
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX
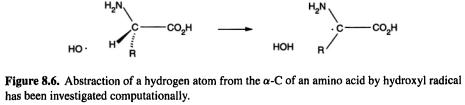
452 Computational Chemistry
oxidation of proteins by hydroxyl radicals may initiate Alzheimer’s disease, cancer, and heart disease. The initial step in the destruction or modification of proteins by hydroxyl radical is likely to be abstraction of a hydrogen atom from the  (Fig. 8.6). In a very thorough study using MP2 (section 5.4) and DFT (chapter 7), Galano et al. (2001) calculated the geometries of the species (amino acid–OH complexes, transition states, and amino acid radicals) involved in the reactions of glycine and alanine (Fig. 8.6, R–H and
(Fig. 8.6). In a very thorough study using MP2 (section 5.4) and DFT (chapter 7), Galano et al. (2001) calculated the geometries of the species (amino acid–OH complexes, transition states, and amino acid radicals) involved in the reactions of glycine and alanine (Fig. 8.6, R–H and  respectively) [20]. The rate constants were also calculated, using partition functions to calculate the preexponential factor (cf. section 5.5.2.2d). This paper provides a good account of how computational chemistry can be used to calculate absolute rate constants for reactions of molecules of moderate size.
respectively) [20]. The rate constants were also calculated, using partition functions to calculate the preexponential factor (cf. section 5.5.2.2d). This paper provides a good account of how computational chemistry can be used to calculate absolute rate constants for reactions of molecules of moderate size.
8.1.3Concepts
There are some very basic concepts in chemistry that have proved to be helpful in rationalizing experimental facts, and which have been taught for perhaps the last fifty years, but which have nevertheless been questioned in the last decade or so; an example is the role of resonance in stabilizing species like carboxylate ions. Some newer concepts, intriguing but not as traditional, have also been scrutinized and questioned; an example is homoaromaticity.
8.1.3.1Resonance vs. inductive effects
Beginning organic chemistry students learn that carboxylic acids are stronger acids than alcohols because of resonance stabilization of the conjugate base (which is more important than the charge-separation resonance in the acid), while resonance does not figure in either an alcohol or its conjugate base. This traditional wisdom was apparently first questioned by Thomas and Siggel, on the basis of photoelectron spectroscopy [21]. They concluded that the relatively high acidity of carboxylic acids is largely inherent in the acid itself, as a consequence of the polarization of the COOH group caused by the electronegative carbonyl group pulling electrons from the hydrogen atom. This idea was taken up by Streitwieser, and applied to other acids, e.g. nitric and nitrous acids, dimethyl sulfoxide and dimethyl sulfone [22]. The results for carbonyl compounds were interpreted in accord with another iconoclastic idea, namely that the carbonyl group is better regarded as 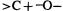 than as
than as  [23]. This polarization interpretation was arrived at largely with the aid of atoms-in-molecules (AIM) analysis of the electron populations on the atoms involved (section 5.5.4), and a simpler variation of AIM (the projection function difference plot) developed by Streitwieser and coworkers [24]. Work by others also supports the view that it is “initial-state electrostatic polarization” that is
[23]. This polarization interpretation was arrived at largely with the aid of atoms-in-molecules (AIM) analysis of the electron populations on the atoms involved (section 5.5.4), and a simpler variation of AIM (the projection function difference plot) developed by Streitwieser and coworkers [24]. Work by others also supports the view that it is “initial-state electrostatic polarization” that is
Literature, Software, Books and Websites 453
largely responsible for the acidity of several kinds of compounds, including carboxylic acids [25]. Streitwieser cautions that “merely reproducing experiment does not teach us much unless the results are analyzed to provide understanding of the important contributions to the numbers in terms of reference systems and simpler models.” [26]. Note that such analysis is presented to a large extent visually (section 5.5.6).
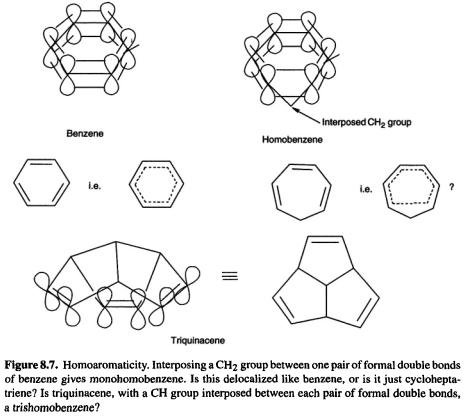
8.1.3.2Homoaromaticity
Aromaticity [27] is associated with the delocalization of (in the simplest version)  electrons (the role of these
electrons (the role of these  electrons in imposing symmetry on the prototypical aromatic species, benzene, is being questioned, but that is another story [28]). A Hückel number of cyclically delocalized electrons confers aromaticity on a molecule (section 4.3.5). The idea behind homoaromaticity (homologous aromaticity) is that if a system is aromatic, then if we interpose one or more atoms between adjacent
electrons in imposing symmetry on the prototypical aromatic species, benzene, is being questioned, but that is another story [28]). A Hückel number of cyclically delocalized electrons confers aromaticity on a molecule (section 4.3.5). The idea behind homoaromaticity (homologous aromaticity) is that if a system is aromatic, then if we interpose one or more atoms between adjacent  orbitals of the
orbitals of the system, provided overlap is not lost, the aromaticity may persist (Fig. 8.7). While there is little doubt about the reality of homoaromaticity in ions, neutral homoaromaticity has been elusive [29].
