
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX

344 Computational Chemistry
where the  are Kronecker deltas
are Kronecker deltas  if the subscripts are the same, zero otherwise). Thus the four-center (i.e.
if the subscripts are the same, zero otherwise). Thus the four-center (i.e.  and three-center (i.e.
and three-center (i.e.  two-electron integrals are ignored, but not the two-center (i.e.
two-electron integrals are ignored, but not the two-center (i.e.  and one-center (i.e.
and one-center (i.e.  twoelectron integrals. The one-center integrals
twoelectron integrals. The one-center integrals  are taken as the difference between the valence-state ionization energy and the electron affinity of the atom bearing
are taken as the difference between the valence-state ionization energy and the electron affinity of the atom bearing  (these valence-state parameters refer to a hypothetical isolated atom in the same hybridization state as in the molecule, and can be found spectroscopically). The two-center integrals
(these valence-state parameters refer to a hypothetical isolated atom in the same hybridization state as in the molecule, and can be found spectroscopically). The two-center integrals  are estimated from
are estimated from  and
and  and the distance between the
and the distance between the 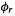 and
and 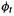
atoms. |
|
|
|
Although the overlap integrals |
are actually calculated for the evaluation of |
||
(Eq. (6.5), the overlap matrix is taken as a unit matrix as far as the matrix |
|||
Roothaan–Hall equations |
go; thus |
or |
and the |
Fock matrix is diagonalized to give the MO coefficients and energy levels without transforming it with an orthogonalizing matrix. That the overlap matrix is a unit matrix is a corollary of the ZDO approximation of Eq. (6.6), from which it follows that the off-diagonal matrix elements are zero; the diagonal elements are of course unity
if normalized |
AO basis functions |
are used. PPP energies are electronic |
||
energies |
or electronic energies |
plus core-core repulsions, |
if |
is added |
(Eq. (6.2)). |
|
|
|
|
The PPP method has been used to calculate the UV spectra of conjugated compounds, especially dyes [13], a task it performs fairly well. The accuracy of these calculations can be improved by incorporating electron correlation (section 5.4), using the configuration interaction (CI) method. The calculations were usually done at a fixed geometry, although an empirical bond length-bond order relation permits optimization of bond length. The classical PPP method is not much used, having evolved into other neglect of differential overlap (NDO) methods, especially those parameterized for spectra, like INDO/S and ZINDO/S (below).
6.2.3 The complete neglect of differential overlap (CNDO) method
The first SE SCF-type method to go beyond just  electrons was the complete neglect of differential overlap method (1965) [14]. This was a general-geometry method, since it is not limited to planar
electrons was the complete neglect of differential overlap method (1965) [14]. This was a general-geometry method, since it is not limited to planar  systems (molecules with conjugated
systems (molecules with conjugated  electron systems, like benzene, are usually planar). Like the other early general-geometry method, the EHM, which appeared in 1963 (section 4.4), CNDO calculations use a minimal valence basis set of Slater-type orbitals, in which each atom has the usual number of valence AOs. The Fock matrix elements are calculated from Eq. (6.1); for a CNDO calculation
electron systems, like benzene, are usually planar). Like the other early general-geometry method, the EHM, which appeared in 1963 (section 4.4), CNDO calculations use a minimal valence basis set of Slater-type orbitals, in which each atom has the usual number of valence AOs. The Fock matrix elements are calculated from Eq. (6.1); for a CNDO calculation  represents the nuclei plus all core electrons,
represents the nuclei plus all core electrons,  is calculated from the coefficients of the valence AOs, and the two-electron repulsion integrals refer to valence electrons.
is calculated from the coefficients of the valence AOs, and the two-electron repulsion integrals refer to valence electrons.
There are two versions of CNDO, CNDO/1 and an improved version, CNDO/2. First
look at CNDO/1. Consider the core integrals |
where both orbitals are the same |
|
(i.e. the same orbital occurs twice in the integral |
|
and are on the |
same atom A. Recall the example of an ab initio calculation on |
(section 5.2.36e). |
|

Semiempirical Calculations 345
Consider, say, element (1,1) of that  matrix. From Eq. (5.116):
matrix. From Eq. (5.116):
Eq. (6.9) can be generalized to a matrix element (r,r) and a molecule with atoms A, B, C,..., giving
where  is a basis function on atom A. The
is a basis function on atom A. The  term in Eq. (7.0) is regarded as the energy of an electron in the AO on A corresponding to the function
term in Eq. (7.0) is regarded as the energy of an electron in the AO on A corresponding to the function  and is taken as the negative of the valence-state ionization energy of such an electron. The integrals in the
and is taken as the negative of the valence-state ionization energy of such an electron. The integrals in the  term are simply calculated as the potential energy of a valence s orbital in the electrostatic field of the core of atom A, B, etc., e.g.
term are simply calculated as the potential energy of a valence s orbital in the electrostatic field of the core of atom A, B, etc., e.g.
where  is the charge on the core of atom B, i.e. the atomic number minus the number of core (non-valence) electrons, and the variable
is the charge on the core of atom B, i.e. the atomic number minus the number of core (non-valence) electrons, and the variable  is the distance of the 2s electron from the center of the core (from the atomic nucleus). The core integrals with different orbitals
is the distance of the 2s electron from the center of the core (from the atomic nucleus). The core integrals with different orbitals  on the same atom (A = B; one-center integrals) or on different atoms are taken as being proportional to the overlap integral of the relevant orbitals:
on the same atom (A = B; one-center integrals) or on different atoms are taken as being proportional to the overlap integral of the relevant orbitals:
The overlap integral here is calculated from the basis functions, although (as for the PPP method, above) the overlap matrix is taken as a unit matrix as far as the matrix Roothaan–Hall equations are concerned. The proportionality constant  is taken as the arithmetic mean of parameters for atoms A and B, these parameters being those that give the best fit of CNDO MO coefficients to those of minimal-basis-set ab initio calculations. Since different AOs on the same atom are orthogonal, when A=B these integrals are zero. Note thatcalculating
is taken as the arithmetic mean of parameters for atoms A and B, these parameters being those that give the best fit of CNDO MO coefficients to those of minimal-basis-set ab initio calculations. Since different AOs on the same atom are orthogonal, when A=B these integrals are zero. Note thatcalculating  from a best-fit to minimal-basis-set ab initio calculations means that CNDO parameterization is not purely empirical, but rather, to some extent attempts to match (low-level) ab initio results. This is a weakness of CNDO and a potential weakness of its successors INDO and NDDO (below). As repeatedly emphasized by Dewar, this deficiency was avoided in his methods (section 6.2.5.1) by consistently parameterizing to match experiment.
from a best-fit to minimal-basis-set ab initio calculations means that CNDO parameterization is not purely empirical, but rather, to some extent attempts to match (low-level) ab initio results. This is a weakness of CNDO and a potential weakness of its successors INDO and NDDO (below). As repeatedly emphasized by Dewar, this deficiency was avoided in his methods (section 6.2.5.1) by consistently parameterizing to match experiment.
As with the PPP method, the two-electron repulsion integrals are evaluated by applying the ZDO approximation to all different orbitals r and s (Eq. (6.6)). Thus the two-electron integrals reduce to  (Eq. (6.8)), i.e. only oneand two-center two-electron integrals are considered. All one-center integrals on the
(Eq. (6.8)), i.e. only oneand two-center two-electron integrals are considered. All one-center integrals on the
