
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
4
Fig. 4.16
a Early stage of chronic alcohol-associated pancreatitis; hypoechoic structure, sporadically interspersed fibrotic areas, and the undulating contour.
Diffuse Pancreatic Change
b Early stage of chronic pancreatitis, hypoechoic lobu- |
c Early stage of chronic pancreatitis with fibrosis. |
lated structure. |
|
Autoimmune Pancreatitis














































Autoimmune pancreatitis was first described in 1961 by Sarle. It occurs as a diffuse (“sausagelike”) focal or segmental form in the pancreatic head, body, or tail. There is diffuse or segmental narrowing of the main pancreatic duct, but usually no calcifications, intraductal calculi, or cysts. In segmental involvement it looks like a carcinoma but there is almost no (or only minimal) duct dilatation. In autoimmune pancreatitis, as in other forms of focal or segmental pancreatitis, an early moderate to distinct enhancement with typical nonhomogeneous vascularization is seen in contrast-enhanced ultrasonography (CEUS) (Fig. 4.17).5,6
Fig. 4.17
a Autoimmune pancreatitis; enlarged organ, diffuse hypoechogenicity.
b Segmental autoimmune pancreatitis; swollen pancreatic tail, diffuse hypoechogenicity.
Hyperechoic Texture
|
|
|
Diffuse Pancreatic Change |
|||
Pancreas |
||||||
|
|
Hypoechoic Texture |
||||
|
|
|
|
|
Large Pancreas |
|
|
|
|
|
|
Small Pancreas |
|
|
|
|
|
|
Hyperechoic Texture |
|
|
|
|
|
|
||
|
|
|
|
|
||
|
|
|
Focal Changes |
|||
|
|
|
||||
|
|
|
|
|
||
|
|
|
Dilatation of the Pancreatic Duct |
|||
|
|
|
||||
Fibromatosis/Lipomatosis
Fibrosis in Hemochromatosis/Cystic Fibrosis
Chronic Pancreatitis
Fibromatosis/Lipomatosis
Lipomatosis. Very few diffuse changes of the pancreas catch the eye as easily as does pancreatic lipomatosis, and therefore this diagnosis is made without hesitation. Compared with the liver, the pancreas demonstrates normal size and an even, intensively hyperechoic texture. This change is found most often in the overweight non-insulin-dependent diabetic patient, but it is unlike the fibrosis of little consequence.
Fibrolipomatosis. The same holds true for fibrolipomatosis, but here the change is coarser and frequently accompanied by enlargement of the gland. Fibrolipomatosis is more common in elderly people, in whom the loss in parenchyma is made up by fibrosis and fatty tissue (Fig. 4.18).
