
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX

340 Computational Chemistry
hierarchy of complexity. The SE methods treated in this chapter are logically regarded as simplifications of the ab initio method, since they use the SCF procedure (chapter 5) to refine the Fock matrix, but do not evaluate these matrix elements ab initio. The SHM was developed (1931) outside the realm of SCF theory (which was invented for atoms: Hartree, 1928 [2]), as the first application of the Schrödinger equation to molecules of reasonable size, and the EHM is a straightforward extension of this. In contrast, the methods of this chapter were created in a conscious attempt to provide practical alternatives to the ab initio approach, the application of which to molecules of reasonable size understandably seemed hopeless in the infancy of electronic computers (the PPP method, one of the first SCF SE methods, was published in 1953, just when the first electronic computers began to be available to chemists [3]). SE calculations are much less demanding than ab initio ones, because parameterization and approximations drastically reduce the number of integrals which must be calculated. The pessimism with which the ab initio approach was viewed is clear in the words of several pioneers of the application of quantum mechanics to chemistry:
C. A. Coulson, 1959: “I see little chance – and even less desirability – of dealing in this accurate manner with systems containing more than 20 electrons...” [4]
M. J. S. Dewar1, 1969: “How then shall we proceed? The answer lies in abandoning attempts to carry out rigorous a priori calculations.” [5].
Neither Coulson nor Dewar could have foreseen the enormous increase in computer power that was to come over the next few decades. What Coulson meant by “even less desirability” was perhaps that the computed results would be too complex to interpret; one factor which has obviated this problem is the visual display of information (sections 5.5.6, 6.3.6). The development of improved algorithms and far faster computers has altered the situation almost out of recognition: computers in 2000 were about one million times faster than in 1959 (computers were said [4] to be 100 000 times faster in 1989 than in 1959, the date of Coulson’s remarks; it seems safe to say that they increased in speed by a factor of 10 in the subsequent decade). A calculation that in 1967 would have taken 200 years can now be run on a cheap computer in less than an hour [6]. Why, then, are SE calculations still used? Because they are still about 100– 1000 times faster than ab initio (chapter 5) or density functional (chapter 7) methods. The increase in computer speed means that we can now routinely examine by ab initio methods moderately large molecules – up to, say, steroids, with about 30 heavy atoms (nonhydrogen atoms), and by semiempirical methods (and faster with MM, chapter 3) huge molecules, like proteins and nucleic acids.
6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
6.2.1Preliminaries
The SE methods we saw in chapter 4 simply construct a Fock matrix and diagonalize it to get molecular orbital (MO) energy levels and MOs (i.e. the coefficients of the basis
1Michael J. S. Dewar, born Ahmednagar, India, 1918. Ph.D. Oxford, 1942. Professor of chemistry at Universities of London, Chicago, Texas at Austin, and University of Florida. Died Florida, 1997.

Semiempirical Calculations 341
functions that make up the MOs). The simple Hückel Fock matrix elements were simply relative energies 0 and –1 (0 and  units, relative to the nonbonding level
units, relative to the nonbonding level  or in the EHM the Fock matrix elements were calculated from ionization energies. A single matrix diagonalization gave the energy levels and MO coefficients. This chapter is concerned with SE methods that are closer to the ab initio method in that the SCF procedure (sections 5.2.3.6d and 5.2.3.6e) is used to refine the energy levels and MO coefficients. As in ab initio calculations each Fock matrix element is calculated from a core integral
or in the EHM the Fock matrix elements were calculated from ionization energies. A single matrix diagonalization gave the energy levels and MO coefficients. This chapter is concerned with SE methods that are closer to the ab initio method in that the SCF procedure (sections 5.2.3.6d and 5.2.3.6e) is used to refine the energy levels and MO coefficients. As in ab initio calculations each Fock matrix element is calculated from a core integral  density matrix elements
density matrix elements  and electron repulsion integrals (rs\tu), (ru\ts):
and electron repulsion integrals (rs\tu), (ru\ts):
As stated above, the following discussion applies to SE methods that use the SCF procedure and so pay some service to Eq. (6.1). As with an ab initio calculation, to initiate the process we need an initial guess of the coefficients, to calculate the density matrix values  the guess can come from a simple Hückel calculation (for a
the guess can come from a simple Hückel calculation (for a  electron theory like the PPP method) or from an extended Hückel calculation (for an all-valence-electron theory, like CNDO and its descendants). The Fock matrix of
electron theory like the PPP method) or from an extended Hückel calculation (for an all-valence-electron theory, like CNDO and its descendants). The Fock matrix of  elements is diagonalized repeatedly to refine energy levels and coefficients.
elements is diagonalized repeatedly to refine energy levels and coefficients.
