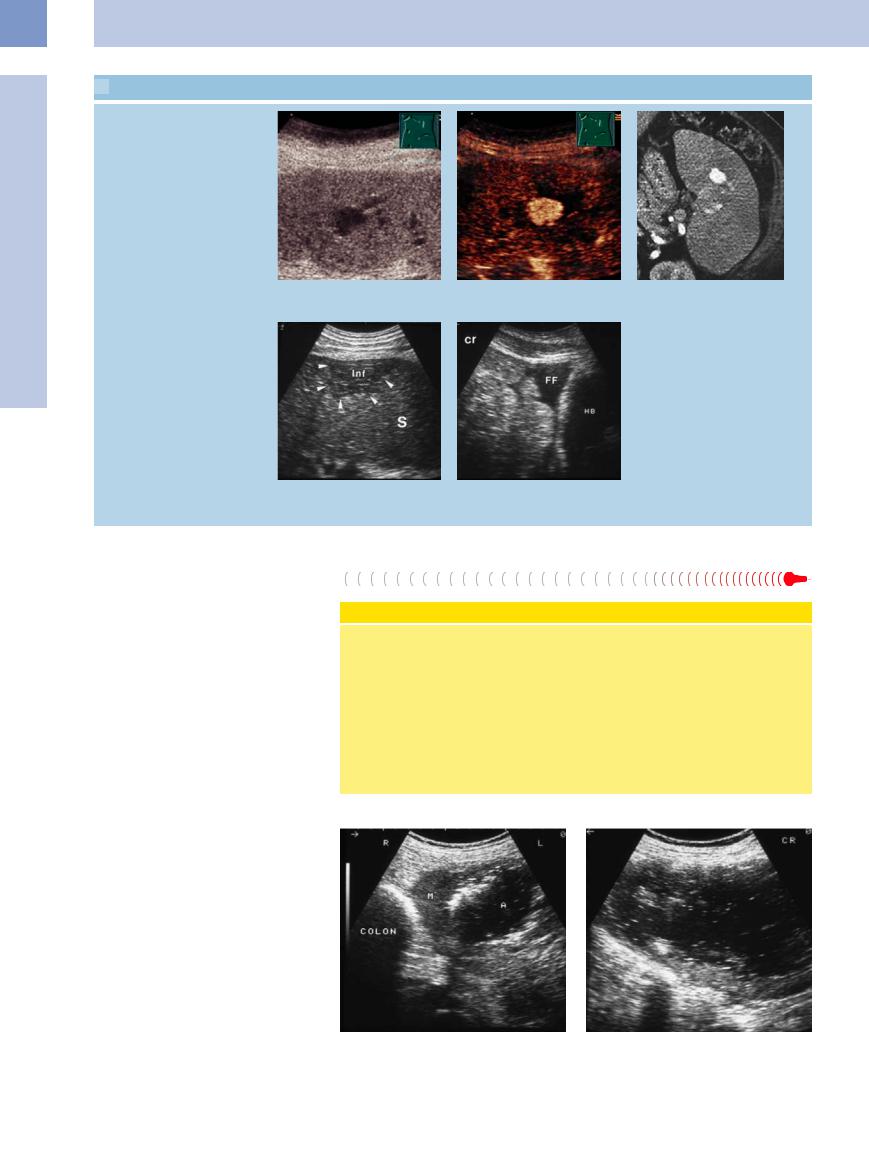
j–l Only an anechoic lesion can be seen in gray-scale ultrasound. CEUS shows a strong signal in the early arterial phase as in intrasplenic pseudoaneurysms. CT shows splenic aneurysms as well.
Free intraperitoneal fluid
m and n Wedge-shaped splenic infarct (inf) with tenuous intraperitoneal fluid (FF) posterior to the urinary bladder (HB). Fine-needle aspiration confirmed the presence of blood within the peritoneal cavity. S = spleen.
