
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX
Ab initio calculations 287
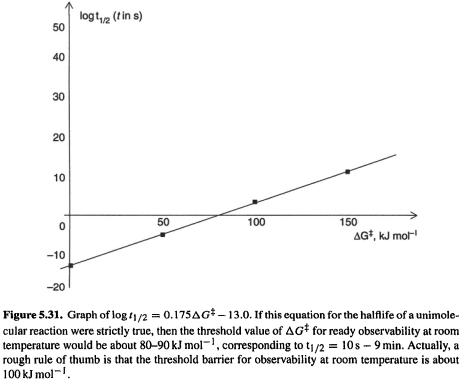
a strong dependence on  Experience shows that in fact the threshold barrier for observing or isolating a compound at room temperature is about
Experience shows that in fact the threshold barrier for observing or isolating a compound at room temperature is about 
5.5.2.3b Energies: concluding remarks
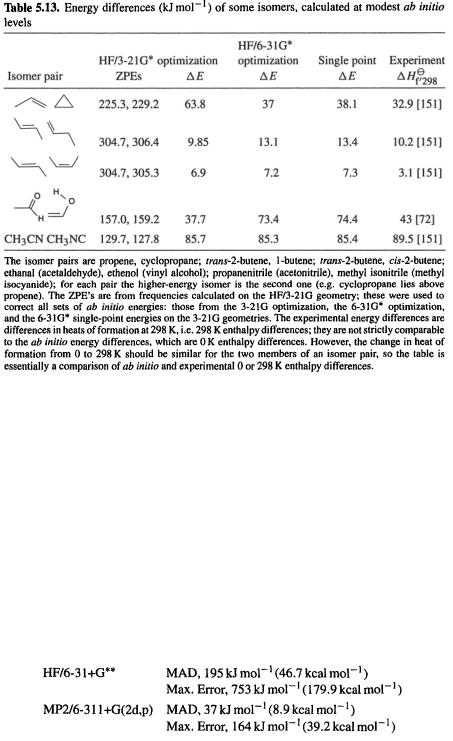
Although we have paid considerable attention to high-accuracy energy methods (CBS and G2), many ab initio studies are limited to obtaining relative energies at a moderate level like MP2/6-31G*, or even HF/6-31G* or HF/3-21G. In comparing the thermodynamic stabilities of isomers (in contrast to calculating an “absolute” thermodynamic parameter like the heat of formation of a compound), simple subtraction of ab initio energies calculated at a modest level (e.g. Fig. 5.24) usually gives at least semiquantitatively reliable results. Some examples of energy differences calculated at modest ab initio levels are shown in Table 5.13. Here 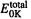 (section 5.2.3.6d), the ZPE-corrected ab initio energy, has been used to calculate energy differences for five pairs of isomers. The ab initio energies are from HF/3-21G-optimized geometries, HF/6-31G*-optimized geometries, and single-point HF/6-31G* calculations on HF/3- 21G-optimized geometries; the ZPE corrections are all from HF/3-21G* frequencies on HF/3-21G-optimized geometries. Although ZPE corrections have been included here, ignoring them for such modest-level calculations on pairs of isomers evidently makes little difference, as the correction is the same for both isomers, to within a few
(section 5.2.3.6d), the ZPE-corrected ab initio energy, has been used to calculate energy differences for five pairs of isomers. The ab initio energies are from HF/3-21G-optimized geometries, HF/6-31G*-optimized geometries, and single-point HF/6-31G* calculations on HF/3- 21G-optimized geometries; the ZPE corrections are all from HF/3-21G* frequencies on HF/3-21G-optimized geometries. Although ZPE corrections have been included here, ignoring them for such modest-level calculations on pairs of isomers evidently makes little difference, as the correction is the same for both isomers, to within a few
(but for comparison of a ground state and a TS the ZPE correction is more likely to be significant, see e.g. Fig. 2.20; and in high-accuracy calculations ZPE’s should always
288 Computational Chemistry
be included). This very small sample does not permit one to draw conclusions about  energies, but this has been discussed in sections 5.3.3 and 5.5.2.2a. The single-point
energies, but this has been discussed in sections 5.3.3 and 5.5.2.2a. The single-point  energies are almost identical to the optimized-geometry ones (correlated single-point energies were discussed in sections 5.4.2 and 5.4.3). Agreement with experiment (the 0 and 298 K enthalpy values are approximately comparable) is good except for ethanal/ethenol; the reported experimental value of
energies are almost identical to the optimized-geometry ones (correlated single-point energies were discussed in sections 5.4.2 and 5.4.3). Agreement with experiment (the 0 and 298 K enthalpy values are approximately comparable) is good except for ethanal/ethenol; the reported experimental value of  for this pair is supported by a high-accuracy energy calculation (CBS-Q, section 5.5.2.2b) that gives a 0 K enthalpy difference of
for this pair is supported by a high-accuracy energy calculation (CBS-Q, section 5.5.2.2b) that gives a 0 K enthalpy difference of  at 298 K, bolstering the assertion that
at 298 K, bolstering the assertion that  changes little from 0 to 298 K). The two
changes little from 0 to 298 K). The two  values are evidently in error by about
values are evidently in error by about 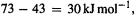 while the
while the 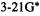 error is only
error is only 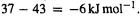 A “higher” level is not guaranteed to give more accurate results, at least not until we reach very high levels. As might be suspected, the relative energies of conformers is also treated quite well by these modest levels [148].
A “higher” level is not guaranteed to give more accurate results, at least not until we reach very high levels. As might be suspected, the relative energies of conformers is also treated quite well by these modest levels [148].
Foresman and Frisch [149], in a chapter with very useful data and recommendations, show large mean absolute deviations (MAD) and enormous maximum errors for HF and even MP2 methods; e.g.
