
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
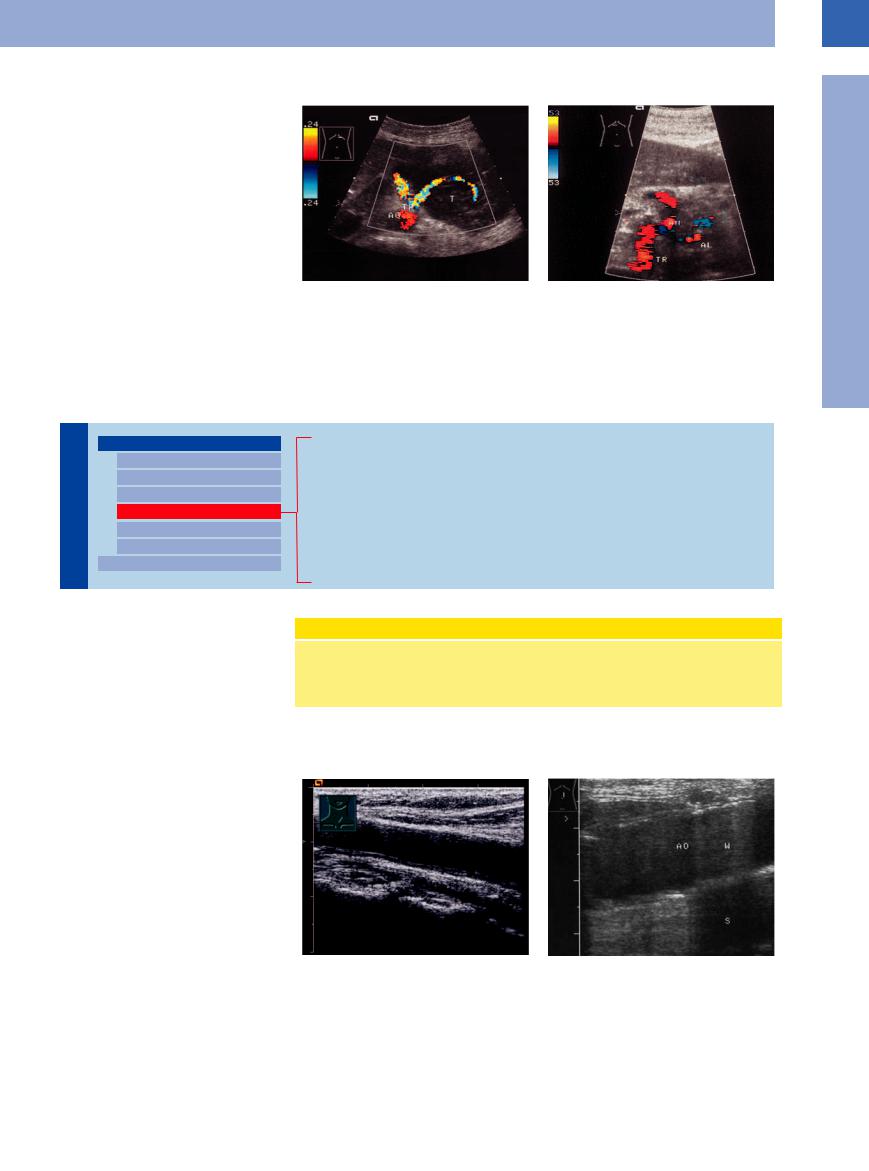
Tumor Stenosis/Infiltration













































Malignant lymphomas tend to compress adjacent vessels (Fig.1.32), while in carcinomas infiltration of the vessel wall is more common.
Fig. 1.32 Malignant lymphoma of the pancreas. |
b Invasion of the celiac trunk and its branches (common |
a Splenic artery coursing through the tumor (T); note the |
hepatic artery, hepatic artery and splenic artery [AL]) by |
distinct compression of the vessel. |
inoperable pancreatic carcinoma: periarterial hypoechoic |
|
masses. AH = hepatic artery; TR = celiac trunk. |
Wall Thickening
Vessels |
Aorta, Arteries |
|||
|
|
|
||
|
|
|
|
Anomalies and Variant Positions |
|
|
|
|
|
|
|
|
|
Dilatation |
|
|
|
|
Stenosis |
|
|
|
|
Wall Thickening |
|
|
|
|
|
|
|
|
|
Intraluminal Mass |
|
|
|
|
Perivascular Mass |
|
|
|
Vena Cava, Veins |
|
|
|
|
||
Early Arteriosclerotic Lesions (High Echogenicity, No Changes of the Wall Surface) Advanced Arteriosclerotic Lesions (Plaques <4 mm without Surface Changes) Complex Arteriosclerotic Lesions (Plaques ≥4 mm)
Protruding Arteriosclerotic Lesions (Plaques with Mobile Parts) White Thrombus
Arteritis
Mönckeberg’s Arteriosclerosis (Medial Calcific Sclerosis) Synthetic Grafts
Most often thickening of the aortic and arterial |
WHO Definition of Atherosclerosis |
|
walls is the result of arteriosclerosis, which in |
||
|
||
turn is rooted in atheromatosis and atheroscle- |
“Atherosclerosis implies a fluid combination of intima changes in the arteries—as compared |
|
rosis. The World Health Organization (WHO) |
with the arterioles—comprising a focal accumulation of lipids, complex carbohydrates, blood |
|
has defined atherosclerosis as follows: |
and its components, fibrous tissue, and calcium deposits, accompanied by changes in the |
|
|
media.” |
Early Arteriosclerotic Lesions (High Echogenicity,
(High Echogenicity, No
No Changes
Changes of the Wall Surface)
of the Wall Surface) 












The early stages of arteriosclerotic lesions are characterized by lipid and cholesterol deposits (lipid plaques and “atheromas” or atherosclerotic plaques, respectively). Subsequently, these plaques may rupture (atherosclerotic ulcers) and become the focus for white thrombi (atherothrombosis).
