
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1. Introduction
- •2. Evaluation of the Craniomaxillofacial Deformity Patient
- •3. Craniofacial Deformities: Review of Etiologies, Distribution, and Their Classification
- •4. Etiology of Skeletal Malocclusion
- •5. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Traumatic Defects
- •6. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Review of Nasal Deformities
- •7. Review of Benign Tumors of the Maxillofacial Region and Considerations for Bone Invasion
- •8. Oral Malignancies: Etiology, Distribution, and Basic Treatment Considerations
- •9. Craniomaxillofacial Bone Infections: Etiologies, Distributions, and Associated Defects
- •11. Craniomaxillofacial Bone Healing, Biomechanics, and Rigid Internal Fixation
- •12. Metal for Craniomaxillofacial Internal Fixation Implants and Its Physiological Implications
- •13. Bioresorbable Materials for Bone Fixation: Review of Biological Concepts and Mechanical Aspects
- •14. Advanced Bone Healing Concepts in Craniomaxillofacial Reconstructive and Corrective Bone Surgery
- •15. The ITI Dental Implant System
- •16. Localized Ridge Augmentation Using Guided Bone Regeneration in Deficient Implant Sites
- •17. The ITI Dental Implant System in Maxillofacial Applications
- •18. Maxillary Sinus Grafting and Osseointegration Surgery
- •19. Computerized Tomography and Its Use for Craniomaxillofacial Dental Implantology
- •20B. Atlas of Cases
- •21A. Prosthodontic Considerations in Dental Implant Restoration
- •21B. Overdenture Case Reports
- •22. AO/ASIF Mandibular Hardware
- •23. Aesthetic Considerations in Reconstructive and Corrective Craniomaxillofacial Bone Surgery
- •24. Considerations for Reconstruction of the Head and Neck Oncologic Patient
- •25. Autogenous Bone Grafts in Maxillofacial Reconstruction
- •26. Current Practice and Future Trends in Craniomaxillofacial Reconstructive and Corrective Microvascular Bone Surgery
- •27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects
- •28. Indications and Technical Considerations of Different Fibula Grafts
- •29. Soft Tissue Flaps for Coverage of Craniomaxillofacial Osseous Continuity Defects with or Without Bone Graft and Rigid Fixation
- •30. Mandibular Condyle Reconstruction with Free Costochondral Grafting
- •31. Microsurgical Reconstruction of Large Defects of the Maxilla, Midface, and Cranial Base
- •32. Condylar Prosthesis for the Replacement of the Mandibular Condyle
- •33. Problems Related to Mandibular Condylar Prosthesis
- •34. Reconstruction of Defects of the Mandibular Angle
- •35. Mandibular Body Reconstruction
- •36. Marginal Mandibulectomy
- •37. Reconstruction of Extensive Anterior Defects of the Mandible
- •38. Radiation Therapy and Considerations for Internal Fixation Devices
- •39. Management of Posttraumatic Osteomyelitis of the Mandible
- •40. Bilateral Maxillary Defects: THORP Plate Reconstruction with Removable Prosthesis
- •41. AO/ASIF Craniofacial Fixation System Hardware
- •43. Orbital Reconstruction
- •44. Nasal Reconstruction Using Bone Grafts and Rigid Internal Fixation
- •46. Orthognathic Examination
- •47. Considerations in Planning for Bimaxillary Surgery and the Implications of Rigid Internal Fixation
- •48. Reconstruction of Cleft Lip and Palate Osseous Defects and Deformities
- •49. Maxillary Osteotomies and Considerations for Rigid Internal Fixation
- •50. Mandibular Osteotomies and Considerations for Rigid Internal Fixation
- •51. Genioplasty Techniques and Considerations for Rigid Internal Fixation
- •52. Long-Term Stability of Maxillary and Mandibular Osteotomies with Rigid Internal Fixation
- •53. Le Fort II and Le Fort III Osteotomies for Midface Reconstruction and Considerations for Internal Fixation
- •54. Craniofacial Deformities: Introduction and Principles of Management
- •55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton
- •56. Calvarial Bone Graft Harvesting Techniques: Considerations for Their Use with Rigid Fixation Techniques in the Craniomaxillofacial Region
- •57. Crouzon Syndrome: Basic Dysmorphology and Staging of Reconstruction
- •58. Hemifacial Microsomia
- •59. Orbital Hypertelorism: Surgical Management
- •60. Surgical Correction of the Apert Craniofacial Deformities
- •Index
33
Problems Related to Mandibular Condylar Prosthesis
Christian Lindqvist, Anna-Lisa Söderholm, and Dorrit Hallikainen
Several different autogenous transplants can be used to restore temporomandibular joint (TMJ) function.1–4 Whenever possible autogenous grafts are always preferred. There are few relative indications for using a condylar implant in arthroplasty.
