
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
“Hepatized” Gallbladder
Gallbladder 














































Viscous bile, rich in cholesterol, within the lumen of the gallbladder may mimic the texture of parenchymal tissue. The typical location and recognition of the gallbladder wall as a continuous margin will prevent the sonographer from misinterpreting this mass as an accessory lobe; this presumed hepatic parenchyma also lacks any kind of vascularization (see
Fig. 3.37b, p.148).
Bile Ducts/Vessels


















































Sediments within dilated bile ducts, thrombosis of branches or the trunk of the portal vein, and branches of the portal vein invaded by tumor may appear isoechoic to the surrounding parenchyma of the liver.
Hyperechoic Masses
Liver

Diffuse Changes in Hepatic
Parenchyma
Localized Changes in Hepatic
Parenchyma
Anechoic Masses
Hypoechoic Masses
Isoechoic Masses
Hyperechoic Masses
Echogenic Masses
Irregular Masses
Differential Diagnosis of Focal Lesions
Hemangioma
Bile Duct Hamartomas
Porphyria
Regenerative Nodules
Hepatocellular Carcinoma
Focal Nodular Hyperplasia
Metastasis
Abscess
Necrosis
Diaphragmatic Slips
Round Ligament of the Liver
Focal Fatty Change
Bile Ducts/Vessels
Hyperechoic liver masses are the typical target structures when diagnosing parenchymal liver pathology. Differential diagnosis is based on essential criteria such as echotexture, delineation, the always present through-transmission of a hyperechoic mass, the interaction of the lesion with vessels and the surface, and the reaction of the surrounding parenchyma.
Hemangioma 




















































The typical hemangioma is a hyperechoic mass |
hemangiomas |
respect adjacent |
structures |
nature. In CT imaging the slow blood flow in |
with a diameter of 10–30 mm, a smooth, clearly |
(Figs. 2.89, 2.90, 2.91). An afferent vessel can |
hemangioma results in the so-called filling-in |
||
defined margin, and good transmission; it ap- |
often be identified. Large hemangiomas display |
phenomenon, and this helps to explain why |
||
pears as a solitary lesion or comes in multiples, |
an increasingly |
inhomogeneous |
echotexture |
most of these hypervascular tumors cannot be |
and will always be found next to a vessel. |
with a nodular hypoechoic center. Palpation |
detected by color-flow Doppler imaging ( |
||
Although the round margin may displace ves- |
with the bare fingertips and the effects of |
2.11, 2.12). |
||
sels, and give the surface a nodular appearance, |
transmitted pulsation will evidence their soft |
|
||
2
Localized Changes in Hepatic Parenchyma
107
2
Liver
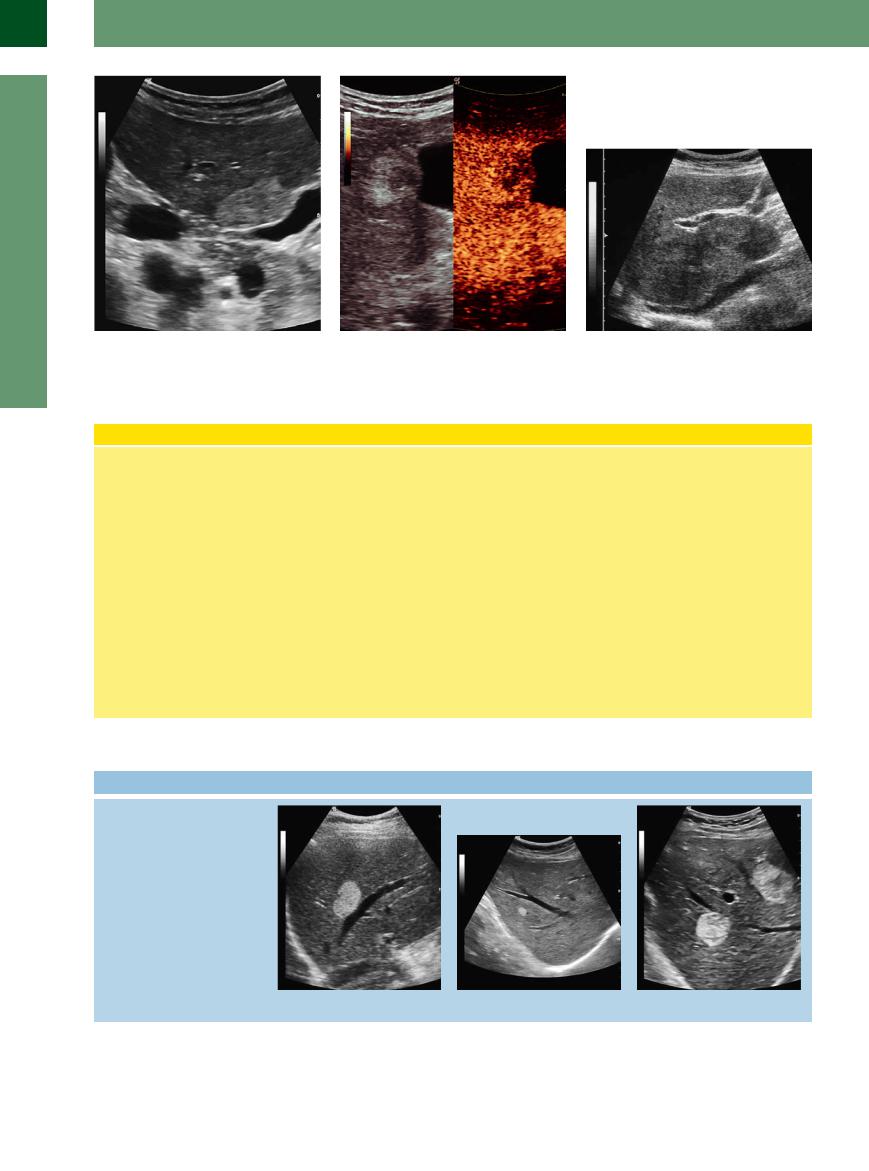
