
- •COMPUTATIONAL CHEMISTRY
- •CONTENTS
- •PREFACE
- •1.1 WHAT YOU CAN DO WITH COMPUTATIONAL CHEMISTRY
- •1.2 THE TOOLS OF COMPUTATIONAL CHEMISTRY
- •1.3 PUTTING IT ALL TOGETHER
- •1.4 THE PHILOSOPHY OF COMPUTATIONAL CHEMISTRY
- •1.5 SUMMARY OF CHAPTER 1
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •2.1 PERSPECTIVE
- •2.2 STATIONARY POINTS
- •2.3 THE BORN–OPPENHEIMER APPROXIMATION
- •2.4 GEOMETRY OPTIMIZATION
- •2.6 SYMMETRY
- •2.7 SUMMARY OF CHAPTER 2
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •3.1 PERSPECTIVE
- •3.2 THE BASIC PRINCIPLES OF MM
- •3.2.1 Developing a forcefield
- •3.2.2 Parameterizing a forcefield
- •3.2.3 A calculation using our forcefield
- •3.3 EXAMPLES OF THE USE OF MM
- •3.3.2 Geometries and energies of polymers
- •3.3.3 Geometries and energies of transition states
- •3.3.4 MM in organic synthesis
- •3.3.5 Molecular dynamics and Monte Carlo simulations
- •3.4 GEOMETRIES CALCULATED BY MM
- •3.5 FREQUENCIES CALCULATED BY MM
- •3.6 STRENGTHS AND WEAKNESSES OF MM
- •3.6.1 Strengths
- •3.6.2 Weaknesses
- •3.7 SUMMARY OF CHAPTER 3
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •4.1 PERSPECTIVE
- •4.2.1 The origins of quantum theory: blackbody radiation and the photoelectric effect
- •4.2.2 Radioactivity
- •4.2.3 Relativity
- •4.2.4 The nuclear atom
- •4.2.5 The Bohr atom
- •4.2.6 The wave mechanical atom and the Schrödinger equation
- •4.3.1 Introduction
- •4.3.2 Hybridization
- •4.3.3 Matrices and determinants
- •4.3.4 The simple Hückel method – theory
- •4.3.5 The simple Hückel method – applications
- •4.3.6 Strengths and weaknesses of the SHM
- •4.4.1 Theory
- •4.4.2 An illustration of the EHM: the protonated helium molecule
- •4.4.3 The extended Hückel method – applications
- •4.4.4 Strengths and weaknesses of the EHM
- •4.5 SUMMARY OF CHAPTER 4
- •REFERENCES
- •EASIER QUESTIONS
- •5.1 PERSPECTIVE
- •5.2.1 Preliminaries
- •5.2.2 The Hartree SCF method
- •5.2.3 The HF equations
- •5.2.3.1 Slater determinants
- •5.2.3.2 Calculating the atomic or molecular energy
- •5.2.3.3 The variation theorem (variation principle)
- •5.2.3.4 Minimizing the energy; the HF equations
- •5.2.3.5 The meaning of the HF equations
- •5.2.3.6a Deriving the Roothaan–Hall equations
- •5.3 BASIS SETS
- •5.3.1 Introduction
- •5.3.2 Gaussian functions; basis set preliminaries; direct SCF
- •5.3.3 Types of basis sets and their uses
- •5.4 POST-HF CALCULATIONS: ELECTRON CORRELATION
- •5.4.1 Electron correlation
- •5.4.3 The configuration interaction approach to electron correlation
- •5.5.1 Geometries
- •5.5.2 Energies
- •5.5.2.1 Energies: Preliminaries
- •5.5.2.2 Energies: calculating quantities relevant to thermodynamics and to kinetics
- •5.5.2.2a Thermodynamics; “direct” methods, isodesmic reactions
- •5.5.2.2b Thermodynamics; high-accuracy calculations
- •5.5.2.3 Thermodynamics; calculating heats of formation
- •5.5.2.3a Kinetics; calculating reaction rates
- •5.5.2.3b Energies: concluding remarks
- •5.5.3 Frequencies
- •Dipole moments
- •Charges and bond orders
- •Electrostatic potential
- •Atoms-in-molecules
- •5.5.5 Miscellaneous properties – UV and NMR spectra, ionization energies, and electron affinities
- •5.5.6 Visualization
- •5.6 STRENGTHS AND WEAKNESSES OF AB INITIO CALCULATIONS
- •5.7 SUMMARY OF CHAPTER 5
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •6.1 PERSPECTIVE
