
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX
Chapter 2
The Concept of the Potential Energy Surface
Everything should be made as simple as possible, but not simpler.
Albert Einstein
2.1 PERSPECTIVE
We begin a more detailed look at computational chemistry with the potential energy surface (PES) because this is central to the subject. Many important concepts that might appear to be mathematically challenging can be grasped intuitively with the insight provided by the idea of the PES [1].
Consider a diatomic molecule AB. In some ways a molecule behaves like balls (atoms) held together by springs (chemical bonds); in fact, this simple picture is the basis of the important method molecular mechanics, discussed in chapter 3. If we take a macroscopic balls-and-spring model of our diatomic molecule in its normal geometry (the equilibrium geometry), grasp the “atoms” and distort the model by stretching or compressing the “bonds,” we increase the potential energy of the molecular model (Fig. 2.1). The stretched or compressed spring possesses energy, by definition, since we moved a force through a distance to distort it. Since the model is motionless while we hold it at the new geometry, this energy is not kinetic and so is by default potential (“depending on position”). The graph of potential energy against bond length is an example of a PES (we will soon see an example of an actual surface rather than the line of Fig. 2.1).
Real molecules behave similarly, but they differ from our macroscopic model in two relevant ways:
1.They vibrate incessantly (as we would expect from Heisenberg’s uncertainty principle: a stationary molecule would have an exactly defined momentum and position) about the equilibrium bond length, so that they always possess kinetic energy (T)
and/or potential energy (V): as the bond length passes through the equilibrium length, V = 0 while at the limit of the vibrational amplitude, T = 0; at all other positions both T and V are nonzero. The fact that a molecule is never actually stationary with zero kinetic energy (it always has zero-point energy (ZPE); section 2.5) is usually shown on potential energy/bond length diagrams by drawing a series of

10 Computational Chemistry
lines above the bottom of the curve (Fig. 2.2) to indicate the possible amounts of vibrational energy the molecule can have (the vibrational levels it can occupy). A molecule never sits at the bottom of the curve, but rather occupies one of the vibrational levels, and in a collection of molecules the levels are populated according to their spacing and the temperature [2]. We will usually ignore the vibrational levels and consider molecules to rest on the actual potential energy curves or (below) surfaces, and
2.Near the equilibrium bond length  the potential energy/bond length curve for a macroscopic balls-and-spring model or a real molecule is described fairly well by
the potential energy/bond length curve for a macroscopic balls-and-spring model or a real molecule is described fairly well by
a quadratic equation, that of the simple harmonic oscillator  where k is the force constant of the spring). However, the potential energy deviates from the quadratic
where k is the force constant of the spring). However, the potential energy deviates from the quadratic  curve as we move away from
curve as we move away from  (Fig. 2.2). The deviations from molecular reality represented by this anharmonicity are not important to our
(Fig. 2.2). The deviations from molecular reality represented by this anharmonicity are not important to our
discussion.
Figure 2.1 represents a one-dimensional (1D) PES (a line is a 1D “surface”) in the 2D graph of E vs. q. A diatomic molecule AB has only one geometric parameter for us to vary, the bond length  Suppose we have a molecule with more than one geometric parameter, e.g. water: the geometry is defined by two bond lengths and a bond angle. If we reasonably content ourselves with allowing the two bond lengths to be the same, i.e. if we limit ourselves to
Suppose we have a molecule with more than one geometric parameter, e.g. water: the geometry is defined by two bond lengths and a bond angle. If we reasonably content ourselves with allowing the two bond lengths to be the same, i.e. if we limit ourselves to  symmetry (two planes of symmetry and a two-fold symmetry axis; see section 2.6) then the PES for this triatomic molecule is a graph of E vs. two geometric parameters,
symmetry (two planes of symmetry and a two-fold symmetry axis; see section 2.6) then the PES for this triatomic molecule is a graph of E vs. two geometric parameters,  the
the  bond length, and
bond length, and 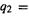 the
the 

The Concept of the Potential Energy Surface 11
bond angle (Fig. 2.3). Figure 2.3 represents a 2D PES (a normal surface is a 2D object) in the 3D-graph; we could make an actual 3D model of this drawing of a 3D graph of
Evs.  and
and 
We can go beyond water and consider a triatomic molecule of lower symmetry, such
as HOF. This has three geometric parameters, the H–O and O–F lengths and the H–O–F angle. To construct a Cartesian PES graph for HOF analogous to that for would require us to plot E vs.
would require us to plot E vs.  and
and  angle H–O–F. We would need four mutually perpendicular axes
angle H–O–F. We would need four mutually perpendicular axes  and since such a 4D graph cannot be constructed in our 3D space we cannot accurately draw it. The HOF PES is a 3D “surface” of more than two dimensions in 4D space: it is a hypersurface, and PESs are sometimes called potential energy hypersurfaces. Despite the problem of drawing a hypersurface, we can define the equation
and since such a 4D graph cannot be constructed in our 3D space we cannot accurately draw it. The HOF PES is a 3D “surface” of more than two dimensions in 4D space: it is a hypersurface, and PESs are sometimes called potential energy hypersurfaces. Despite the problem of drawing a hypersurface, we can define the equation  as the PES for HOF, where f is the function that describes how E varies with the q’s, and treat the hypersurface mathematically. For example, in the AB diatomic molecule PES (a line) of Fig. 2.1 the minimum potential energy geometry is the point at which
as the PES for HOF, where f is the function that describes how E varies with the q’s, and treat the hypersurface mathematically. For example, in the AB diatomic molecule PES (a line) of Fig. 2.1 the minimum potential energy geometry is the point at which  On the
On the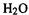 PES (Fig. 2.3) the minimum energy geometry is defined by the point Pm, corresponding to the equilibrium values of
PES (Fig. 2.3) the minimum energy geometry is defined by the point Pm, corresponding to the equilibrium values of and
and  at this point
at this point  Although hypersurfaces cannot be faithfully rendered pictorially, it is very useful to a computational chemist to develop an intuitive understanding of them. This can be gained with the aid of diagrams like Figs 2.1 and 2.3, where we content ourselves with a line or a 2D surface, in effect using a slice of a multidimensional diagram. This can be understood by analogy: Fig. 2.5 shows how 2D slices can be made of the 3D diagram for water.
Although hypersurfaces cannot be faithfully rendered pictorially, it is very useful to a computational chemist to develop an intuitive understanding of them. This can be gained with the aid of diagrams like Figs 2.1 and 2.3, where we content ourselves with a line or a 2D surface, in effect using a slice of a multidimensional diagram. This can be understood by analogy: Fig. 2.5 shows how 2D slices can be made of the 3D diagram for water.

12 Computational Chemistry