Splenic Abscess 









Microabscess. This is usually pyogenic. On ultrasound, a macroabscess is visualized as a smooth or irregularly shaped, primarily hypoechoic lesion that is delineated from the normal parenchymal texture. However, as for hepatic abscess, there is a wide spectrum of ultrasound morphology of these splenic abscesses, which can be traced back to the difference in the number of acoustically relevant echo boundaries. These morphologies include:
●Completely anechoic abscess resembling a cyst with posterior acoustic enhancement
●Hypoechoic abscess with different degree of textural echo pattern ( 5.8a–c)
5.8a–c)
●Markedly hyperechoic abscess, sometimes gaseous, with or without posterior acoustic enhancement (Fig. 5.29)
Differentiating splenic abscess from other focal masses (hematoma, infarct, cyst, tumor) by ultrasound criteria for contour and texture alone, not taking into account any clinical parameters, is fraught with problems. Since splenic abscess has a mortality of up to 100% if left untreated, a tentative diagnosis should be confirmed by ultrasound-guided fine-needle aspiration (3–4 Fr) with bacteriological and cytological work-up ( 5.8 d–f). If it is an abscess, the diagnostic procedure can be expanded to definitive treatment in terms of quantitative evacuation; an abscess less than 8–10 cm across may be aspirated repeatedly, while catheter drainage is recommended for abscess with
5.8 d–f). If it is an abscess, the diagnostic procedure can be expanded to definitive treatment in terms of quantitative evacuation; an abscess less than 8–10 cm across may be aspirated repeatedly, while catheter drainage is recommended for abscess with
Pathogenesis
One of the explanations for the rather low incidence of splenic abscess is the high degree of phagocytosis in the spleen. Hematogenous bacterial spread accounts for approximately 75% of all abscess formation, bacterial endocarditis being the most common cause (possibly via infected splenic infarct).
Superinfection of hematoma in splenic hemorrhage or a liquefying infarct accounts for only 15% of cases.
Other predisposing factors are diabetes, immunosuppression, and neoplastic disea- se—cancer of the stomach, colon, and pancreas. These cancers tend to invade the spleen, with secondary infection after the original organ is perforated.
A distinction has to be made between macroabscesses and microabscesses.
Fig. 5.29a and b Large complex splenic abscess (A) with intralesional gas. M = spleen.
220
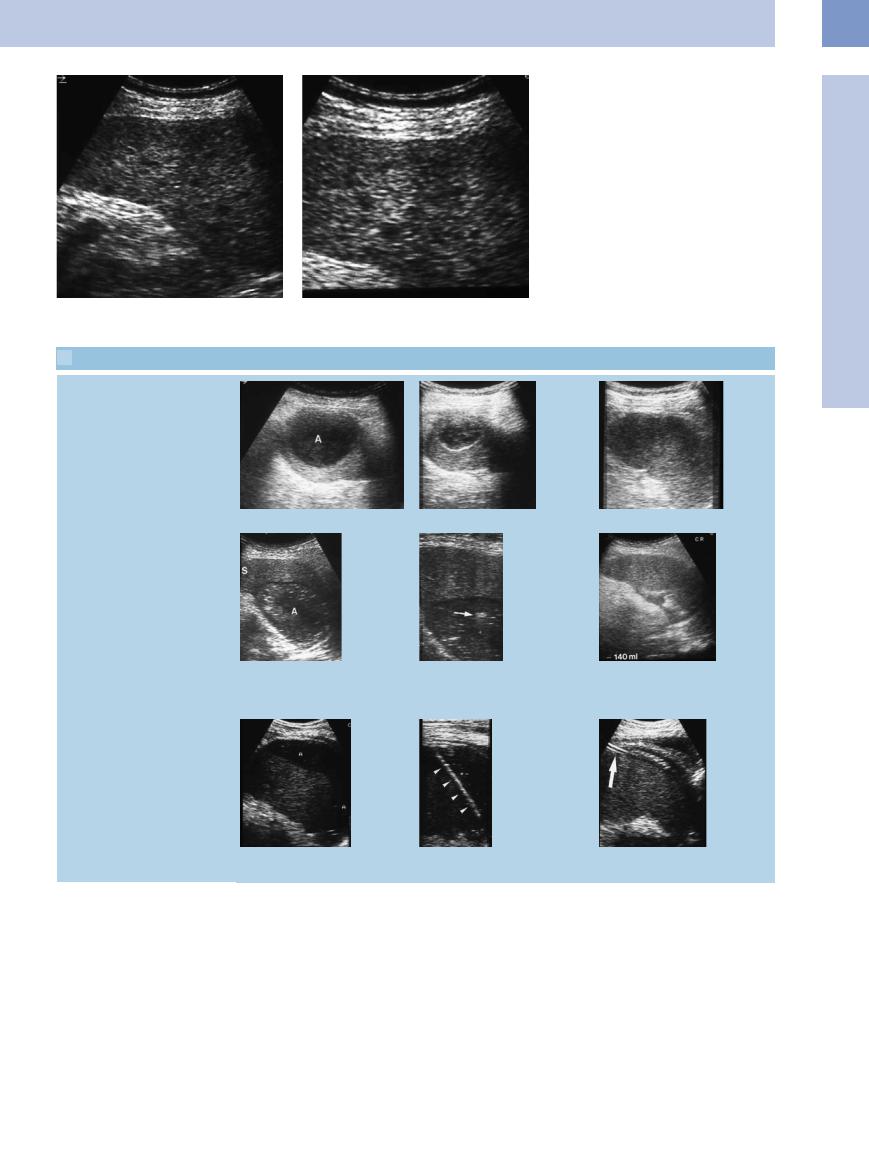
Fig. 5.30a and b Diffuse micronodular splenic invasion in a patient with systemic hematological malignancy, in complete remission, with septic temperature; highly indicative of splenic candidiasis.