There is a significant correlation between the complexity of the aortic plaques and a risk of embolization: more than 4 mm thickening of the plaques increases the risk of cerebral infarction more than ninefold.12
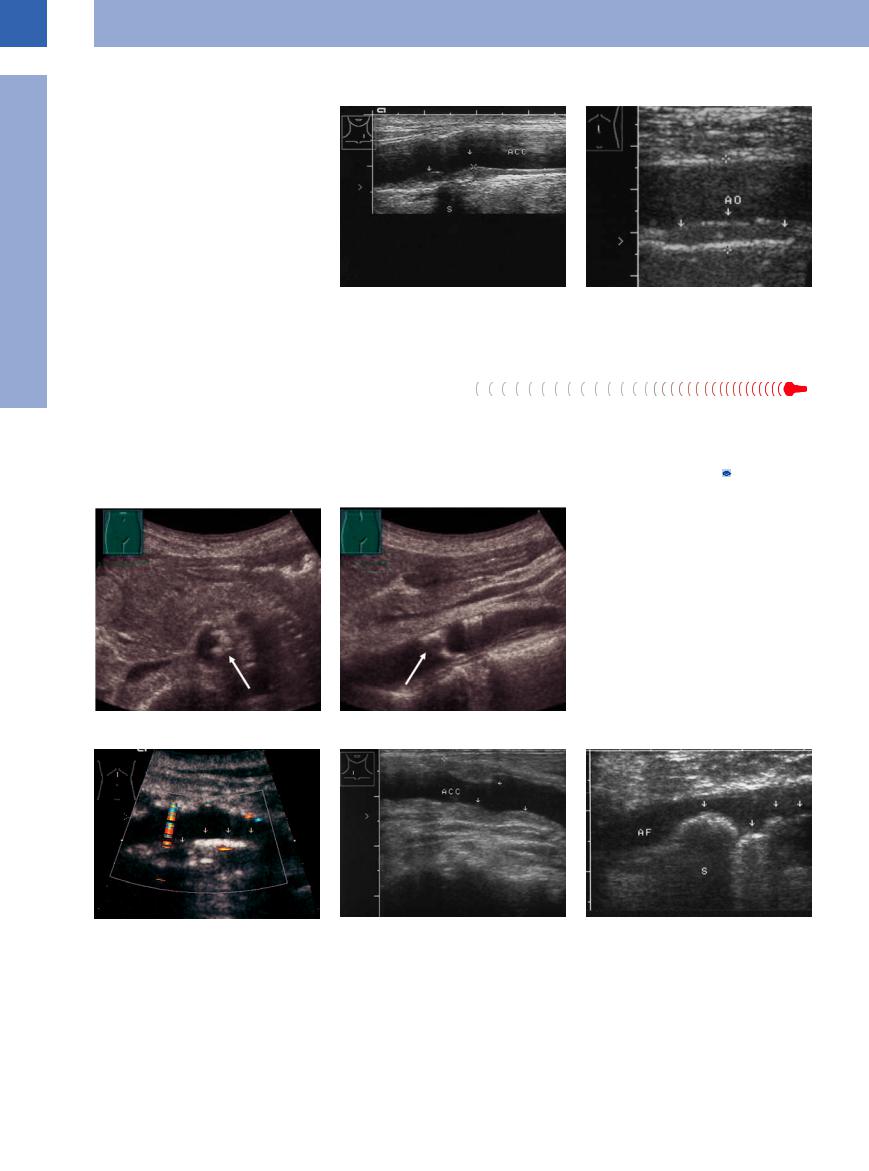
The different phases and stages of arteriosclerosis result in a variety of sonographic structures in and at the wall of the vessels; however, this always will imply a thickening of the wall. The earliest morphological sign in ultrasonography of these alterations taking place within the intima and media is a widening of the intima–media complex1 (Fig.1.33, Fig.1.34).