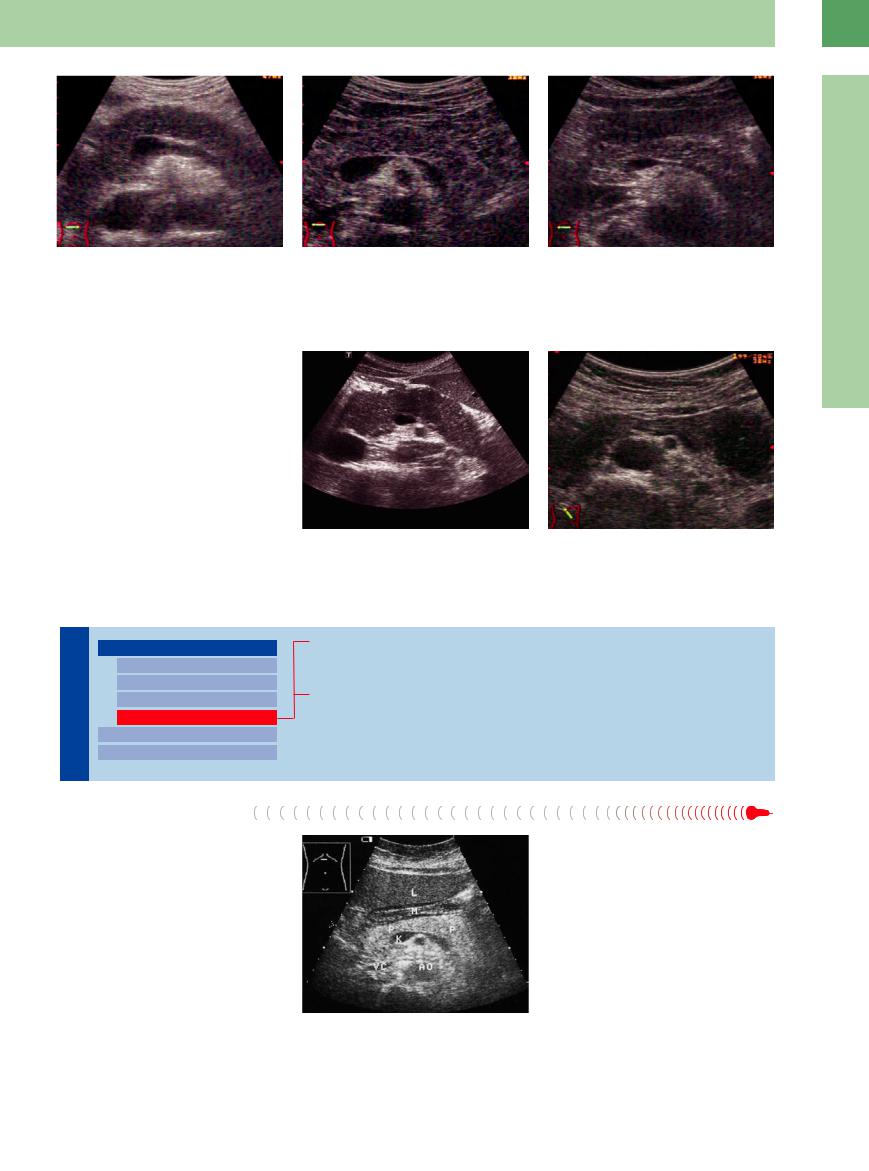
Fig. 4.18 Fibrolipomatosis of the pancreas (P): hyperechoic, somewhat coarse texture. M = stomach; K = confluence of portal vein; VC = vena cava; AO = aorta; L = liver.
175

4
Pancreas
sound cannot differentiate the various causes of pancreatic fibrosis (Fig. 4.19).
Fig. 4.19 Fibrosis of the pancreas (calipers, P). VL = splenic |
b In hemochromatosis. |
vein; AO = aorta; L = liver. |
|
a In chronic alcohol-associated pancreatitis (calipers). |
|
Chronic Pancreatitis







Parenchymal changes. Chronic pancreatitis is characterized by fibrotic parenchymal scarring, particularly in the acinar epithelium; with increasing fibrosis, only ectatic excretory ducts, pancreatic islets, obliterated vessels, and some residual parenchyma will remain. In more than 40% of cases this process of fibrosis will be focal within the pancreatic tail or will appear as “groove pancreatitis” or divisum pancreatitis in the head of the pancreas. The frequency of calcification (intraductal calculi) varies, focal necrosis being present in roughly 10% of patients and inflammatory postpancreatitis pseudocysts in 40%. Dilatation of the primary and secondary ductsmanifestsitselfasretentioncysts,which in turn are sequelae to intrapancreatic secretory obstruction and ductal ectasia.