 5.8 Treatment of Splenic Abscess
5.8 Treatment of Splenic Abscess
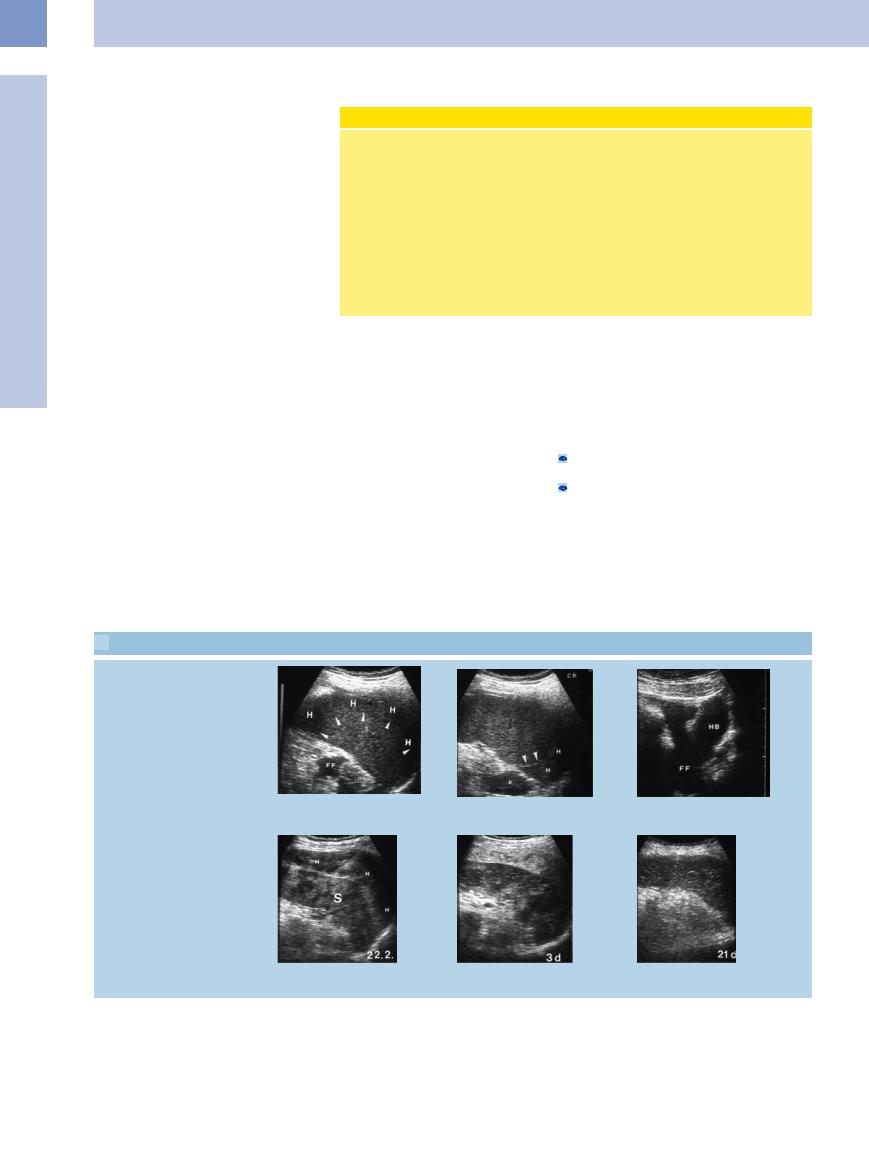
Repeated aspiration
5
Focal Changes of the Spleen
a Hypoechoic splenic abscess (A); 80 mL. b and c Resolution 4 weeks later after two aspiration cycles.