Fig. 2.89 Typical echoic hemangioma, smooth-margined, homogeneous hemangioma in segment V of the right liver lobe with compression of the gallbladder neck.
Fig. 2.90 Hemangioma as in Fig. 2.89. CEUS: peripheral nodular enhancement in the arterial phase, progressing in a centripetal direction to partial or complete fill-in.
Fig. 2.91 Giant hemangiomas. They can be demonstrated in all of segments IV and I as isoechoic masses.
Liver Hemangioma
Pathology and histology. Hemangioma is the most frequently encountered benign liver tumor, occurring in 0.4–7.3% of autopsies. Most often they are solitary (90%) and 1–3 cm in diameter, but 7% are classified as giant hemangioma (>4–5 cm). They consist of interconnected cavernous vascular spaces, whose walls are lined with a fibrous matrix and delicate epithelium, the spaces being filled with blood and clots. Hemangiomas are soft, compressible tumors that may grow in size by enlargement of their spaces. Such enlargement has been reported for estrogen replacement therapy and pregnancy. Degenerative changes will result in fibrosis of the connective-tissue septa and calcification.
Ultrasound. The typical (95%) sonographic image of a hemangioma is a hyperechoic mass measuring 1–3 cm in diameter, with a smooth, clearly defined margin and good through-transmission. The fact that the echogenicity may vary, depending on the plane of scanning, is another typical characteristic. Hemangiomas always arise in the vicinity of a vessel, which their bulge may displace. In many cases an afferent vessel can be demonstrated. In CT imaging the slow blood flow in the hemangioma results in the so-called filling-in phenomenon, and this helps to explain the paradox that color-flow Doppler scanning usually will not detect these hypervascular tumors. CEUS studies are required to demonstrate the filling-in effect and to better character-
ize the lesion by taking advantage of stimulated acoustic emission (SAE). During CEUS typical nodular arterial pooling of contrast agent occurs in hemangioma. The filling-in phenomena (sometimes partial) can take several minutes and can be documented. Both traits are characteristic and have a sensitivity of 98% if both occur simultaneously.
Atypical variants. Atypical hemangiomas (ca. 5%) exhibit an inhomogeneous echotexture in many cases, while others demonstrate hyperechoic textural changes and quite often an echogenic margin and ultrasound signal attenuation due to increased fibrosis and calcification.
 2.11 Hemangiomas
2.11 Hemangiomas
Sharply circumscribed hyperechoic mass
a–c Typical hemangiomas: hyperechoic, smooth contour and clearly defined, good through-transmission, frequently in contact with vessels.