The divergence from the ab initio method lies in (1) treating only valence or electrons, i.e. in the meaning of the “core,” (2) the mathematical functions used to expand the MOs (the basis set functions), (3) how the core and two-electron repulsion integrals are evaluated, and (4) the treatment of the overlap matrix. These approximations are discussed in detail by Dewar [7]. An excellent yet compact survey of the principles behind all the major SE methods is given by Levine [8], and SE methods have also been reviewed by Thiel [9]; a detailed exposition of the basic (pre-1970) theory behind these methods can be found in the book by Pople and Beveridge [10]. Expanding on points (1)–(4):
electrons, i.e. in the meaning of the “core,” (2) the mathematical functions used to expand the MOs (the basis set functions), (3) how the core and two-electron repulsion integrals are evaluated, and (4) the treatment of the overlap matrix. These approximations are discussed in detail by Dewar [7]. An excellent yet compact survey of the principles behind all the major SE methods is given by Levine [8], and SE methods have also been reviewed by Thiel [9]; a detailed exposition of the basic (pre-1970) theory behind these methods can be found in the book by Pople and Beveridge [10]. Expanding on points (1)–(4):
(1) Treating only valence or  electrons, i.e. the meaning of the “core”. In an ab initio calculation
electrons, i.e. the meaning of the “core”. In an ab initio calculation  is the kinetic energy of an electron moving in the force-field of the atomic nuclei, plus the potential energy of attraction of the electron to these atomic nuclei: the electron is moving under the influence of a positive core composed of atomic nuclei. SE calculations handle at most valence electrons (the PPP method handles only
is the kinetic energy of an electron moving in the force-field of the atomic nuclei, plus the potential energy of attraction of the electron to these atomic nuclei: the electron is moving under the influence of a positive core composed of atomic nuclei. SE calculations handle at most valence electrons (the PPP method handles only  electrons), so each element of the core becomes an atomic nucleus plus its core electrons (for the PPP method, a nucleus with the core electrons plus all
electrons), so each element of the core becomes an atomic nucleus plus its core electrons (for the PPP method, a nucleus with the core electrons plus all  valence electrons). Instead of a cloud of all the electrons moving in a framework of nuclei, we have a cloud valence electrons (for the PPP method,
valence electrons). Instead of a cloud of all the electrons moving in a framework of nuclei, we have a cloud valence electrons (for the PPP method,  electrons) moving in a framework of atomic cores (atomic core = nucleus + valence electrons, or for PPP, nucleus + all electrons that don’t contribute to the
electrons) moving in a framework of atomic cores (atomic core = nucleus + valence electrons, or for PPP, nucleus + all electrons that don’t contribute to the  system). The SCF SE energy is calculated in a manner analogous to that of an ab initio calculation of the Hartree-Fock energy (cf. Eq. (5.149)), but n in Eq. (6.2) is not half the total number of electrons, but rather half the number of valence electrons (half the number of
system). The SCF SE energy is calculated in a manner analogous to that of an ab initio calculation of the Hartree-Fock energy (cf. Eq. (5.149)), but n in Eq. (6.2) is not half the total number of electrons, but rather half the number of valence electrons (half the number of  electrons for a PPP calculation), i.e. n is the number of MOs formed from the those electrons being included in the basis set.
electrons for a PPP calculation), i.e. n is the number of MOs formed from the those electrons being included in the basis set.  is the valence electronic
is the valence electronic  electronic for the PPP method) energy, rather than the total electronic energy, and
electronic for the PPP method) energy, rather than the total electronic energy, and 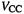 is the core-core repulsion, rather than
is the core-core repulsion, rather than

342 Computational Chemistry
the nucleus–nucleus repulsion:
Treating the core electrons in effect as part of the atomic nuclei means that we need basis functions only for the valence electrons. With a minimal basis set (section 5.3.3) an ab initio calculation on ethene,  needs five basis functions
needs five basis functions 
 for each carbon and one basis function (1s) for each hydrogen, a total of 14 basis functions, while a SE calculation needs four functions for each carbon and one for each hydrogen, for a total of 12; for cholesterol,
for each carbon and one basis function (1s) for each hydrogen, a total of 14 basis functions, while a SE calculation needs four functions for each carbon and one for each hydrogen, for a total of 12; for cholesterol,  the numbers of basis functions are 181 and 154 for ab initio and SE, respectively. In both cases the SE calculation needs only about 85% as many basis functions as an ab initio calculation; the SE basis set advantage is small compared to minimal basis set ab initio calculations, but large compared to ab initio calculations with split valence and split valence plus polarization (section 5.3.3) basis sets. For ethene, comparing a
the numbers of basis functions are 181 and 154 for ab initio and SE, respectively. In both cases the SE calculation needs only about 85% as many basis functions as an ab initio calculation; the SE basis set advantage is small compared to minimal basis set ab initio calculations, but large compared to ab initio calculations with split valence and split valence plus polarization (section 5.3.3) basis sets. For ethene, comparing a  ab initio calculation with a minimal basis SE calculation, the numbers of basis functions are 38 and 12, a ratio of 32%; for cholesterol, 497 and 154, a ratio of 31%. SE calculations use only a minimal basis set and hope to compensate for this by parameterization of the two-electron integrals (below).
ab initio calculation with a minimal basis SE calculation, the numbers of basis functions are 38 and 12, a ratio of 32%; for cholesterol, 497 and 154, a ratio of 31%. SE calculations use only a minimal basis set and hope to compensate for this by parameterization of the two-electron integrals (below).