Fig. 1.33 Markedly thickened intima–media complex |
Fig. 1.34 Early stage of a complex arteriosclerotic lesion: |
(0.14 mm, cursors). |
nascent calcification at the anterior aortic wall, mirrored |
|
echo (W, resonance artifact). Compared to the remainder |
|
of the aorta (AO) this segment appears to generate an |
|
echo. |
1
Aorta, Arteries
19
1
Vessels
Advanced Arteriosclerotic Lesions (Plaques < 4 mm without
without  Surface Changes)
Surface Changes)

















The sonographic sign of advanced arteriosclerotic lesions is a thickened arterial wall. It may appear as a hypoechoic or—as in most cases—a hyperechoic structure. If there is no shadowing, this increased echogenicity could be explained by cholesterol deposits. On the other hand, in complex lesions there will be fibrosis leading to echogenic caps covering the plaques (Fig.1.35).
|
Fig. 1.35 |
b Advanced arteriosclerotic lesion: localized thickening |
|
a Soft plaques: small, soft, nonshadowing plaque (arrow) |
of the wall seen as a hypoechoic band covered by an |
|
in the common carotid artery. Broadening of the intima– |
echogenic (fibrous) cap (arrows). AO = aorta. |
|
media complex, consistent with early atherosclerosis. |
|
Complex Arteriosclerotic Lesions (Plaques ≥ 4 mm) |
|
|
Secondary calcification, fibrosis, and plaque |
truding hypoechoic areas, or in the case of |
the lumen or systemic embolization (Figs.1.35, |
rupture (arteriosclerotic ulcer) with superim- |
secondary calcification a severely hyperechoic |
1.36, 1.37, 1.38, 1.39). Because of the turbulence, |
posed white thrombi lead to a complex lesion. |
area. Consecutive shadowing may block the lu- |
color-flow Doppler produces numerous color |
The ultrasound image shows irregular wall |
men. Complex lesions protrude into the lumen |
changes from red to blue (“confetti phenom- |
thickening with a complex structure and pro- |
to varying degrees and result in narrowing of |
enon”) (Fig.1.28, Fig.1.46b, 1.3b). |
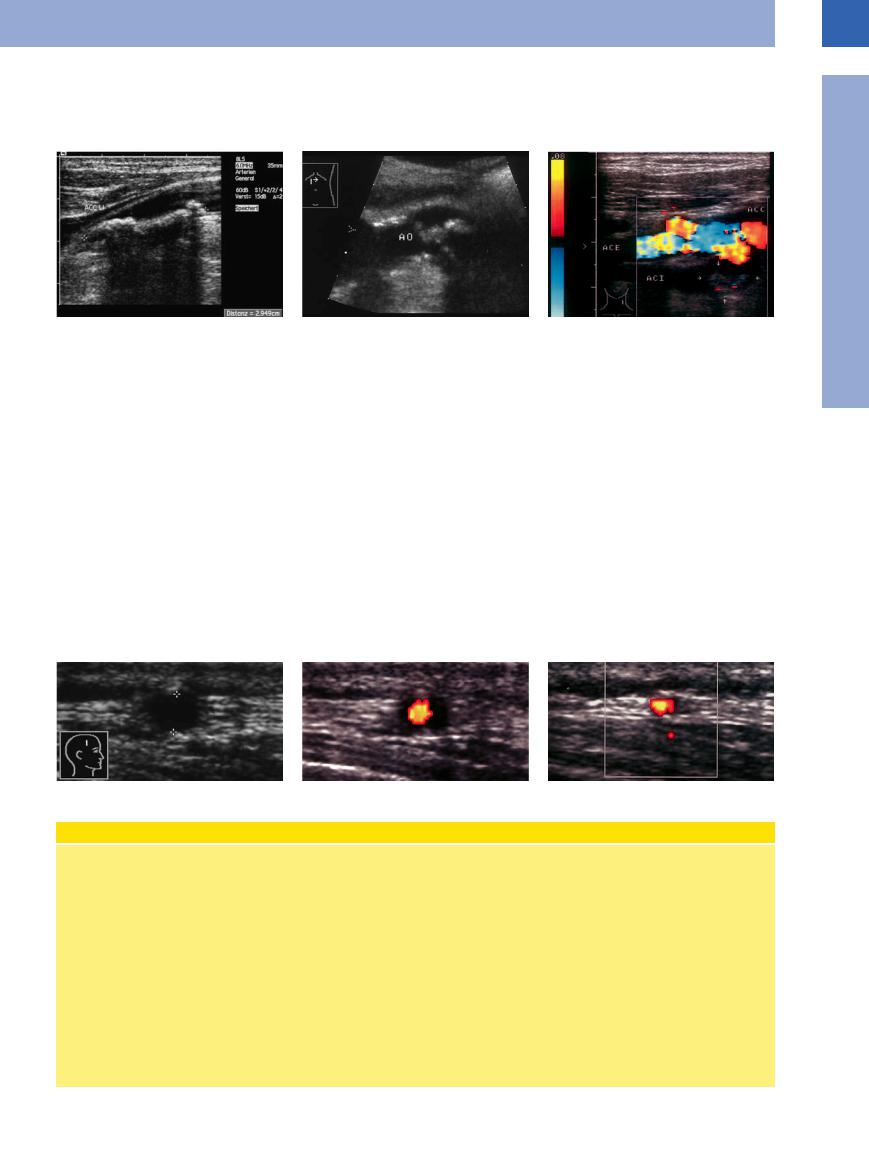
Fig. 1.36 a and b Complex arteriosclerotic plaques ≥ 4 mm (arrows) of the aorta.