Ultrasound findings. As in siderosis, here too ultrasound can demonstrate the increased echogenicity induced by the fibrosis, but in chronic pancreatitis these fibrotic areas tend to be mottled or cloddy. They may be loosely distributed or bunched together. Additional calcification results in zones of posterior shadowing. Calcifying chronic pancreatitis demonstrates finely or roughly distributed hard echoes. Since the frequent shadowing zones are most often wide, they will conceal the deeper structures such as the retroperitoneal vessels. The pancreas will be difficult to identify because the landmark structures cannot be visualized and the contour of the organ is blurred. Together with the subsequent atrophy, this compounds the fact that ultrasound studies of the pancreas have a sensitivity of only 70–80%. For EUS it is 80–100%, and therefore higher, as in the ERCP (Fig. 4.20; see also Figs. 4.15c, 4.46, 4.47).7,8 Pseudocysts and dilatation of the duct tend to result in a more heterogeneous texture (see below).
If all morphological changes are present, chronic pancreatitis is characterized by the fol-
Classification of Chronic Pancreatitis (Marseille 1984)3
Clinical characteristics. Recurrent or persistent abdominal pain; sometimes without pain.
Morphological characteristics:
●Irregular sclerosis with focal, segmental, or diffuse destruction and permanent loss of exocrine parenchyma of the gland.
●Dilatation of varying degree of various ductal segments.
●Protein deposits, calcification (intraductal calculi).
●Edema, focal necrosis, inflammatory cells, and cysts and pseudocysts are found to a varying degree.
●The islets of Langerhans will be affected much later.
●The following descriptive morphological terms are used:
1.Chronic pancreatitis with focal necrosis
2.Chronic pancreatitis with segmental or diffuse fibrosis
3.Chronic pancreatitis with or without calcification (calculi)
Course. The irreversible morphological changes will lead to a definite progressive loss of the exocrine and endocrine pancreatic function.
Special case: Obstructive chronic pancreatitis
Morphological characteristics:
●Dilatation of the ductal tree upstream of the obstruction in a major duct (e. g., narrowing as in pancreas divisum or scarring)
●Diffuse atrophy of the parenchyma and regularly diffuse fibrosis
●Calculi almost never present
Course. After the obstruction has been cleared, the morphological and functional changes will usually resolve completely.
lowing ultrasound criteria (see Fig. 4.24,  4.1; see also Fig. 4.55):
4.1; see also Fig. 4.55):
●Fibrosis
●Calcification/intraductal stones
●Microcysts and macrocysts
●Ectatic duct
●Atrophy in the terminal stage
Most chronic pancreatitis does not exhibit all of the above criteria but rather only fibrosis, dilated ducts, or a combination of two or more of these criteria. In acute episodes the sonographic signs of acute pancreatitis may also be present.
The 1984 Marseille classification3 of chronic pancreatitis rests on the clinical and morphological aspects.
Imaging modalities play a vital role in the diagnosis of chronic pancreatitis. On account of its possible side effects, endoscopic retrograde pancreatography (ERP) hasbeenabandoned as a diagnostic baseline method in favor of other imaging methods such as EUS or magnetic resonance cholangiopancreatography (MRCP). Nevertheless, ERCP is a decisive procedure for planning interventional therapeutic options. The guidelines for assessing the severity of chronic pancreatitis also take these different imaging modalities into account (Table 4.2).
176

Since the different forms of chronic pancreatitis can be differentiated morphologically, and furthermore since there may be focal change, by itself or concomitant with the underlying disease, chronic pancreatitis may be demonstrated in a number of sonographic variants, depending on the predominant histopathological changes.  4.1 depicts possible sonographic images and variants.
4.1 depicts possible sonographic images and variants.
Differentiation. Misdiagnosis may result from pancreatic fibrolipomatosis/lipomatosis due to fatty and fibrotic deposits in elderly obese diabetic patients, as well as scattering, refraction, and ultrasound absorption by adjacent/superimposed tissue. These factors will blur the margins and texture of the organs and could lead to the mistaken ultrasound diagnosis of chronic pancreatitis. In CDS stones calculi (calcium car-
bonate) can be differentiated from fibrolipomatosis by the “twinkling artifact” with high pulse repetition frequency (PRF) (color-rich “confetti phenomenon” in the region of the shadowing) (Table 4.3, Fig. 4.21; see also
Fig. 4.47b).