- •6.2 THE BASIC PRINCIPLES OF SCF SE METHODS
- •6.2.1 Preliminaries
- •6.2.2 The Pariser-Parr-Pople (PPP) method
- •6.2.3 The complete neglect of differential overlap (CNDO) method
- •6.2.4 The intermediate neglect of differential overlap (INDO) method
- •6.2.5 The neglect of diatomic differential overlap (NDDO) method
- •6.2.5.2 Heats of formation from SE electronic energies
- •6.2.5.3 MNDO
- •6.2.5.7 Inclusion of d orbitals: MNDO/d and PM3t; explicit electron correlation: MNDOC
- •6.3 APPLICATIONS OF SE METHODS
- •6.3.1 Geometries
- •6.3.2 Energies
- •6.3.2.1 Energies: preliminaries
- •6.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •6.3.3 Frequencies
- •6.3.4 Properties arising from electron distribution: dipole moments, charges, bond orders
- •6.3.5 Miscellaneous properties – UV spectra, ionization energies, and electron affinities
- •6.3.6 Visualization
- •6.3.7 Some general remarks
- •6.4 STRENGTHS AND WEAKNESSES OF SE METHODS
- •6.5 SUMMARY OF CHAPTER 6
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •7.1 PERSPECTIVE
- •7.2 THE BASIC PRINCIPLES OF DENSITY FUNCTIONAL THEORY
- •7.2.1 Preliminaries
- •7.2.2 Forerunners to current DFT methods
- •7.2.3.1 Functionals: The Hohenberg–Kohn theorems
- •7.2.3.2 The Kohn–Sham energy and the KS equations
- •7.2.3.3 Solving the KS equations
- •7.2.3.4a The local density approximation (LDA)
- •7.2.3.4b The local spin density approximation (LSDA)
- •7.2.3.4c Gradient-corrected functionals and hybrid functionals
- •7.3 APPLICATIONS OF DENSITY FUNCTIONAL THEORY
- •7.3.1 Geometries
- •7.3.2 Energies
- •7.3.2.1 Energies: preliminaries
- •7.3.2.2 Energies: calculating quantities relevant to thermodynamics and kinetics
- •7.3.2.2a Thermodynamics
- •7.3.2.2b Kinetics
- •7.3.3 Frequencies
- •7.3.6 Visualization
- •7.4 STRENGTHS AND WEAKNESSES OF DFT
- •7.5 SUMMARY OF CHAPTER 7
- •REFERENCES
- •EASIER QUESTIONS
- •HARDER QUESTIONS
- •8.1 FROM THE LITERATURE
- •8.1.1.1 Oxirene
- •8.1.1.2 Nitrogen pentafluoride
- •8.1.1.3 Pyramidane
- •8.1.1.4 Beyond dinitrogen
- •8.1.2 Mechanisms
- •8.1.2.1 The Diels–Alder reaction
- •8.1.2.2 Abstraction of H from amino acids by the OH radical
- •8.1.3 Concepts
- •8.1.3.1 Resonance vs. inductive effects
- •8.1.3.2 Homoaromaticity
- •8.2 TO THE LITERATURE
- •8.2.1 Books
- •8.2.2 The Worldwide Web
- •8.3 SOFTWARE AND HARDWARE
- •8.3.1 Software
- •8.3.2 Hardware
- •8.3.3 Postscript
- •REFERENCES
- •INDEX

54 Computational Chemistry
constants are in fact generally roughly proportional to bond order (double bonds and triple bonds are about two and three times as stiff, respectively, as the corresponding single bonds). Some forcefields account for the variation of bond order with conformation (twisting p orbitals out of alignment reduces their overlap) by performing a simple PPP molecular orbital calculation (chapter 6) to obtain the bond order.
A sophisticated forcefield might also consider H/H nonbonded interactions explicitly, rather than simply subsuming them into methyl/methyl interactions (combining atoms into groups is the feature of a united atom forcefield). Furthermore, nonbonding interactions between polar groups need to be accounted for in a field not limited to hydrocarbons. These are usually handled by the well-known potential energy/electrostatic charge relationship
which has also been used to model hydrogen bonding [11].