108
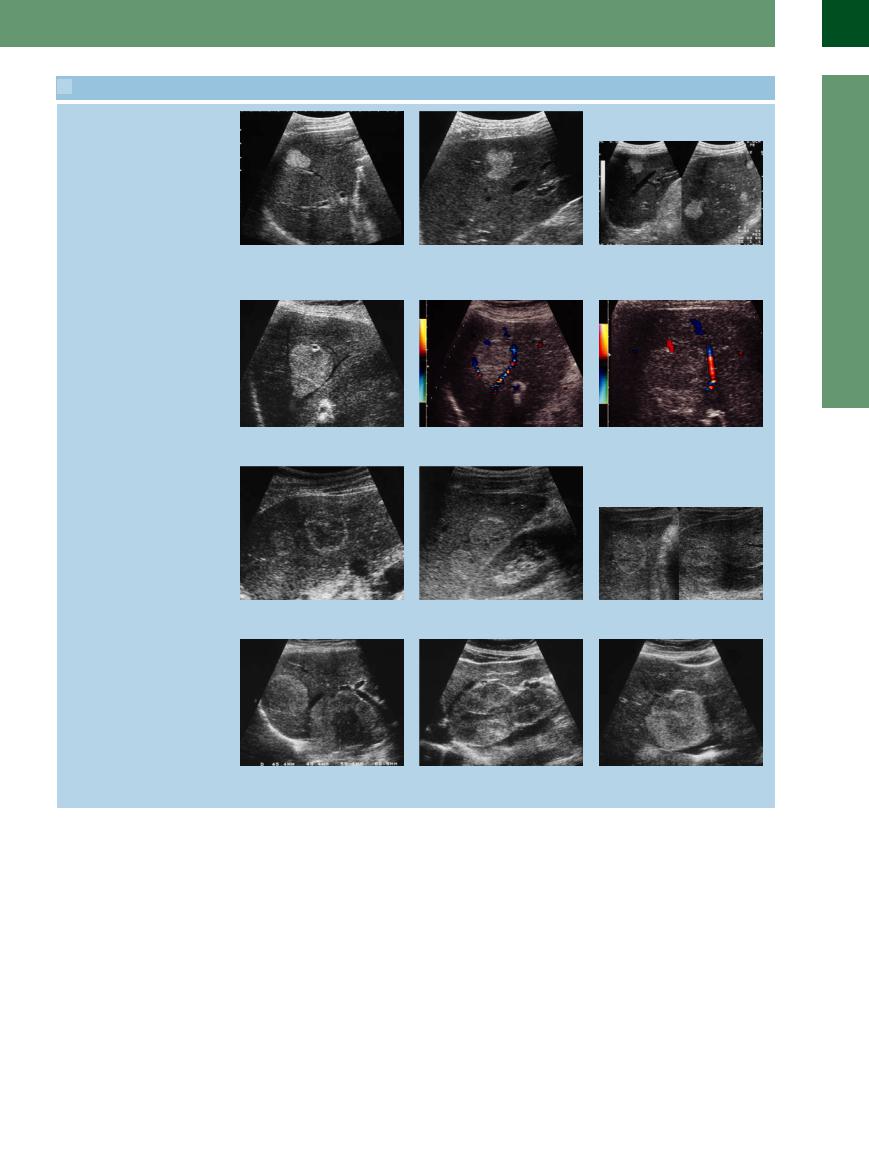
 2.11 Hemangiomas (Continued)
2.11 Hemangiomas (Continued)
d–f Typical hemangiomas: hyperechoic, smooth contour and clearly defined, good through-transmission, frequently in contact with vessels.
Color duplex findings
g–i Demonstration of the vascular pedicle; curved displacement but no further interference with the other vessels. Color-flow Doppler scanning cannot identify the blood-filled cisterns because the flow is too slow.
j–l The same hemangiomas displaying varying degrees of echogenicity depending on the time as well as the plane of scanning.
Giant hemangiomas
m–o The characteristics of giant hemangiomas resemble those of the smaller variant: hyperechoic, smooth contour and clearly defined, good through-transmission; the small hypoechoic centers and nodules within are quite typical. Frequently, these hemangiomas will break the surface of the liver, and their soft texture can be demonstrated on palpation or compression.