For various reasons, autogenous transplantation may be contraindicated. Transplants usually require maxillomandibular fixation (MMF), although fixation of the graft with lag-screw technique can shorten the period of immobilization.5 When any fixation between the jaws implies a risk because of the patient’s general condition, another method of arthroplasty should be chosen. The same holds for situations in which removal of a rib should not be undertaken for the same reason. Other relative contraindications for autogenous arthroplasty might be extremely large osseous ankylotic masses and, in certain cases, reankylosis after costochondral transplantation.
Another type of problem arises when, in addition to the condyle, large segments of the mandible have to be reconstructed. For example, in tumor surgery, when mandibular resection with exarticulation of the condyle is necessary, an allogeneic prosthesis might be the best method for primary reconstruction. The same holds for traumatic cases in which the condyle is avulsed or highly fragmented, and primary restoration of mandible and joint functions by osteosynthesis is impossible.
During the 10-year period 1984–1994, 31 condylar prostheses were placed in 13 male and 11 female patients at the Department of Oral and Maxillofacial Surgery, Helsinki University Central Hospital, Helsinki, Finland. The mean age of the 24 patients was 49 years (range, 39 to 89 years). Two essentially different implant types were used. In 12 patients there was mainly joint pathology (posttraumatic or rheumatoid ankylosis, or condylar tumor), and in 12 the condyle had to be reconstructed along with the extracapsular mandibular segments that had been removed or destroyed because of a malignant tumor or extensive trauma.
In the first group, a condylar prosthesis was used (Figure 33.1a). Four patients with severe posttraumatic osseous ankylosis and four with bilateral ankylosis owing to rheumatoid
arthritis had contraindications for an autogenous arthroplasty. In three of these patients, earlier rib arthroplasty had failed and reankylosis developed. A gap arthroplasty was not considered to be sufficient for permanent relief of the ankylotic situation in any of these cases.
In the second group, a reconstruction plate with condylar head was used (Figure 33.1b). In two traumatic cases, both of which represented shotgun injuries, there was no possibility of retaining the multiple bone fragments, including the mandibular condyle. A similar reconstruction was also performed in 11 tumor cases, in which, because of tumor extension, hemimandibulectomy and condylar exarticulation was indicated.
In surgery for TMJ ankylosis, stainless steel AO/ASIF condylar prostheses were used. In the cases in which, in addition to the condylar process, segments of extra-articular mandibular bone also had to be reconstructed, AO/ASIF reconstruction plates including the condylar head were installed.
A combined preauricular, hemicoronal or bicoronal, and retromandibular approach was used in the ankylosis operations. After substantial removal of the ankylotic mass, a new fossa was created in the region of the damaged mandibular condyle. No attempt was made, however, to remove the condylar process totally. Instead, we prefer that some condylar bone is left in the region of the former glenoid fossa (Figure 33.2). A unilateral coronoidectomy at the minimum was always performed. A correctly sized condylar prosthesis was attached to the ramus and angular region with 2.7-mm bicortical screws. Whenever possible, temporary MMF was utilized during insertion of the implant.
In tumor and trauma surgery, MMF must always be applied intraoperatively. In edentulous patients, Erich arch bars can be preoperatively attached to the patients’ complete dentures, which are then affixed to the maxilla and mandible by screws and or wires. Thereby, the mandible is immobilized during plate bending and insertion. Effort is taken to remove the condyle carefully to preserve the disk intact within the fossa.