A subtler problem with the naive forcefield developed here is that stretching, bending, torsional, and nonbonded terms are not completely independent. For example, the butane torsional potential energy curve (Fig. 3.5) does not apply precisely to all  systems, because the barrier heights will vary with the length of the central C–C bond, obviously decreasing (other things being equal) as the bond is lengthened, since there will be a decrease in the interactions (whatever causes them) between the
systems, because the barrier heights will vary with the length of the central C–C bond, obviously decreasing (other things being equal) as the bond is lengthened, since there will be a decrease in the interactions (whatever causes them) between the  and
and  on one of the carbons of the central C–C and those on the other carbon. This could be accounted for by making the k’s of Eq. (3.25) a function of the X–Y length. Actually, partitioning the energy of a molecule into stretching, bending, etc. terms is somewhat formal; e.g. the torsional barrier in butane can be considered to be partly due to nonbonded interactions between the methyl groups. It should be realized that there is no one, right functional form for an MM forcefield (see, e.g. [1b]); accuracy, versatility, and speed of computation are the deciding factors in devising a forcefield.
on one of the carbons of the central C–C and those on the other carbon. This could be accounted for by making the k’s of Eq. (3.25) a function of the X–Y length. Actually, partitioning the energy of a molecule into stretching, bending, etc. terms is somewhat formal; e.g. the torsional barrier in butane can be considered to be partly due to nonbonded interactions between the methyl groups. It should be realized that there is no one, right functional form for an MM forcefield (see, e.g. [1b]); accuracy, versatility, and speed of computation are the deciding factors in devising a forcefield.
3.2.3A calculation using our forcefield
Let us apply the naive forcefield developed here to comparing the energies of two 2,2,3,3-tetramethylbutane  i.e. t-Bu-Bu-t) geometries. We compare the energy of structure 1 (Fig. 3.8) with all the bond lengths and angles at our “natural” or standard values (i.e. at the STO-3G values we took as the equilibrium bond lengths and angles in section 3.2.2) with that of structure 2, where the central C–C bond has been stretched from 1.538 to 1.600 Å, but all other bond lengths, as well as the bond angles and dihedral angles, are unchanged. Figure 3.8 shows the nonbonded distances we need, which would be calculated by the program from bond lengths, angles and dihedrals. Using Eq. (3.1):
i.e. t-Bu-Bu-t) geometries. We compare the energy of structure 1 (Fig. 3.8) with all the bond lengths and angles at our “natural” or standard values (i.e. at the STO-3G values we took as the equilibrium bond lengths and angles in section 3.2.2) with that of structure 2, where the central C–C bond has been stretched from 1.538 to 1.600 Å, but all other bond lengths, as well as the bond angles and dihedral angles, are unchanged. Figure 3.8 shows the nonbonded distances we need, which would be calculated by the program from bond lengths, angles and dihedrals. Using Eq. (3.1):

Molecular Mechanics 55
For structure 1
Bond stretch contribution cf. structure with 
Bond stretch contribution cf. structure with 
Bond bend contribution cf. structure with 
Bond bend contribution cf. structure with 
Torsional contribution cf. structure with no gauche–butane interactions
nonbonding contribution cf. structure with noninteracting 
Actually, nonbonding interactions are already included in the torsional term (as gauche–butane interactions); we might have used an ethane-type torsional function and accounted for  interactions entirely with nonbonded terms. However, in
interactions entirely with nonbonded terms. However, in

56 Computational Chemistry
comparing calculated relative energies the torsional term will cancel out.
For structure 2
Bond stretch contribution cf. structure with 
Bond stretch contribution cf. structure with
Bond bend contribution cf. structure with 
Bond bend contribution cf. structure with 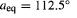
Torsional contribution cf. structure with no gauche–butane interactions.
The stretching and bending terms for structure 2 are the same as for structure 1, except for the contribution of the central C–C bond; strictly speaking, the torsional term should be smaller, since the opposing 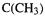 groups have been moved apart.
groups have been moved apart.
nonbonding contribution cf. structure with noninteracting
So the relative energies are calculated to be
This crude method predicts that stretching the |
central C/C bond of 2,2,3,3- |
tetramethylbutane from the approximately normal |
length of 1.583 Å |