4
Diffuse Pancreatic Change
Fig. 4.20 Micronodular calcification with shadowing (S) in |
b Plain radiograph. |
chronic calcifying pancreatitis. |
|
a Sonographic image. M = stomach; AO = aorta; L = liver. |
|
Fig. 4.21 Misdiagnosis of chronic pancreatitis (P = pancreas). In this case, ultrasound cannot assess the possible presence of coarse, cloddy fibrosis. CT: normal pancreas (no clinical symptoms). Probable interference by intervening peripancreatic fat.
Table 4.2 Classification of chronic pancreatitis by imaging modality (Cambridge 1984)4
Pancreas/ |
ERCP |
Ultrasound or CT |
pancreatitis |
|
|
Normal |
Good-quality visualization of the entire gland without pathological signs |
|
Questionable |
< 3 pathological branches |
1 pathological finding = pancreatic duct |
|
|
2–4 mm |
Mild |
> 3 pathological branches |
2 pathological findings of |
|
|
● Cysts < 10 mm |
|
|
● Irregular duct |
|
|
● Acute focal pancreatitis |
|
|
● Parenchymal heterogenicity |
|
|
● Increased echogenicity of the duct wall |
|
|
● Irregular contour of head/body |
Moderate |
All of the above signs plus |
All of the above pathological signs |
|
pathological pancreatitis |
|
Severe |
All of the above signs plus one or more of the following: |
|
|
● Cyst > 10 mm |
|
|
● Intraductal voids |
|
|
● Calculi/pancreatic calcification |
|
|
● Duct obstruction (stricture) |
|
|
● Severe dilatation or irregularity of duct |
|
|
● Adjacent organs a ected (demonstrated on ultrasound or CT) |
|
Table 4.3 Differentiating chronic pancreatitis and fibrolipomatosis
Chronic |
Fibrolipomatosis |
pancreatitis |
|
Hyperechoic, coarse |
Hyperechoic, fine |
texture |
texture |
Inhomogeneities |
Homogeneous |
because of: |
structure |
●Microcysts/ macrocysts
●Parenchymal calcification/ intraductal calculi
●Obstruction/ stricture of duct, dilated duct
Increased/decreased |
Normal size |
size (atrophy) |
|
Indurated |
No or little indu- |
|
ration (palpating |
|
finger/propagated |
|