2
Localized Changes in Hepatic Parenchyma
109
2
Liver
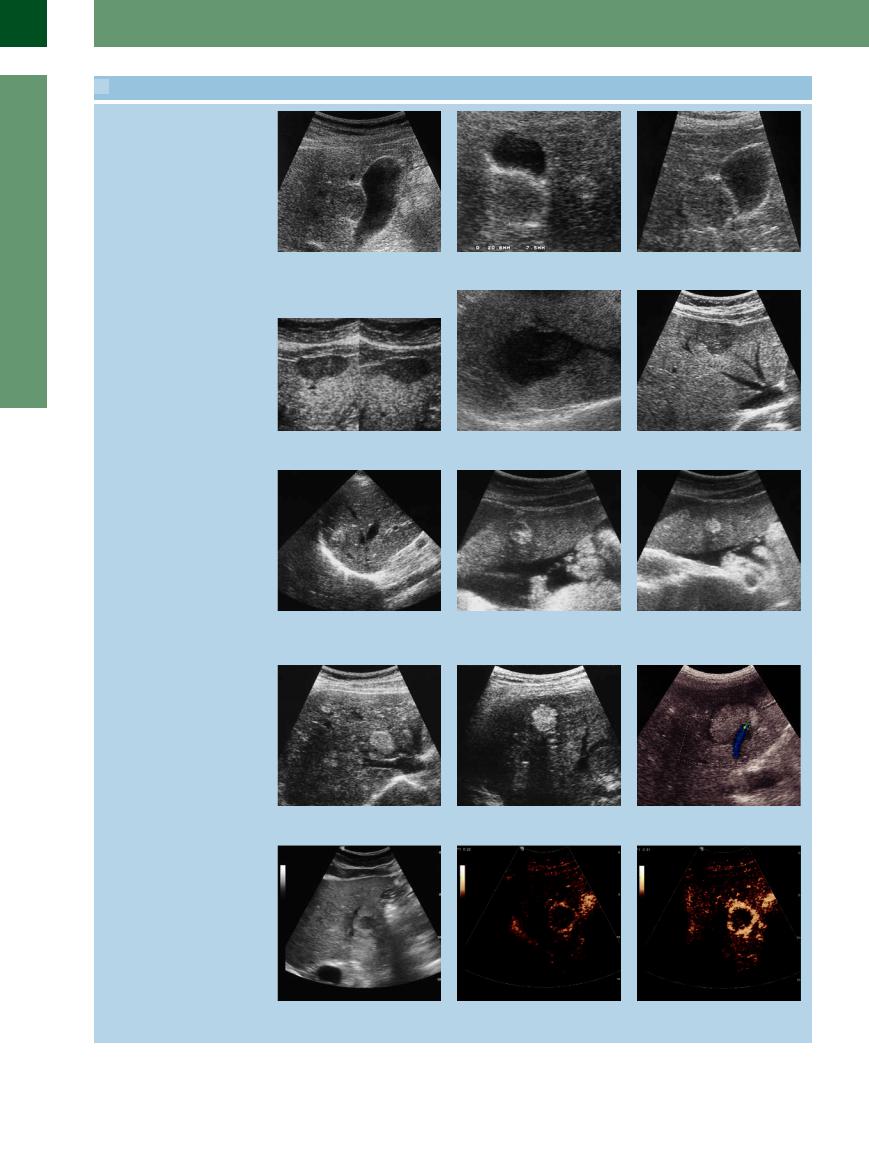
 2.12 Atypical Hemangiomas. Differential Diagnosis and Behavior in CEUS
2.12 Atypical Hemangiomas. Differential Diagnosis and Behavior in CEUS
Atypical hemangiomas
a–c Isoechoic hemangioma in segment V of the right hepatic lobe in the gallbladder fossa. In b there is another small hemangioma.
d Hypoechoic hemangioma. Note the un- e Hypoechoic hemangioma. |
f Hypoechoic hemangioma. |
usual buckling of the liver surface. |
|
Differential diagnosis of hemangiomas
g Metastasis of myosarcoma. |
h and i Falciform ligament. |
j Hemangioma adjacent to multiple small cholangiofibromas.
k HCC; note the distinctive signal attenu- |
l Metastasis of esophageal cancer. |
ation of the lesion. |
|
In CEUS, hemangiomas demonstrate a characteristic behavior; this makes them readily recognizable
m In grayscale, atypical hemangioma.
n–r Typical peripheral nodular enhancement in the arterial phase, progressing in a centripetal direction. The typical CEUS features of a hemangioma are peripheral nodular enhancement in the arterial phase, progressing centripetally.