Abscess drainage without catheter
d Complex splenic abscess (A) within the hilum. S = spleen.
e In ultrasound-guided fine-needle aspira- f Complete evacuation of the material. tion of the abscess the echo of the tip of
the needle is seen within the mass (arrow).
Catheter drainage of abscess
g Large abscess (A) capping the spleen.
h Puncture and placement of the guidewire.
i The catheter is placed by Seldinger technique and the abscess is drained.
a diameter in excess of 8–10 cm ( 5.8 g–i). Splenectomy should be reserved for very special cases. Since the parenchymal trauma carries with it the risk of potential hemorrhage, any interventional procedures in splenic abscess should involve close cooperation with the surgeons at an early stage.
5.8 g–i). Splenectomy should be reserved for very special cases. Since the parenchymal trauma carries with it the risk of potential hemorrhage, any interventional procedures in splenic abscess should involve close cooperation with the surgeons at an early stage.
Microabscess. Microabscesses within the spleen, or liver, are uncommon and are seen primarily in immunocompromised patients.
The prevalence is about 26% of all splenic abscesses. The most important etiology is that of chronic disseminated candidiasis, manifested as hepatosplenic candidiasis; microabscess as part of bacterial infection, such as miliary tuberculosis or infection with Pneumocystis jiroveci, is infrequent (Fig. 5.30).
Establishing a prognosis in candida abscess is impossible unless the clinical picture is considered. It is not possible to differentiate a hypoechoic mass from micronodular invasion, as
in malignant lymphoma or leukemia, by sonographic morphology alone. The diagnosis is confirmed by clinical parameters, following up the antimycotic treatment by ultrasound, and sometimes even ultrasound-guided fineneedle aspiration biopsy; because of the concurrent antimycotic therapy, cytohistology mostly demonstrates the presence of necrosis and granulation. Frequently, calcification will be seen as residual scar after splenic microabscess.
221
5
Spleen
Splenic Trauma 



















































Sonographic findings. Ultrasound can visualize the following alterations indicative of splenic trauma:
●Hematoperitoneum: free intraperitoneal, perisplenic, perihepatic fluid and/or fluid in the rectovesical pouch or rectouterine pouch in primary splenic rupture with hemorrhage into the peritoneal cavity.
●Subcapsular splenic rupture: liquid zones (anechoic, isoechoic, or hyperechoic) between the parenchyma and capsula of the spleen.
●Organ injury: destruction of the parenchymal texture, with visualization of textural nonhomogeneity or splenic laceration of varying severity as direct indication of tissue lesions and/or hemorrhage.
Subcapsular splenic rupture. External trauma leading to subcapsular splenic rupture ( 5.9a–f) frequently results in the hemorrhage between parenchyma and capsula capping the organ, primarily along the convex diaphragmatic surface; this can produce a double contour on ultrasound examination. Subcapsular hemorrhage around the splenic hilum is less common.
5.9a–f) frequently results in the hemorrhage between parenchyma and capsula capping the organ, primarily along the convex diaphragmatic surface; this can produce a double contour on ultrasound examination. Subcapsular hemorrhage around the splenic hilum is less common.
As in the post-traumatic subcapsular hemorrhage of the liver, it has to be pointed out that the acute subcapsular splenic hemorrhage may also be distinctly hyperechoic and could easily be overlooked on cursory examination. Sometimes an enlarged left hepatic lobe may mimic a subcapsular mass.
Ultrasound Grading System in Splenic Trauma
Grade 1
Subcapsular hematoma, thickness <3 cm, and/or intraparenchymal lesion <3 cm diameter, splenic capsule unbroken.