aortic pulse) |
Elastography: indu- |
No color changes |
ration color-coded |
|
blue |
|
177
4
Pancreas
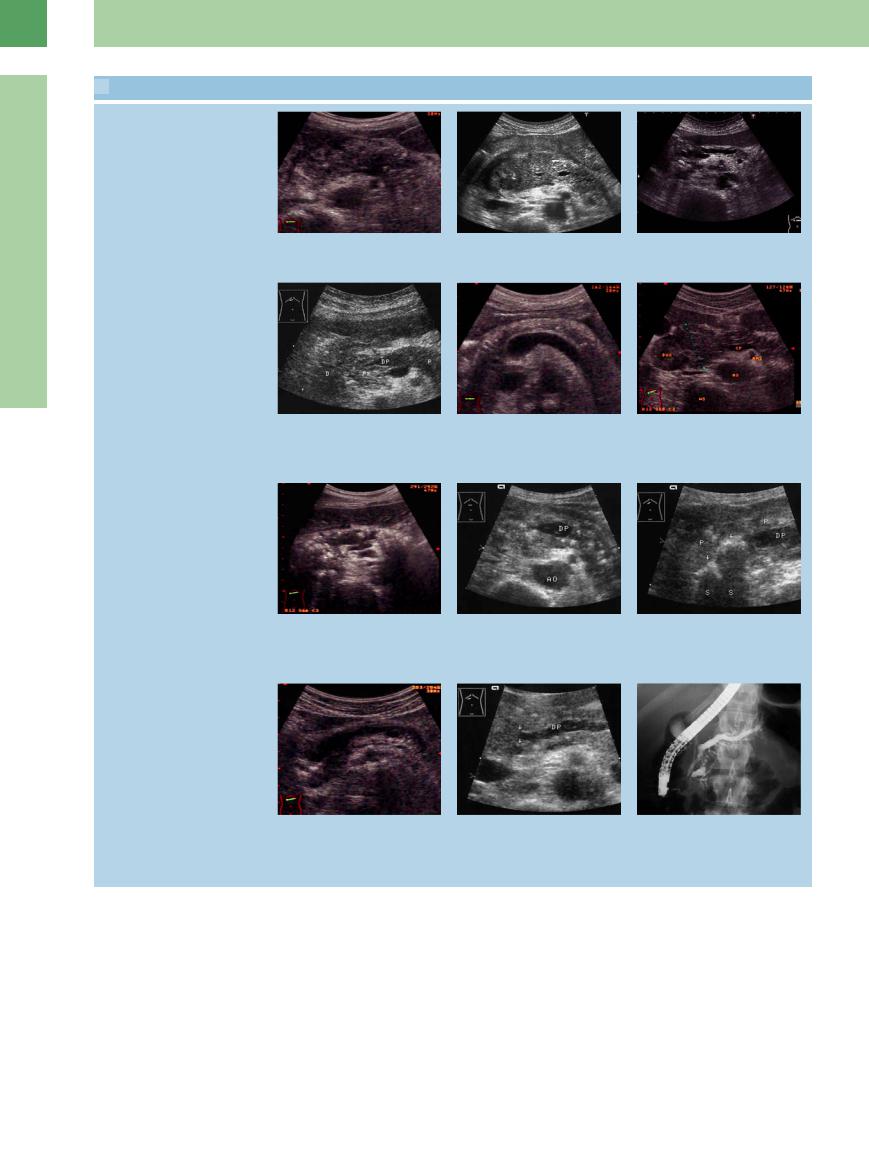
 4.1 Chronic Pancreatitis on Ultrasound
4.1 Chronic Pancreatitis on Ultrasound
Chronic pancreatitis with focal necrosis
Chronic pancreatitis with segmental or diffuse fibrosis
Chronic pancreatitis with calcification/ calculi
Obstructive chronic pancreatitis (special variant)
a Acute episode of chronic pancreatitis with focal necrosis (anechoic area).
d Fibrosis of the pancreatic head (PK), stenosis of the pancreatic duct (DP); normal body and tail of the pancreas. D = duodenum; P = pancreas.
g Calcification in the dilated main duct and in parenchyma, atrophy.
j Chronic obstructive pancreatitis (for the last 5 years) due to intraductal papillary mucinous neoplasm in the head.
b Acute episode of chronic pancreatitis with focal necrosis in the head and tail.
e Fibrosis in amyloidosis.
h Same patient as in d with segmental CP 3.5 years later: calcification of the entire pancreas. DP = pancreatic duct;
AO = aorta.
k Chronic obstructive pancreatitis in pancreas divisum: relative stenosis of the anterior accessory duct of Santorini. In the pancreatic head two ducts are detectable (arrows). DP = pancreatic duct.
c Acute episode of chronic pancreatitis with focal necrosis in the head, dilated pancreatic duct.
f Fibrosis of the pancreas with segmental head enlargement (cursors). DUO = duodenum, AMS = superior mesenteric artery, AO = aorta, WS = spinal column, CF = venous confluence.
i Same patient: oblique right epigastric view: coarse, cloddy calcification or intraductal calculi (arrows, shadowing S) along the pancreatic duct in the head. P = pancreas; DP = pancreatic duct.
l Same patient as in k, pancreas divisum: prominently dilated stubby pancreatic duct terminating in a cul de sac; dilated accessory duct (of Santorini), which has taken over as primary drain via the minor papilla (see Fig. 4.44b).
178