Fig. 1.37 Advanced, complicated (“complex”) atherosclerotic lesion: pronounced wall thickening (posterior) with a hypoechoic band of atheromatosis, bordered on the luminal side by hyperechoic plaque. “Stable plaque” with smooth margins. Note the twinkling artifact on the wall.
Fig. 1.38 Complex arteriosclerotic lesion of the common carotid artery (ACC): extensive white thrombi (arrows) mimicking thickening of the wall but still resulting in significant narrowing of the lumen.
Fig. 1.39 Protuberant plaque with risk of embolization. Hyperechoic, well-circumscribed plaques (arrows), probably at low risk for embolization. AF = femoral artery; S = shadowing.
20
Protruding Arteriosclerotic
Arteriosclerotic Lesions (Plaques with Mobile Parts)
Lesions (Plaques with Mobile Parts)

























Once the lesions become superimposed by |
intraluminal mass of sometimes irregular |
white thrombi, the wall will bulge into the lu- |
structure (Fig.1.40; see also Fig.1.46, Fig.1.47). |
men, and these thrombi appear as hypoechoic |
|
1
Aorta, Arteries
Fig. 1.40 |
b Complex structure with an irregular surface in the |
a Protuberant complex plaque (cursors) in the left CCA |
distal aorta (AO), presumably at greater risk for emboli- |
with cranially attached platelet thrombus, risk of emboli- |
zation. |
zation. |
|
White Thrombus 


















































Intraluminal white thrombi are most common |
sequela of the underlying arteriosclerosis |
in aneurysms. They do not represent a true |
(Fig.1.16b; see also Fig.1.46). |
thickening of the wall but nevertheless are a |
|
Arteritis 























































There are clinical and histological differences between the various types of arteritis. Figure 1.41 illustrates the sonographic characteristics of one such type. Figure 1.22e and Fig.1.41 demonstrate the characteristic signs of arteritis in B-mode scanning and color Doppler sonography. The most reliable sign is the hypoechoic, concentric wall thickening as a dark halo sur-
rounding the colorful lumen in giant-cell arteritis, with a sensitivity of about 80% and a specificity of more than 90%. With the equally frequent indication of stenoses and occlusions, the sensitivity increases, so that a biopsy is only necessary in the rare case of negative sonography.6
c Hyperechoic plaque with a regular surface (arrows; internal carotid artery, ACI); mild to moderate embolization risk. ACE = external carotid artery.
There are characteristic signs of temporal arteritis that can be visualized by color duplex ultrasonography; the most specific sign is a dark halo on the artery wall, other signs are stenosis or occlusions of temporal artery segments.
Fig. 1.41a–c Temporal arteritis. Hypoechoic wall thickening (cursors), in CDS no detectable lumen, regressed in response to treatment (b, c). Clinically polymyalgia, short-term cortisone therapy.
Classification of Arteritis
●Panarteritis nodosa. Complex, aggressive autoimmune inflammation, particularly of the smaller and medium-sized arteries: Involvement of all layers of the wall, in particular the adventitia (”periarteritis”).
●Giant-cell arteritis. Giant-cell containing, aggressive autoimmune inflammation of the medium-sized and large arteries, particularly the supraaortic arteries and their branches (temporal artery), as well as the abdominal and pelvic arteries and vessels supplying muscles and the extremities. The disease manifests with polymorphic clinical symptoms and is prevalent in patients over the age of
50. Women are affected approximately twice as frequently as men.
●Takayasu arteritis. Giant-cell arteritis and Takayasu arteritis have the common feature that they manifest not only with local symptoms and signs of peripheral or cerebral perfusion dysfunction, but also with general symptoms including hypostenia, weight loss, fever, and nonspecific signs of inflammation. Takayasu arteritis is accompanied by stenoses and secondary thrombotic occlusions, or by aneurysms at the aortic arch and the supraaortic arteries (and less frequently at the other branches of the aorta and at the pulmonary arteries). At the subcla-
vian artery it can lead to hypoperfusion of the arms (“pulseless disease”), occurring primarily in young women.
