
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX

86 Computational Chemistry
the photon, and  is the kinetic energy of the free electron. Rearranging Eq. (4.5):
is the kinetic energy of the free electron. Rearranging Eq. (4.5):
Thus a plot of the kinetic energies of the electrons  vs. the frequency
vs. the frequency 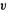 of the light should be a straight line of positive slope (h; this is one way to find Planck’s
of the light should be a straight line of positive slope (h; this is one way to find Planck’s
constant) intersecting the axis at a positive value |
as experiment indeed |
showed (Fig. 4.3). |
|
Planck’s explanation of the blackbody radiation curves (1900 [4]) and Einstein’s explanation of the facts of the photoelectric effect (1905 [7]) indicated that the flow of energy in physical processes did not take place continuously, as had been believed, but rather jerkily, in discrete jumps, quantum by quantum. The contributions of Planck and Einstein were the signal developments marking the birth of quantum theory and the transition from classical to modern physics.
4.2.2 Radioactivity
Brief mention of radioactivity is in order because it, along with quantum mechanics and relativity, transformed classical into modern physics. Radioactivity was discovered by Becquerel in 1896. However, an understanding of how materials like uranium and radium could emit, over the years, a million times more energy than would be permitted by chemical reactions, had to await Einstein’s special theory of relativity (section 4.2.3), which showed that a tiny, unnoticeable decrease in mass represented the release of a large amount of energy.

Introduction to Quantum Mechanics 87
4.2.3 Relativity
Relativity is relevant to computational chemistry because it must often be explicitly taken into account in accurate calculations involving atoms heavier than about chlorine or bromine (see below) and because, strictly speaking, the Schrödinger equation, the fundamental equation of quantum chemistry, is an approximation to a relativistic equation, the Dirac6 equation.
Relativity was discovered in by Einstein in 1905, when he formulated the special theory of relativity, which deals with nonaccelerated motion in the absence of significant gravitational fields (general relativity, published by Einstein in 1915, is concerned with accelerated motion and gravitation). Special relativity predicted a relationship between mass and energy, the famous  equation and, of more direct relevance to computational chemistry, showed that the mass of a particle increases with its velocity, dramatically so near the velocity of light. In heavier elements the inner electrons are moving at a significant fraction of the speed of light, and the relativistic increase in their masses affects the chemistry of these elements (actually, some physicists do not like to think in terms of rest mass and relativistic mass, but that is a controversy that need not concern us here). In computational chemistry relativistic effects on electrons are usually accounted for by what are called effective core potentials or pseudopotentials (section 5.3.3).
equation and, of more direct relevance to computational chemistry, showed that the mass of a particle increases with its velocity, dramatically so near the velocity of light. In heavier elements the inner electrons are moving at a significant fraction of the speed of light, and the relativistic increase in their masses affects the chemistry of these elements (actually, some physicists do not like to think in terms of rest mass and relativistic mass, but that is a controversy that need not concern us here). In computational chemistry relativistic effects on electrons are usually accounted for by what are called effective core potentials or pseudopotentials (section 5.3.3).
4.2.4 The nuclear atom
The “nuclear atom” is the picture of the atom as a positive nucleus surrounded by negative electrons. Although the idea of atoms in speculative philosophy goes back to at least the time of Democritus,7 the atom as the basis of a scientifically credible theory emerges only in nineteenth century, with the rationalization by Dalton8 in 1808 of the law of definite proportions. Nevertheless, atoms were regarded by many scientists of the positivist school of Ernst Mach as being at best a convenient hypothesis, despite the success of the atomistic Maxwell-Boltzmann9 kinetic theory of gases and it was not until 1908, when Perrin’s10 experiments confirmed Einstein’s atomistic analysis of
6 Paul Adrien Maurice Dirac, born Bristol, England, 1902. Ph.D. Cambridge, 1926. Professor, Cambridge, Dublin Institute for Advanced Studies, University of Miami, Florida State University. Nobel prize in physics 1933 (shared with Schrödinger). Known for his mathematical elegance, for connecting relativity with quantum theory, and for predicting the existence of the positron. Died Tallahassee, Florida, 1984.
7 Democritus, Greek philosopher, born Abdera, Thrace (the eastern Balkans) ca. 470 B.C. Died ca. 370 B.C.
8John Dalton, born Eaglesfield, England, 1766. Considered the founder of quantitative chemical atomic theory: law of definite proportions, pioneered determination of atomic weights. Cofounder of British Association for the Advancement of Science. Died Manchester, England, 1844.
9 Ludwig Boltzmann, born Vienna 1844. Ph.D. Vienna. Professor Graz, Vienna. Developed the kinetic theory of gases independently of Maxwell (viz., Boltzmann constant’s, k). Firm supporter of the atomic theory in opposition to Mach and Ostwald, helped develop concept of entropy (S). Died Duino, Austria (now in Italy), 1906 (suicide incurred by depression). Inscribed on gravestone: 
10 Jean Perrin, born Lille, France, 1870. Ph.D. École Normal Supérieure, Paris. Professor University of Paris. Nobel prize in physics 1923. Died New York, 1942.