Grade 2
Subcapsular hematoma, thickness ≥ 3 cm, and/or intraparenchymal lesion >3 cm diameter, splenic capsule unbroken.
Grade 3
Splenic fragmentation, avascular spleen, or flow phenomena (arteriovenous pseudo-
aneurysms) in liquefied intraparenchymal areas.
A retrospective study of 30 patients with splenic rupture demonstrated that “watchful waiting” was justified in grade 1 patients (n = 6), while surgery became necessary in all grade 3 trauma cases (n = 8). Patients with grade 2 splenic trauma (n = 16) had to undergo surgery in 62% of cases (n = 9).
Organ injury. A careful ultrasound study will be able to successfully demonstrate most cases of organ injury, although sometimes shortterm follow-up will become necessary. Injuries to the spleen are recognized by:
●Irregularly defined hypoechoic, isoechoic, as well as hyperechoic lesions of the usually
homogeneous parenchymal |
texture |
( |
5.9 g–l) |
|
|
● Destruction of the splenic |
contour |
( |
5.9 m–o) |
|
|
CEUS delineates subcapsular hemorrhages and intraparenchymal lesions better than B-scan (
5.9 p,q).
Treatment. In traumatic and nontraumatic splenic rupture, the current regimen calls for preservation of the spleen, if at all possible. This depends on the extent of the injury, for which there are several grading systems. These are founded on ultrasound studies and have become quite useful as additional parameters in the decision how to tailor treatment. However, imaging modalities cannot be the sole foundation on which to rest the decision for or against surgery. The clinical picture and laboratory work-up still play the primary role here. Repeated short-term (possibly even hourly) follow-up ultrasound studies have proved to be very useful.
 5.9 Splenic Trauma
5.9 Splenic Trauma
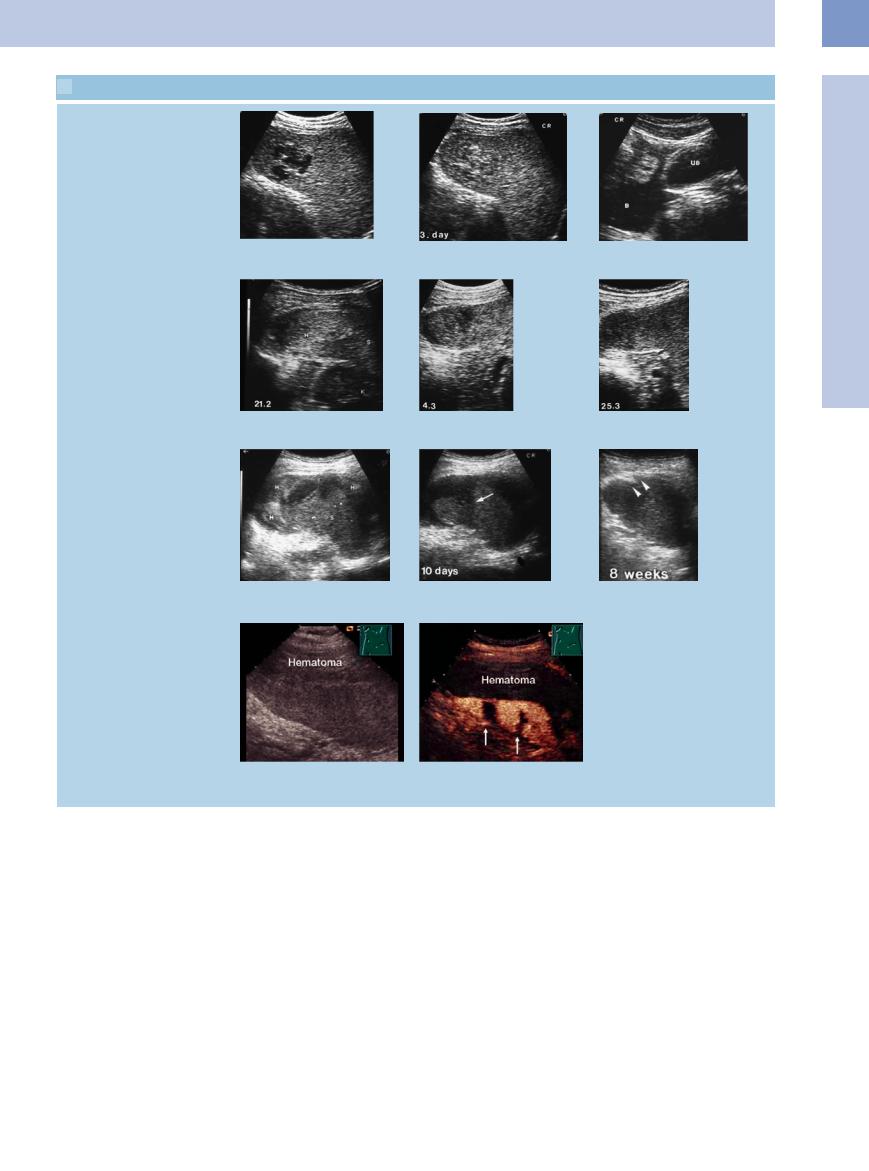
Subcapsular hematoma
a–c Almost isoechoic subcapsular hematoma (H) in mononucleosis. Free fluid (FF) in rectouterine pouch. HB = bladder; K = kidney; S = spleen.