system, provided overlap is not lost, the aromaticity may persist (Fig. 8.7). While there is little doubt about the reality of homoaromaticity in ions, neutral homoaromaticity has been elusive [29].
454 Computational Chemistry
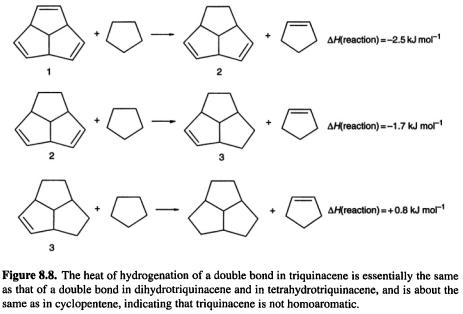
One molecule that might be expected to be homoaromatic, if the phenomenon can exist in neutral species, is triquinacene (Fig. 8.7): the three double bonds are held rigidly in an orientation which appears favorable for continuous overlap with concomitant cyclic delocalization of six  electrons. Indeed, its potential aromaticity was one of the reasons cited for the synthesis of this compound [30]. A measurement of the heat of hydrogenation of triquinacene found a value
electrons. Indeed, its potential aromaticity was one of the reasons cited for the synthesis of this compound [30]. A measurement of the heat of hydrogenation of triquinacene found a value  lower than that for each of the next two steps (leading to hexahydrotriquinacene) [31]. This was taken as proof of homoaromaticity in the triene, i.e. that the compound was
lower than that for each of the next two steps (leading to hexahydrotriquinacene) [31]. This was taken as proof of homoaromaticity in the triene, i.e. that the compound was  stabler than expected for an unstabilized species (note that this is a small stabilization energy compared to the resonance energy of benzene, a recent computational estimate of which is
stabler than expected for an unstabilized species (note that this is a small stabilization energy compared to the resonance energy of benzene, a recent computational estimate of which is  However, a recent experimental and computational study of this question led to the conclusion that triquinacene is not homoaromatic [33]. This was shown by (a) redetermination of its heat of formation (which had been calculated from the heat of hydrogenation in the earlier work [31]) using the measured heat of combustion, (b) by calculation of the heat of hydrogenation of a double bond in triquinacene and in its diand tetrahydro derivatives (1, 2, 3, Fig. 8.8), and by (c) calculation of magnetic properties of the triene and related molecules. The heat of formation was about
However, a recent experimental and computational study of this question led to the conclusion that triquinacene is not homoaromatic [33]. This was shown by (a) redetermination of its heat of formation (which had been calculated from the heat of hydrogenation in the earlier work [31]) using the measured heat of combustion, (b) by calculation of the heat of hydrogenation of a double bond in triquinacene and in its diand tetrahydro derivatives (1, 2, 3, Fig. 8.8), and by (c) calculation of magnetic properties of the triene and related molecules. The heat of formation was about  higher than the previously reported [31] value, removing the supposed stabilization energy. The heats of hydrogenation of the double bonds were calculated with the aid of homodesmotic reactions, a kind of isodesmic reaction (section 5.5.2.2a) which preserves the number of each kind of bond, and so in which correlation errors should cancel well; for 1, 2, and 3 the calculated hydrogenation energies of a double bond are all essentially the same, showing that a double bond of 1 is an ordinary cyclopentene double bond. Note that using cyclopentane (Fig. 8.8) rather than, say, ethane – which would also preserve bond types – to (conceptually) hydrogenate 1, 2,
higher than the previously reported [31] value, removing the supposed stabilization energy. The heats of hydrogenation of the double bonds were calculated with the aid of homodesmotic reactions, a kind of isodesmic reaction (section 5.5.2.2a) which preserves the number of each kind of bond, and so in which correlation errors should cancel well; for 1, 2, and 3 the calculated hydrogenation energies of a double bond are all essentially the same, showing that a double bond of 1 is an ordinary cyclopentene double bond. Note that using cyclopentane (Fig. 8.8) rather than, say, ethane – which would also preserve bond types – to (conceptually) hydrogenate 1, 2,