The condylar head might be placed slightly inferior to the fossa ( 5 mm) to reduce the risk of glenoid fossa erosion.
377

378 |
C. Lindqvist, A.-L. Söderholm, and D. Hallikainen |
a |
b |
FIGURE 33.1 (a) Three different sizes of AO/ASIF condylar prostheses. (b) Three different sizes of AO/ASIF reconstruction plates including the condylar head. Currently, 2.4 mm titanium plates with condylar heads are available for use (see Chapter 22).
Radiologic Examination
Radiologic evaluation should include a panoramic radiograph and Townes’ view. Other images are obtained as appropriate with respect to the diagnostic problem. Lateral panoramic views of both joints (Zonarc, Instrumentarium, Finland), lateral and posteroanterior detailed panoramic views of the operated joint, as well as tomography in the lateral and posteroanterior projection can also be undertaken. We have obtained detailed images and tomograms using Scanora (Orion Corp., Soredex, Helsinki, Finland) equipment.
The radiographs should be evaluated for displacement of the prosthetic condyle and bone resorption in the glenoid fossa and the ramus area (in the region of the screw fixation). Heterotopic bone formation can be recorded and graded according to Brooker (Table 33.1).6 New bone formation within the fossa is recorded separately and not considered as heterotopic bone (Figure 33.3).
The prosthesis can be regarded as displaced from the glenoid fossa when more than two thirds of its articular surface is incongruent with the joint groove (lateromedially or anteroposteriorly).
of severe glenoid fossa resorption. In the first case, 21 months after alloarthroplasty undertaken for a benign condylar tumor, radiologic examination revealed large amounts (Gr IV) of heterotopic bone around the metallic condyle. The patient was
Ankylosis
In 1992 we published a follow-up on 19 patients with a total of 23 condylar prostheses7). The follow-up time was 29 months on average. During this period, two implants had to be removed, one because of reankylosis and the other because
FIGURE 33.2 Postoperative radiograph showing AO prosthesis in place. No attempt was made to remove all the hypertrophic bone from the temporal fossa.
33. Problems Related to Mandibular Condylar Prosthesis
TABLE 33.1 Modified classification of heterotopic bone formation in the temporomandibular joint area according to Brooker.
Class |
Description |
IIslands of bone within the soft tissues about the temporomandibular joint.
IIBone spurs from the condyle or the joint groove leaving at least one third of the joint capsular area free.
IIIBone spurs from the condyle or the joint groove extending over more than two thirds of the joint capsular area.
IV |
Apparent bony ankylosis of the temporomandibular joint. |
Determination of the classification based on lateral and posteroanterior images.
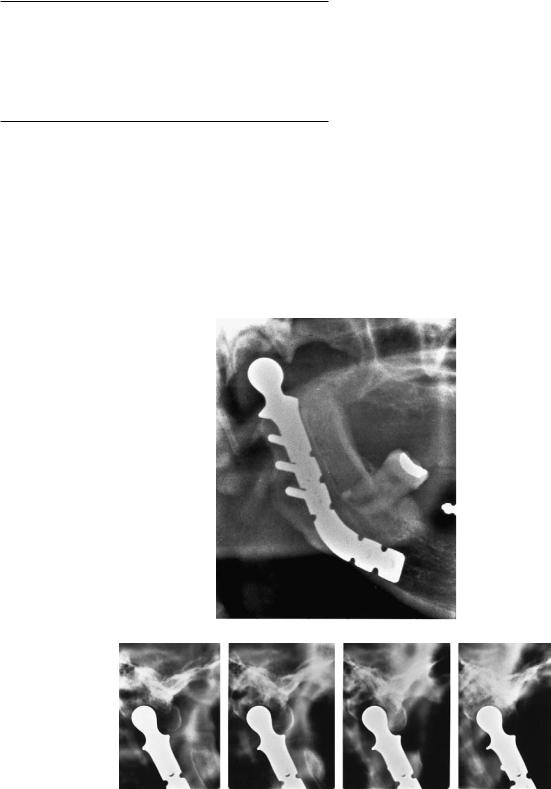
unable to open her mouth for more than 4 to 5 mm (Figure 33.4a–c). After removal of the implant a costochondral arthroplasty was performed without any subsequent complications (Figure 33.4d). Two years after the second operation, maximal incisal opening (MIO) was 45 mm.