d–f Subcapsular hematoma (H) in chickenpox sepsis. During the course, changing echogenicity of the hematoma with resolution. S = spleen.
222
 5.9 Splenic Trauma (Continued)
5.9 Splenic Trauma (Continued)
Intraparenchymal splenic trauma
g–i Intraparenchymal splenic trauma with increasing echogenicity over time. In addition, free fluid (probably blood: B) in rectouterine pouch. UB = urinary bladder.
j–l Intraparenchymal, irregularly defined echogenic defect after blunt abdominal trauma, resolving eventually. H = hematoma; S = spleen.
m–o Large complex subcapsular splenic hemorrhage (H) with demarcation of a parenchymal laceration (arrow); eventually resolved but with scarring. S = spleen.
p and q A subcapsular hemorrhage can be seen in gray-scale ultrasound. In CEUS, the subcapsular hemorrhage is demarcated by nonenhancement. In addition, two other intraparenchymal lacerations are detectable (arrows).
5
Focal Changes of the Spleen
223
5
Spleen
Splenic Metastasis








Splenic metastasis is detected in approximately 7% of autopsies. Metastasis in the spleen is far less common than in the liver, the hypothesis being that the spleen is equipped with a special immunological microenvironment.
Ultrasound appearance. Classification of splenic metastasis is along the same lines as in the liver, the splenic parenchyma being the in-vivo reference. The metastases can be classified as being anechoic, hypoechoic, isoechoic, hyperechoic, and complex. The most common sonographic types demonstrated in the spleen are hypoechoic metastases. Larger metastases may exhibit central colliquation.
All metastases may present with or without a halo, which can be regarded as typical of, but not specific to, hepatic and splenic malignancy (splenic metastases can be isoechoic or even hyperechoic in comparison to splenic tissue
and are discussed here for systematic reasons ( 5.10a–f).
5.10a–f).
Metastasis in direct extension. Splenic metastasis may also arise by direct extension of pancreatic, colonic, and gastric cancer ( 5.11). In rare cases, malignancies of the lung or diaphragm may break through and invade the spleen in continuity. Also peritoneal metastasis may encase and invade the spleen.
5.11). In rare cases, malignancies of the lung or diaphragm may break through and invade the spleen in continuity. Also peritoneal metastasis may encase and invade the spleen.
Complications. Complications may be localized perforation, abscess, splenic hemorrhage, and rupture ( 5.10e–i). Calcification of splenic metastasis has also been reported as well as tumor-induced splenic vein thrombosis.
5.10e–i). Calcification of splenic metastasis has also been reported as well as tumor-induced splenic vein thrombosis.