●Allergic vasculitis. This affects arterioles and venules and occurs in aggressive autoimmune diseases, active hepatitis, and bacterial diseases. It can also be drug-related.
●Secondary forms of arteritis. Mesoaortitis syphilitica—chronic bacterial (Treponema pallidum) inflammation of the aortic wall with granulomas, ulcerous destruction of the media, and aortic aneurysm formation; late sequelae are fibrosis and scarring.
21
1
Vessels
Mönckeberg’s Arteriosclerosis (Medial Calcific Sclerosis)
Arteriosclerosis (Medial Calcific Sclerosis)





























This appears as a distinctive late effect of diabetes in large, medium-sized, and small arteries, especially at the iliofemoral axis but also at the upper extremities (pseudohypertension). Mönckeberg’s arteriosclerosis is a horse- shoe-shaped calcification of the tunica elastica in the media and may be demonstrated as such on radiographic films and in ultrasonography.
Because of the loss of elasticity in the arterial wall, the pedal pulses are no longer palpable and Doppler-controlled blood pressure measurements will yield extremely high values, above 260 mmHg (this will also be true in case of upper limb involvement). The loss of elasticity leads to an increased pulsatility with
elevated systolic pressure. The sensitivity of ultrasound is up to 73%.
Ultrasound images will show circular beaded, hyperechoic thickening of the wall with incomplete shadowing. In color-flow Doppler scanning, it becomes evident that Mönckeberg’s arteriosclerosis does not result in stenosis of the vessel (Fig.1.42).
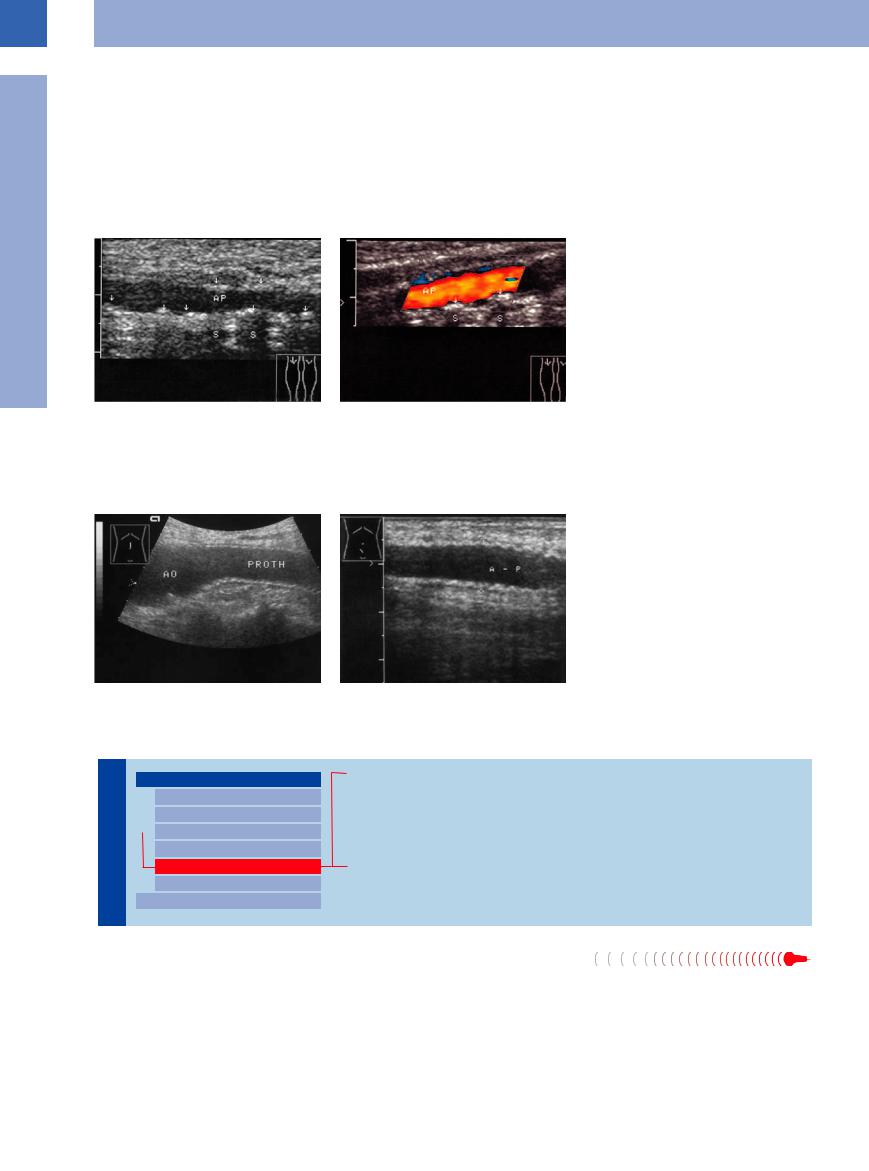
Fig. 1.42 Mönckeberg’s arteriosclerosis. Clinical symptoms: diabetic neuropathic plantar ulcer, palpable pedal pulses, Doppler blood pressure above 300 mmHg. AP = popliteal artery.
a B-mode image: diffuse spots of calcification in the popliteal artery wall (arrows), sometimes with shadowing
(S).
b Color-flow Doppler scan: no significant stenosis. Strong reflections off the wall may account for segmental blanking of the color signals.