(2) The basis set functions. In SE methods the basis functions correspond to atomic orbitals (valence AOs or 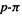 AOs), while in ab initio calculations this is strictly true only for a minimal basis set, since an ab initio calculation can use many more basis functions than there are conventional AOs. The SCF-type SE methods we are considering in this chapter use Slater functions, rather than approximating Slater functions as sums of Gaussian functions (section 5.3.2). Recall that the only reason ab initio calculations use Gaussian, rather than the more accurate Slater, functions, is because calculation of the electron–electron repulsion two-electron integrals is far faster with Gaussian functions (section 5.3.2). In SE calculations these integrals have been parameterized into the
AOs), while in ab initio calculations this is strictly true only for a minimal basis set, since an ab initio calculation can use many more basis functions than there are conventional AOs. The SCF-type SE methods we are considering in this chapter use Slater functions, rather than approximating Slater functions as sums of Gaussian functions (section 5.3.2). Recall that the only reason ab initio calculations use Gaussian, rather than the more accurate Slater, functions, is because calculation of the electron–electron repulsion two-electron integrals is far faster with Gaussian functions (section 5.3.2). In SE calculations these integrals have been parameterized into the
calculation (see below). Mathematical |
forms of the basis functions are still needed, |
to calculate overlap integrals |
for although these methods treat the overlap |
matrix as a unit matrix, some overlap integrals are evaluated (approximate MO theory has some apparent logical contradictions [7]) and used to help calculate core integrals and electron-repulsion integrals. As in ab initio calculations linear combinations of the basis functions are used to construct MOs, which in turn are multiplied by spin functions and used to represent the total molecular wavefunction as a Slater determinant (section 5.2.3.1).
(3) The integrals. The core integrals and the two-electron repulsion integrals (electron-repulsion integrals), Eq. (6.1), are not calculated from first principles (i.e. not from an explicit Hamiltonian and basis functions, as illustrated in section 5.2.3.6e), but rather many integrals are taken as zero, and those that are used are evaluated in an empirical way from the kinds of atoms involved and their distances apart. Recall that calculation of the two-electron integrals, particularly the threeand four-center ones (those involving three or four different atoms) takes up most of the time in an ab initio calculation. The integrals to be ignored (set equal to zero) are determined from the extent to which differential overlap is neglected. Differential overlap dS is the

Semiempirical Calculations 343
differential of the overlap integral (e.g. section 4.3.3) S:
SE methods differ amongst themselves in (amongst other ways) the criteria for setting dS = 0, i.e. for applying zero differential overlap (ZDO).
(4) The overlap matrix. SCF-type SE methods take the overlap matrix as a unit matrix, S = 1, so S vanishes from the Roothaan–Hall equations  without the necessity of using an orthogonalizing matrix to transform these equations into standard eigenvalue form
without the necessity of using an orthogonalizing matrix to transform these equations into standard eigenvalue form  (which latter enables the Fock matrix to be diagonalized to give the MO coefficients and energy levels; sections 4.4.3, 4.4.1, and 5.2.3.6b).
(which latter enables the Fock matrix to be diagonalized to give the MO coefficients and energy levels; sections 4.4.3, 4.4.1, and 5.2.3.6b).
6.2.2 The Pariser-Parr-Pople (PPP) method
The first SE SCF-type method to gain widespread use was the PPP method (1953) [11,12]. Like the SHM, PPP calculations are limited to  electrons, with the other electrons forming a
electrons, with the other electrons forming a  framework to hold the atomic
framework to hold the atomic 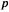 orbitals in place. The Fock matrix elements are calculated from Eq. (6.1); for a PPP calculation
orbitals in place. The Fock matrix elements are calculated from Eq. (6.1); for a PPP calculation  represents the nuclei plus all non-
represents the nuclei plus all non-  -system electrons,
-system electrons,  is calculated from the coefficients of those p AOs contributing to
is calculated from the coefficients of those p AOs contributing to  and the two-electron repulsion integrals refer to electrons in the
and the two-electron repulsion integrals refer to electrons in the  system. The one-center core integrals
system. The one-center core integrals  are estimated empirically from the ionization energy of a 2p AO and (see below) the two-electron integral
are estimated empirically from the ionization energy of a 2p AO and (see below) the two-electron integral 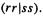 The two-center core integrals
The two-center core integrals 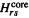 are calculated from
are calculated from
where k is an empirical parameter chosen to give the best agreement with experiment of the wavelength of UV absorption bands, and the overlap integral 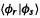 is calculated from the basis functions, with the proviso that if
is calculated from the basis functions, with the proviso that if  and
and  are on atoms that are not connected then the integral is taken as zero.
are on atoms that are not connected then the integral is taken as zero.
The two-electron integrals are evaluated by applying the ZDO approximation (above) to all different orbitals r and s:
From Eq. (6.6) and the definition of the two-electron integral
it follows that (1) for  (rs|tu) = 0, and (2) for r =s and t = u, (rs|tu) = (rr|tt). Both cases are taken into account by writing
(rs|tu) = 0, and (2) for r =s and t = u, (rs|tu) = (rr|tt). Both cases are taken into account by writing