88 Computational Chemistry
Brownian motion that the reality of atoms was at last accepted by such eminent holdouts as Boltzmann’s opponent Ostwald.11
The atom has an internal structure; it is thus not “atomic ” in the Greek sense and is more than the mere restless particle of kinetic theories of gases or of Brownian motion. This was shown by two lines of work: the study of the passage of electricity through gases and the behavior of certain solutions. The study of the passage of electricity through gases at low pressure was a very active field of research in the nineteenth century and only a few of the pioneers in what we can now see as the incipient field of subatomic physics will be mentioned here. The observation by Plücker in 1858 of a fluorescent glow near the cathode on the glass walls of a current-carrying evacuated tube was one of the first inklings that particles might be elicited from atoms. That these were indeed particles rather than electromagnetic rays was indicated by Crookes in the 1870s, by showing that they could be deflected by a magnet. Goldstein showed in 1886 the presence of particles of opposite charge to those emitted from the cathode, and christened the latter “cathode rays.” That the cathode rays were negative particles was proved by Perrin in 1895, when he showed that they imparted a charge to an object on which they fell. Further evidence of the particle nature of cathode rays came at around the same time from Thomson,12 who showed (1897) that they are deflected in the expected direction by an electric field. Thomson also measured their mass-to-charge ratio and from the smallest possible value of charge in electrochemistry calculated the mass of these particles to be about 1/1837 of the mass of a hydrogen atom. Lorentz later applied the name “electron” to the particle, adopting a term that had been appropriated from the Greek by Stoney for a unit of electric current  amber, which acquires a charge when rubbed). Thomson has been called the discoverer of the electron.
amber, which acquires a charge when rubbed). Thomson has been called the discoverer of the electron.
It was perhaps Thomson who first suggested a specific structure for the atom in terms of subatomic particles. His “plum pudding” model (ca. 1900), which placed electrons in a sea of positive charge, like raisins in a pudding, accorded with the then-known facts in evidently permitting electrons to be removed under the influence of an electric potential. The modern picture of the atom as a positive nucleus with extranuclear electrons was proposed by Rutherford13 in 1911. It arose from experiments in which alpha particles from a radioactive sample were shot through very thin gold foil. Most of the time the particles passed through, but occasionally one bounced back, indicating that the foil was mostly empty space, but that present were particles which were small and, compared to the mass of the electron (which was much too light to stop an alpha particle), massive. From these experiments emerged our picture of the atom as consisting of a small, relatively massive positive nucleus surrounded by electrons: the nuclear atom.
11 Wilhelm Friedrich Ostwald, German chemist, born Riga, Latvia, 1853. Ph.D. Dorpat, Estonia. Professor Riga, Leipzig. A founder of physical chemistry, opponent of the atomic theory till convinced by the work of Einstein and Perrin. Nobel prize in chemistry 1909. Died near Leipzig, 1932.
12 Sir Joseph John Thomson, born near Manchester, 1856. Professor, Cambridge. Nobel prize in physics 1906. Knighted 1908. Died Cambridge, 1940.
13 Ernest Rutherford (Baron Rutherford), born near Nelson New Zealand, 1871. Studied at Cambridge under J. J. Thomson. Professor McGill University (Montreal), Manchester, and Cambridge. Nobel prize in chemistry 1908 for work on radioactivity, alpha particles, and atomic structure. Knighted 1914. Died London,
1937.