Synthetic Grafts
Grafts



















































Stents and synthetic grafts also exhibit the |
their walls have such a unique corrugated |
are easily identifiable (Fig.1.31a, Fig.1.43; see |
characteristics of wall thickening. However, |
echogenic appearance in ultrasound that they |
also Fig.1.50). |
|
|
Fig. 1.43 Dacron graft (prosthesis). |
|
|
a Synthetic aortoiliac bypass: echogenic, thickened cor- |
|
|
rugated wall of the graft. AO = aorta. |
|
|
b Enlarged detail of an iliac graft: typical corrugated |
|
|
appearance of the graft wall (in color-flow Doppler scan- |
|
|
ning and spectral analysis a peak systolic velocity above |
|
|
300 cm/s implies a highly significant stenosis of the |
|
|
graft). A – P = artery graft. |
Intraluminal Mass
Vessels |
|
|
Aorta, Arteries |
|
|
|
|
|
|
|
Anomalies and Variant Positions |
|
|
|
|
|
|
|
Dilatation |
|
|
|
Stenosis |
|
|
|
|
|
|
|
Wall Thickening |
|
|
|
Intraluminal Mass |
|
|
|
Perivascular Mass |
|
|
|
Vena Cava, Veins |
|
|
Aortic/Arterial Embolism
Protruding Arteriosclerotic Plaques
White Thrombi
Endovascular Stent
Intimal Dissection
Aortic/Arterial Embolism
Embolism 























Complete embolic obstruction of the aorta or of |
lacks any impedance and thus is difficult to |
a major artery is always a serious event, some- |
define within the low-level structures of the |
times life-threatening. Duplex scanning is a |
artery (in contrast to venous thrombosis) |
rapid and precise imaging modality in the di- |
(Fig.1.44). Knowing the clinical symptoms, a |
agnosis of vascular occlusion (see below). |
hypoechoic mass may be presumed to be an |
In B-mode imaging, the actual embolus is |
embolus; this suspicion is confirmed by an- |
hard to see as an intraluminal mass since it |
giography and color-flow duplex scanning, |
where the decrease, and even cessation, in flow velocity becomes evident. Figure 1.45 shows the incidence of embolic events at various locations in the arterial tree.11
22
1
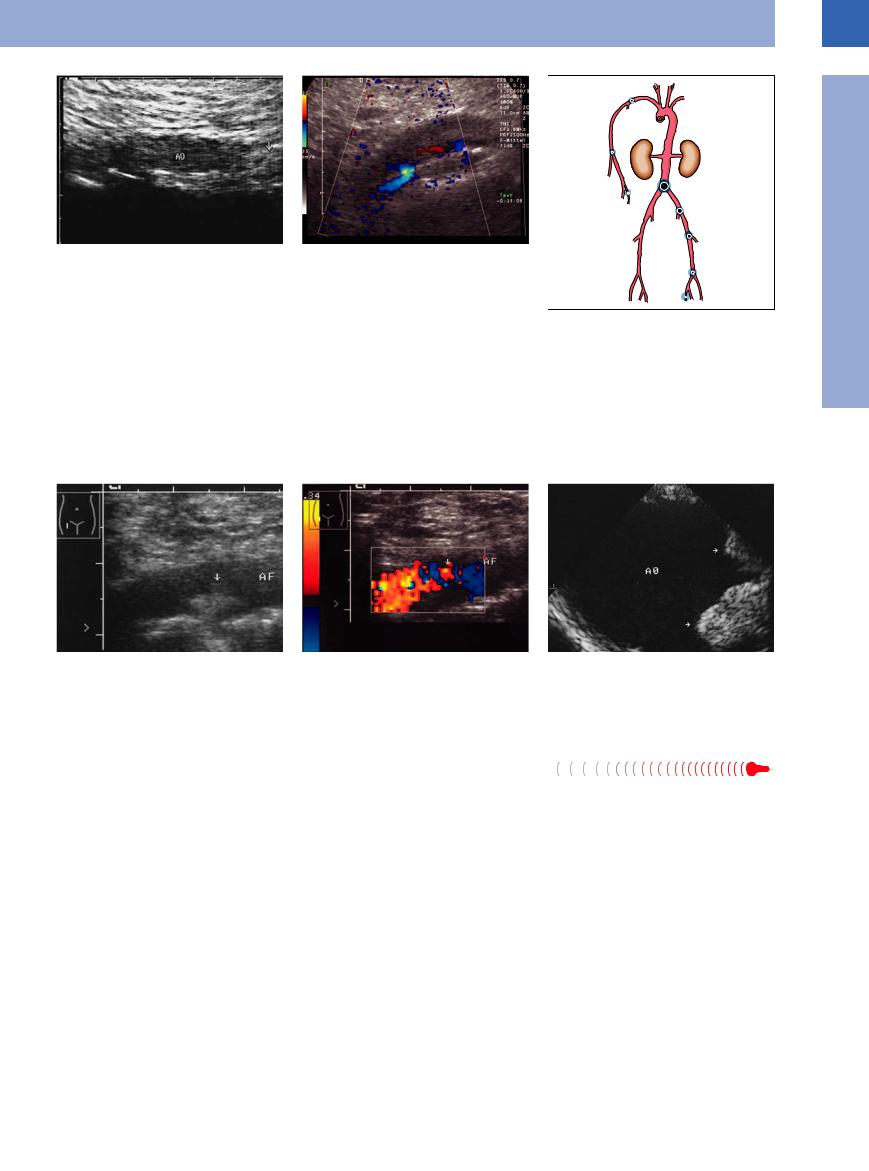
Fig. 1.44
