
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX

68 Computational Chemistry
3.5 FREQUENCIES CALCULATED BY MM
Any method that can calculate the energy of a molecular geometry can in principle calculate vibrational frequencies, since these can be obtained from the second derivatives of energy with respect to molecular geometry (section 2.5), and the masses of the vibrating atoms. Some commercially available MM programs, for example the MMFF as implemented in SPARTAN [13], can calculate frequencies. Frequencies are useful (section 2.5) (1) for characterizing a species as a minimum (no imaginary frequencies) or a transition state or higher-order saddle point (one or more imaginary frequencies),
(2)for obtaining zero-point energies to correct frozen-nuclei energies (section 2.2), and
(3)for interpreting or predicting infrared spectra.

Molecular Mechanics 69
(1) Characterizing a species. This is not often done with MM, because MM is used mostly to create input structures for other kinds of calculations, and to study known (often biological) molecules. Nevertheless MM can yield information on the curvature of the potential energy surface (as calculated by that particular forcefield, anyway) at the point in question. For example, the MMFF-optimized geometries of 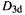 (staggered) and
(staggered) and  (eclipsed) ethane (Fig. 3.3) show, respectively, no imaginary frequencies and one imaginary frequency, the latter corresponding to rotation about the C/C bond. Thus the MMFF (correctly) predicts the staggered conformation to be a minimum, and the eclipsed to be a transition state connecting successive minima along the torsional reaction coordinate. Again, calculations on cyclohexane conformations with the MMFF correctly give the boat an imaginary frequency corresponding to a twisting motion leading to the twist conformation, which latter has no imaginary frequencies (Fig. 3.10). Although helpful for characterizing conformations, particularly hydrocarbon conformations, MM is less appropriate for species in which bonds are being formed and broken. For example, the symmetrical
(eclipsed) ethane (Fig. 3.3) show, respectively, no imaginary frequencies and one imaginary frequency, the latter corresponding to rotation about the C/C bond. Thus the MMFF (correctly) predicts the staggered conformation to be a minimum, and the eclipsed to be a transition state connecting successive minima along the torsional reaction coordinate. Again, calculations on cyclohexane conformations with the MMFF correctly give the boat an imaginary frequency corresponding to a twisting motion leading to the twist conformation, which latter has no imaginary frequencies (Fig. 3.10). Although helpful for characterizing conformations, particularly hydrocarbon conformations, MM is less appropriate for species in which bonds are being formed and broken. For example, the symmetrical  species in the
species in the reaction, with equivalent C/F partial bonds, is incorrectly characterized by the MMFF as a minimum rather than a transition state, and the C/C bonds are calculated to be 1.289 Å long, cf. the value of ca. 1.8 Å from methods known to be trustworthy for transition states.
reaction, with equivalent C/F partial bonds, is incorrectly characterized by the MMFF as a minimum rather than a transition state, and the C/C bonds are calculated to be 1.289 Å long, cf. the value of ca. 1.8 Å from methods known to be trustworthy for transition states.
(2)Obtaining zero-point energies (ZPEs). ZPEs are essentially the sum of the energies of each normal-mode vibration. They are added to the raw energies (the frozennuclei energies, corresponding to the stationary points on a Born-Oppenheimer surface; section 2.3) in accurate calculations of relative energies using ab initio (chapter 5) or DFT (chapter 7) methods. However, the ZPEs used for those corrections are usually obtained from an ab initio or DFT calculation.
(3)Infrared spectra. The ability to calculate the energies 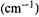 and relative intensities of molecular vibrations amounts to being able to calculate infrared spectra. MM as
and relative intensities of molecular vibrations amounts to being able to calculate infrared spectra. MM as
such cannot calculate the intensities of vibrational modes, since these involve changes in dipole moments (section 5.5.3), and dipole moment is related to electron distribution, a concept that lies outside MM. However, approximate intensities can be calculated by
assigning dipole moments to bonds or charges to atoms, and such methods have been implemented in MM programs [31], although MM programs that calculate intensities are not yet widely used. Figures 3.14–3.17 compare the IR spectra of acetone, benzene, dichloromethane and methanol, calculated with the MM3 program6 with the experimental spectra, and with spectra calculated by the ab initio  method; the data for Figs 3.14–3.17 are in Tables 3.5–3.8 (in chapters 5–7 spectra for these four molecules, calculated by ab initio, SE, and density functional methods, are also given). The MP2 spectra generally match experiment better than the MM3, although the latter method furnishes a rapid way of obtaining approximate IR spectra. For a series of related compounds, MM3 might be a reasonable way to quickly investigate trends in frequencies and intensities. Extensive surveys of MMFF and MM4 frequencies showed that MMFF root-mean-square errors are ca.
method; the data for Figs 3.14–3.17 are in Tables 3.5–3.8 (in chapters 5–7 spectra for these four molecules, calculated by ab initio, SE, and density functional methods, are also given). The MP2 spectra generally match experiment better than the MM3, although the latter method furnishes a rapid way of obtaining approximate IR spectra. For a series of related compounds, MM3 might be a reasonable way to quickly investigate trends in frequencies and intensities. Extensive surveys of MMFF and MM4 frequencies showed that MMFF root-mean-square errors are ca.  and MM4 errors
and MM4 errors  [5b].
[5b].
6 The MM3 frequencies and intensities were kindly provided by Dr. J. -H. Lii of the Department of Chemistry of the University of Georgia, Athens, Georgia, USA.

70 Computational Chemistry

Molecular Mechanics 71