Hypoechoic Hypervascularized Mass
Round masses are sometimes detected as incidental findings in color Doppler ultrasound ( 5.12 g–i) and show an increased vascularization in CEUS (
5.12 g–i) and show an increased vascularization in CEUS ( 5.12j–m). These hypervasculated tumors show no growth according to present observations and most likely correspond to vascular tumors, such as hemangioma. A follow-up exam by ultrasound is necessary.7
5.12j–m). These hypervasculated tumors show no growth according to present observations and most likely correspond to vascular tumors, such as hemangioma. A follow-up exam by ultrasound is necessary.7
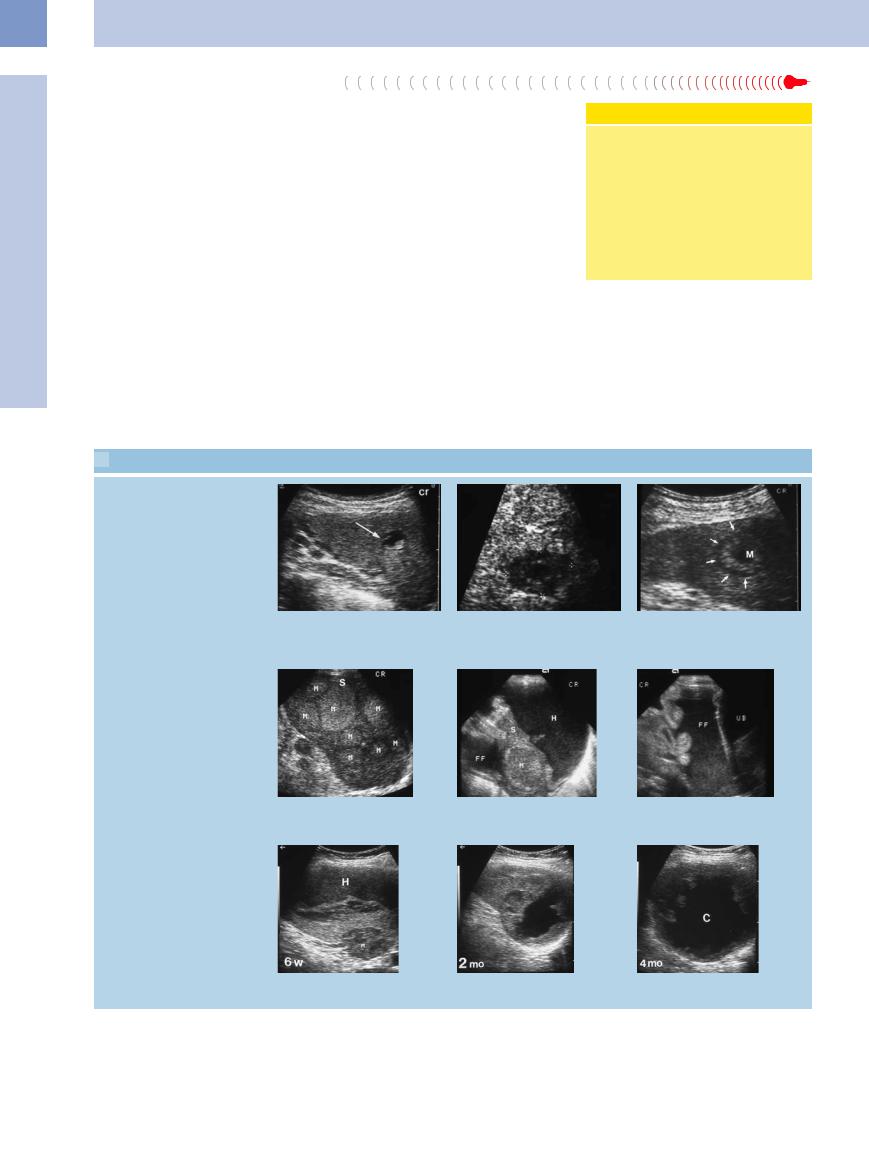
 5.10 Splenic Metastasis
5.10 Splenic Metastasis
Hypoechoic/complex
a Splenic metastasis. |
b Hypoechoic splenic mass with central |
|
liquefaction in colon cancer: splenic |
|
metastasis. |
Hyperechoic
c Hyperechoic splenic mass (M) with hypoechoic margin (“halo”) and central liquefaction in colon cancer: splenic metastasis.
d Splenic metastasis (M). |
e and f Splenic metastasis (M) in ENT malignancy. Large intrasplenic hematoma (H); at |
|
the same time a large amount of free intraperitoneal fluid (FF) can be demonstrated. |
|
The weak, emaciated patient refused surgery. UB = urinary bladder; S = spleen. |
Bleeding, liquefaction
g–i Malignant melanoma. Slowly resolving splenic metastasis (M) and subcapsular hematoma (H); in addition, formation of a liquefied splenic metastasis.
224