a Aortic saddle embolus (AO): weakly echogenic mass (arrow) cephalad of the aortic bifurcation. Clinical findings: mitral valve defect, acute pulmonary edema, pain in the lower extremities. Emergency operation without preoperative angiography.
Aorta, Arteries
b Acute occlusion of the aorta, CDS: no flow detectable (except marginal).
Fig. 1.45 Incidence of arterial embolism.
Protruding Arteriosclerotic
Arteriosclerotic Plaques
Plaques 








































Protruding arteriosclerotic plaques represent circumscribed thickening of the arterial wall (see above) but appear as an intraluminal mass. If a white thrombus becomes superimposed on a ruptured arteriosclerotic plaque, its
sonographic appearance is that of a homogeneous, hypoechoic intraluminal mass. Such plaque–thrombus complexes increase the risk of arterial occlusion (myocardial infarction), rupture, and microembolism. They also make
the vessels prone to sclerosis and calcification, in which case ultrasonography will show them as heterogeneous irregular structures (Fig.1.40,
Fig.1.46, Fig.1.47).
Fig. 1.46 Complex protruding lesion of the femoral artery |
b Color-flow duplex scan: there are blank areas within the |
(AF). |
flow; the “confetti phenomenon” indicates significant |
a B-mode image: echogenic white thrombus (arrow). |
stenosis. Clinical diagnosis was a neuroischemic diabetic |
|
foot syndrome (in these lesions platelet inhibition is man- |
|
datory). |
White Thrombi 




























A white or pale thrombus arises from a lesion of |
found in atherosclerotic lesions and aneur- |
the endothelium, leading to turbulence and |
ysms. |
decreased flow velocity. These clots spread in |
In ultrasound they appear as layered hypo- |
layers of “white” thrombi (platelets) and “red” |
echoic bands. Sometimes they become reper- |
thrombi (fibrin and red blood cells), and are |
fused again or they wall off anechoic spaces |
Fig. 1.47 Atheromatous plaques (arrows) protruding into the lumen, echogenic arteriosclerotic wall thickening of the descending aorta (AO); TEE search for source of embolism.
without flow in the interior or at the margin (serosanguineous fluid) (Fig.1.48, Fig.1.49).