110
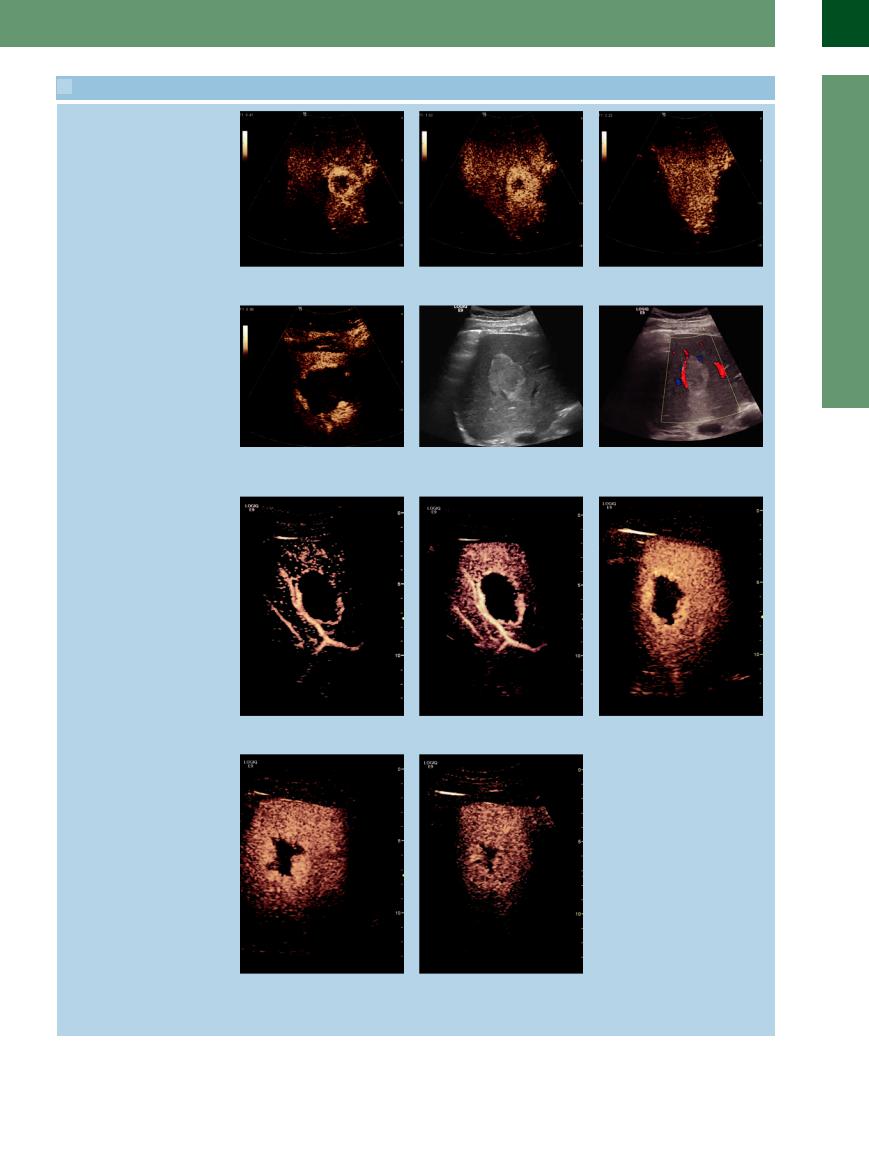
 2.12 Atypical Hemangiomas. Differential Diagnosis and Behavior in CEUS (Continued)
2.12 Atypical Hemangiomas. Differential Diagnosis and Behavior in CEUS (Continued)
p–r Progressing in a centripetal direction to complete filling-in.
s Another hemangioma with a typical pe- t Typical hemangioma, gray-scale image. |
u Color Doppler sonograph of t. |
ripheral nodular enhancement in the early |
|
arterial phase. |
|
v–z CEUS features. v–x Peripheral nodular enhancement progressing in the center with partial fill-in.