Introduction to Quantum Mechanics 89
Rutherford gave the name proton (from Greek |
primary or first) to the least |
massive of these nuclei (the hydrogen nucleus). |
|
There is another thread to the development of the concept of the atom as a composite of subatomic particles. The enhanced effect of electrolytes (solutes that provide an electrically conducting solutions) on boiling and freezing points and on the osmotic pressure of solutions led Arrhenius14 in 1884 to propose that these substances exist in water as atoms or groups of atoms with an electric charge. Thus sodium chloride in solution would not, as was generally held, exist as NaCl molecules but rather as an a positive sodium “atom” and a negative chlorine “atom”; the presence of two particles instead of the expected one accounted for the enhanced effects. The ability of atoms to lose or gain charge hinted at the existence of some kind of subatomic structure, and although the theory was not warmly received (Arrhenius was almost failed on his Ph.D. exam), the confirmation by Thomson (ca. 1900) that the atom contains electrons made acceptable the concept of charged atoms with chemical properties quite different from those of the neutral ones. Arrhenius’ was awarded the Nobel prize for his Ph.D. work.
4.2.5 The Bohr atom
The nuclear atom as formulated by Rutherford faced a serious problem: the electrons orbit the nucleus like planets orbiting the Sun. An object engaged in circular (or elliptical) motion experiences an acceleration because its direction is changing and thus its velocity, which unlike speed is a vector, is also changing. An electron in circular motion about a nucleus would experience an acceleration toward the nucleus, and since from Maxwell’s equations of electromagnetism an accelerated electric charge radiates away energy, the electron should lose energy by spiralling in toward the nucleus, ending up there, with no kinetic and potential energy; calculations show this should happen in a fraction of a second [8].
A way out of this dilemma was suggested by Bohr15 in 1913 [9,10]. He retained the classical picture of electrons orbiting the nucleus in accord with Newton’s laws, but subject to the constraint that the angular momentum of an electron must be an integral multiple of 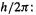
where m is the electron mass,  is the electron velocity, r is the radius of electron orbit, and h is the Planck’s constant. Equation (4.7) is the Bohr postulate, that electrons can defy Maxwell’s laws provided they occupy an orbit whose angular momentum (i.e. an orbit of appropriate radius) satisfies Eq. (4.7). The Bohr postulate is not based on a whim, as most textbooks imply, but rather follows from: (1) the Plank equation Eq. (4.3),
is the electron velocity, r is the radius of electron orbit, and h is the Planck’s constant. Equation (4.7) is the Bohr postulate, that electrons can defy Maxwell’s laws provided they occupy an orbit whose angular momentum (i.e. an orbit of appropriate radius) satisfies Eq. (4.7). The Bohr postulate is not based on a whim, as most textbooks imply, but rather follows from: (1) the Plank equation Eq. (4.3),  and (2) starting with an orbit of large radius such that the motion is essentially linear and classical physics applies, as no acceleration is involved, then extrapolating
and (2) starting with an orbit of large radius such that the motion is essentially linear and classical physics applies, as no acceleration is involved, then extrapolating
14Svante Arrhenius, born near Uppsala, Sweden, 1859. Ph.D. University of Stockholm. Nobel prize in chemistry 1903. Professor Stockholm. Died Stockholm 1927.
15Niels Bohr, born Copenhagen, 1885. Ph.D. University of Copenhagen. Professor, University of Copenhagen. Nobel prize in physics 1922. Founder of the “Copenhagen school” interpretation of quantum theory. Died Copenhagen, 1962.

90 Computational Chemistry
to small-radius orbits. The fading of quantum-mechanical equations into their classical analogues as macroscopic conditions are approached is called the correspondence principle [11].
Using the postulate of Eq. (4.7) and classical physics, Bohr derived an equation for the energy of an orbiting electron in a one-electron atom (a hydrogen-like atom, H or 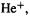 etc.) in terms of the charge on the nucleus and some constants of nature. Starting
etc.) in terms of the charge on the nucleus and some constants of nature. Starting
with the total energy of the electron as the sum of its kinetic and potential energies:
where Z is the nuclear charge (1 for H, 2 for He, etc.), e is the charge on the electron,  is the permitivity of the vacuum. Using force = mass × acceleration:
is the permitivity of the vacuum. Using force = mass × acceleration:
i.e.
So from Eq. (4.8)
From Eqs (4.7) and (4.10):
So from Eqs (4.11) and (4.12):
Equation (4.13) expresses the total (kinetic plus potential) energy of the electron of a hydrogen-like atom in terms of four fundamental quantities of our universe: electron charge, electron mass, the permittivity of empty space, and Planck’s constant. From Eq. (4.13) the energy change involved in emission or absorption of light by a hydrogenlike atom is simply
where  is the energy of a state characterized by quantum number
is the energy of a state characterized by quantum number  minus the energy of a state characterized by quantum number
minus the energy of a state characterized by quantum number  Note that from Eq. (4.13) the total energy increases (becomes less negative) as n increases (= 1,2, 3,...), so higherenergy states are associated with higher quantum numbers n and
Note that from Eq. (4.13) the total energy increases (becomes less negative) as n increases (= 1,2, 3,...), so higherenergy states are associated with higher quantum numbers n and  corresponds to absorption of energy and
corresponds to absorption of energy and  to emission of energy. The Planck relation between the amount of radiant energy absorbed or emitted and its frequency
to emission of energy. The Planck relation between the amount of radiant energy absorbed or emitted and its frequency 
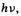 Eq. (4.3)), Eq. (4.14) enables one to calculate the frequencies of spectroscopic absorption and emission lines for hydrogen-like atoms. The agreement with experiment is excellent, and the same is true too for the calculated ionization energies of hydrogenlike atoms
Eq. (4.3)), Eq. (4.14) enables one to calculate the frequencies of spectroscopic absorption and emission lines for hydrogen-like atoms. The agreement with experiment is excellent, and the same is true too for the calculated ionization energies of hydrogenlike atoms  for
for  in Eq. (4.14)).
in Eq. (4.14)).