23
1
Vessels
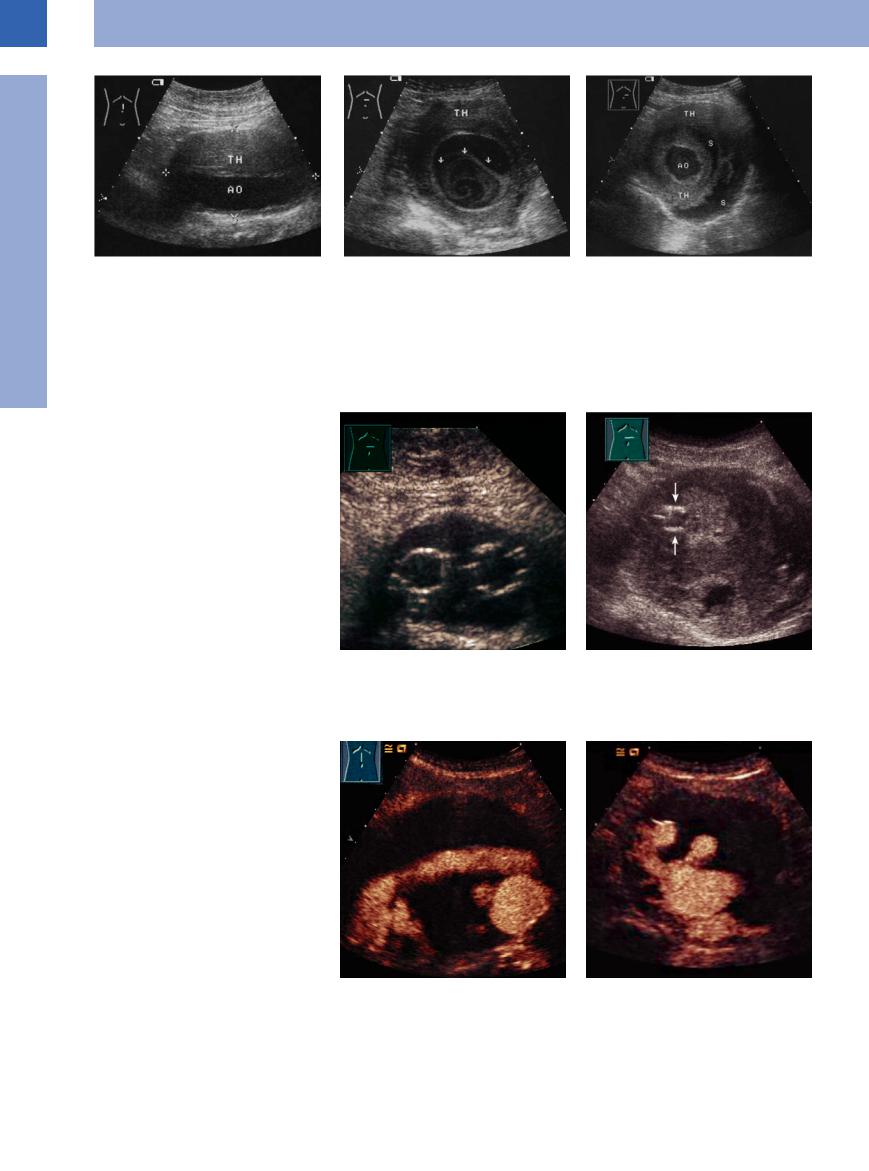
Fig. 1.48 White thrombus (TH) within a saccular aortic aneurysm: at first glance, the shape of the aorta (AO) seems to be regular while the vessels appears to be surrounded by a tumor; however, these are white thrombi within the aneurysmatic lumen.
Fig. 1.49 White thrombi within a saccular aortic aneurysm, staggered transverse views.
a Marginal layered thrombus (TH) and swirled thrombus (arrow) with the crescent-shaped anechoic remaining lumen; both clots are covered by an echogenic dissecting membrane (in aortic dissection this would be the intima).
b More caudad there is a regular, circular, central lumen of the aorta (AO). It is surrounded by alternating layers of white thrombi (TH) and serosanguineous fluid (S).
Endovascular Stent 

















































Depending on the situation involved, vascular grafts for aortic/arterial repair require open surgery or endovascular stenting. In the latter case, ultrasonography delineates these stents as echogenic reflecting bands (Fig.1.31,
Fig.1.43, Fig.1.50).
“Endoleaks” are the most common complication after endovascular aortic aneurysm repair (up to 45%). Their presence is to be assumed when the aneurysm shows no sign of diminishing (retrograde arterial “feeding”) after stent therapy, which then requires con- trast-enhanced ultrasound diagnosis. Endoleaks also lead to an enlargement of the aneurysm with the risk of rupture. Current classification describes four types of endoleaks:
●Type I, resulting from incomplete sealing of the stent graft to the attachment sites (type Ia at the upper site, type Ib at the lower site)
●Type II, determined by retrograde flow from aortic collateral vessels (type IIa retrograde flow of the inferior mesenteric artery, type IIb usually of vertebral arteries)
●Type III, caused by graft disruption
●Type IV, due to an abnormal porosity of the graft structure.
The diagnosis is usually made by angio-CT, but can also be made by contrast-enhanced ultrasound, which is very sensitive13 (Fig.1.31a,
Fig.1.50, Fig.1.51a).
Fig. 1.50
a Intraluminal grafts of a prosthetic-treated aortic aneurysm: the bifurcated endograft is visible (see also
Fig. 1.15b, p. 8).
b Endoleak of the aortal graft? Graft inserted 6 years previously, currently upper abdominal pain on pressure. B-mode: echogenic walls of the graft (arrows) surrounded by a heterogeneous thrombotic material implying a thrombosed aneurysm.
c and d Longitudinal and transverse section. CEUS: endoleak after endovascular grafting. CEUS is a reliable diagnostic procedure in diagnosing endoleaks.
24