y Peripheral nodular enhancement pro- |
z Enhancement progressing in the center |
gressing in the center with partial fill-in. |
with partial fill-in has markedly improved |
|
the accurate diagnosis of hemangiomas, |
|
which is now possible in about 95% of |
|
cases.3,4 |
2
Localized Changes in Hepatic Parenchyma
111
2
Liver
Bile Duct Hamartomas
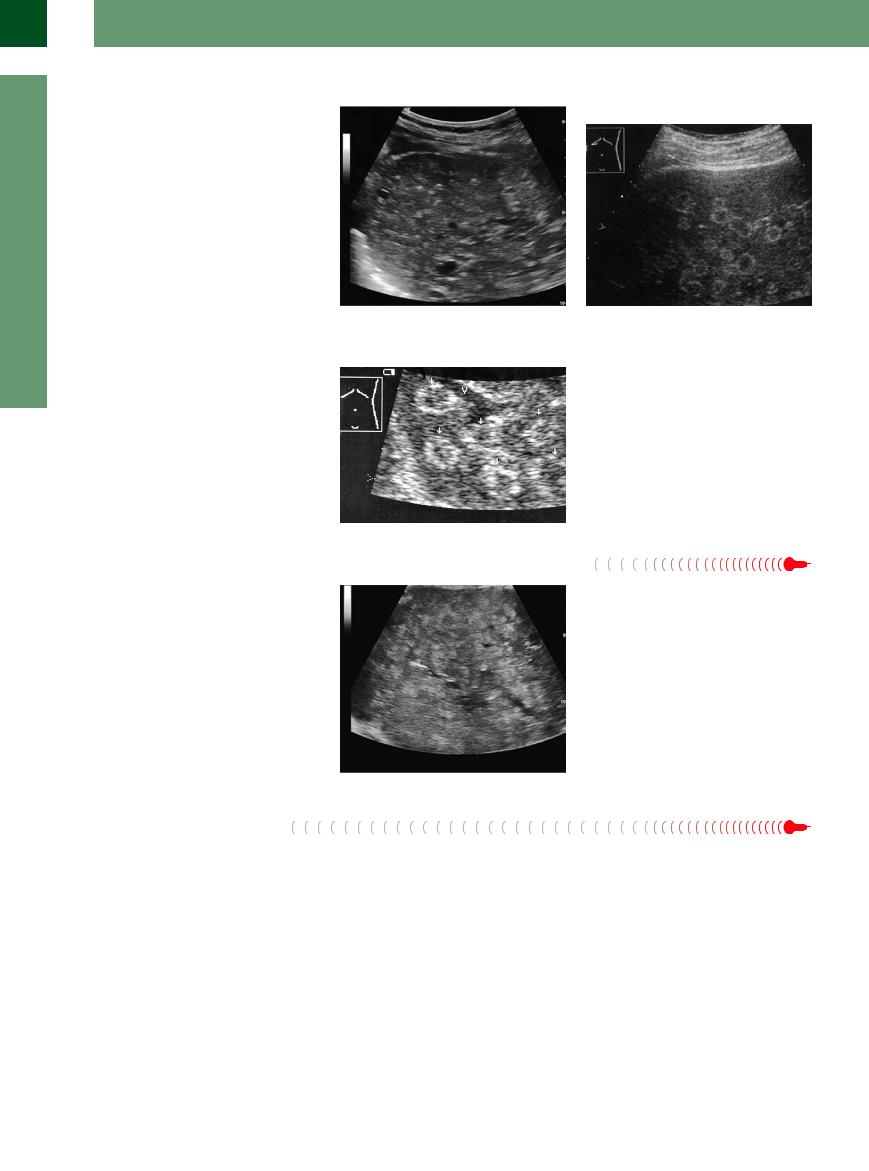
Hamartomas 















































Bile duct hamartomas (so-called Meyenburg complexes) appear as very small hyperechoic nodules, diffusely and unevenly distributed in the parenchyma, usually < 5 mm in diameter (Fig. 2.92a,b). (The echogenic masses in porphyria are similar; see below.)
Fig. 2.92
a Cholangiofibromas are benign bile duct hamartomas, which are small echoic particles demonstrable in the liver. b Differential diagnosis of hepatic hemangioma. Multiple round “masses” with hypoechoic centers, initially misinterpreted as multiple hemangiomas: chronic hepatic porphyria. Color duplex image shows vessels passing freely through the lesions (which resolved completely in 6 years).
Porphyria 






















































In hepatic porphyria, hyperechoic lesions may |
Fig. 2.93 Chronic hepatic porphyria (porphyria cutanea |
|
be seen in an otherwise normal parenchyma. |
tarda): disseminated, echogenic target lesions (arrows), |
|
These pseudotumors are characterized by the |
no longer detectable several years later (misinterpreted |
|
initially as multiple hemangiomas). Clinical presentation: |
||
fact that they do not induce changes in the |
||
history of alcohol abuse, signs of porphyria. V = hepatic |
||
texture of the tissue or the course of vessels, |
||
vein. |
||
contours, segmental interfaces, and surfaces |
|
|
(Fig. 2.93). |
|
Regenerative Nodules

























Multiacinal regenerative nodules occur in chronic liver diseases as a sign of parenchymal hypergeneration. They can range from 2 mm up to a few centimeters in size.
In cirrhotic or inflammatory parenchyma, there are mainly multiple small nodular zones that could be either slightly hyperechoic, isoechoic, or even hypoechoic to the surrounding tissue. They should be kept in mind as a rare, but significant, differential diagnosis when considering hyperechoic masses (Fig. 2.94).
Fig. 2.94 Regenerative nodules are normally some millimeters in size, in special cases even some centimeters. They appear as echoic modules in the presence of an earlier disease; in this case hepatitis B cirrhosis.
Hepatocellular Carcinoma
Carcinoma
Any hyperechoic mass in a cirrhotic liver must be suspected of HCC, since about one-third of all HCC will present as such a hyperechoic lesion ( 2.10 g–i). Frequently these lesions attenuate the through-transmission and exhibit posterior shadowing. Color-flow Doppler imaging shows these masses to have significant hypervascularization. CEUS has improved the detection of HCC from 52% to 67%.
2.10 g–i). Frequently these lesions attenuate the through-transmission and exhibit posterior shadowing. Color-flow Doppler imaging shows these masses to have significant hypervascularization. CEUS has improved the detection of HCC from 52% to 67%.
Focal Nodular Hyperplasia 













































Although FNH may be more echogenic than the |
spoke-like architecture with its central vessel, |
vascularization, radial centrifugal flow, central |
surrounding parenchyma, the most important |
and its vascularization (enhanced marginal |
vessel, vascular pedicle of the mass). |
criteria are structural changes, such as the |
|
|
112

Metastasis






















































Hyperechoic metastatic masses in the liver vary in size and may occur in any segment. Although conclusions on the etiology of the primary tumor cannot be drawn from the echogenicity of these masses, it is not uncommon to find hyperechogenic metastases of colon carcinomas in the liver. They are characterized by the textural differences in comparison to the normal parenchyma. If the metastatic mass presents as a hyperechoic lesion, the tumor activity in the lesion may be regarded as low (Fig. 2.95, Fig. 2.96); only a hypoechoic rim indicates the zone of active growth.
Fig. 2.95 Large colon cancer metastasis in the liver. |
Fig. 2.96 The same colon cancer metastasis as in |
|
Fig. 2.95, CEUS: unmasked hyperechoic mass as a meta- |
|
stasis. |
Abscess
The hyperechoic image of an abscess is due to the textural transformation and organization. Usually, this type of abscess is found as a solitary mass and lacks the typical concomitant clinical symptoms (Fig. 2.97, Fig. 2.98).
Fig. 2.97 Abscess in the right liver lobe with gas bubbles in |
Fig. 2.98 Abscess, inspissated by pus, therefore echoic |
a case of cholangiosepsis; additionally, pneumobilia. |
structure. |
Necrosis 













Tumoror inflammation-induced necrosis may yield a coarsely inhomogeneous and hyperechoic mass (Fig. 2.99). The increased echogenicity may also be due to local compression. Apart from this hyperechoic necrosis, additional complications can induce liquid areas that may in turn be due to sequestered bile, hemorrhage, or liquefaction of necrotic material.
Fig. 2.99 Centrally, a necrotic metastasis of gastric cancer; the tumor activity is visible by the hypoechoic rim.
Diaphragmatic Slips
Slips

















































Diaphragmatic incisures at the dome of the |
triangular lesions. They will always demon- |
may be more or less pronounced, depending |
liver may present as hyperechoic curved or |
strate a connection with the diaphragm and |
on the respiratory motion. |
2
Localized Changes in Hepatic Parenchyma
113