In the other patient, who suffered from severe juvenile
a
b
379
rheumatoid arthritis, there was erosion of the glenoid fossa, resulting in perforation of the middle cranial fossa 10 months after arthroplasty. The implant was removed and exchanged for a rib (Figure 33.5). As the dura was exposed the fossa was also reconstructed with a cortical bone transplant. Later, the other joint was also affected and replaced with a rib. The other 9 joints functioned satisfactorily (Table 33.2). The mean MIO for all ankylosis patients was, however, only 22.8 mm. Radiologically, three condyles were not in a correct position with respect to the glenoid fossa. Bone resorption in at least some part of the fossa was diagnosed in 8 of the 11 joints. Heterotopic bone formation was seen in eight cases (Table 33.2). In three of them excessive new bone had been formed in the glenoid fossa and late resorption of the new bony surface was recorded.
Another type of complication has also been noted during later follow-up of three patients. A 63-year-old female patient with severe rheumatic ankylosis (RA) had bilateral TMJ arthroplasty in 1988 (Figure 33.6a,b). Because of the changed
FIGURE 33.3 (a) Right condyle immediately after alloarthroplasty owing to posttraumatic ankylosis. Prosthesis in correct position in the glenoid fossa. Some condylar bone left in fossa. (b) Right lateral to-
mograms 6 years after alloarthroplasty. Note significant amount of heterotopic bone formation anterior to the condylar head. Restriction of mouth opening.

380 |
C. Lindqvist, A.-L. Söderholm, and D. Hallikainen |
a |
b |
c |
d |
FIGURE 33.4 (a) Panoramic image after right TMJ alloarthroplasty because of benign condylar tumor. (b) Nine months later, signs of heterotopic bone formation around the condylar head. (c) Twelve months later (12 months after arthroplasty) a significant amount of
ectopic bone is present. Patient was unable to open her mouth more than 4 or 5 mm. (d) Panoramic image 2 years after removal of prosthesis and costochondral arthroplasty. MIO 45 mm.
33. Problems Related to Mandibular Condylar Prosthesis
form of the mandible combined with the prebent angle of the AO prosthesis, the condylar head was erroneously placed in front of the articular eminence. This led to pain and dysfunction. The prosthesis was changed, and the angle was completely straightened. Only in this position was it possible for the prosthetic head to hit the fossa correctly (Figure 33.6c,d). This consideration for mandibular deformation and standard prosthesis forms should be taken into account when treating patients with RA.
Malignant Tumors and Trauma
The 11 patients who had condylar reconstruction plates placed were followed up for an average of 25 months. Three plates had to be removed because of infection. In 1 patient the plate fractured and was exchanged for a similar one (Table 33.3, patient A). Three patients died during follow-up, 2 of whom had a functional alloplastic joint. The mean MIO for the remaining 6 patients, alive at the end of follow-up with the implant in place, was 31.5 mm.
Radiologically, the condyle was found to be displaced in four cases. The displacement, when occurring, was usually lateral and caudal. In only two patients was the condylar head in an exact position, centered deep within the glenoid fossa (Figure 33.7). In these two, however, there was bone erosion in the skull base. In four joints, heterotopic new bone formation was recorded.
Condylar Position, Resorption,
and Heterotopic Bone
Of the total of 23 TMJ half-prostheses, only 16 postoperatively were initially found to be situated in the glenoid fossa. Bone resorption developed during follow-up in 10 cases (43%), and in 1 a perforation to the skull base occurred 10 months after insertion of the implant (initial resorption of the fossa had already been diagnosed 2 months postoperatively). The first evidence of resorption for all 10 cases was recorded between 2 months and 3 years. Late resorption occurred in 3 cases where the condyle was partly incongruent with the joint groove (Table 33.2, patient B; Table 33.3, patients B and D). In 3 cases the resorption did not take place until new bone formation in the fossa had formed a bony surface in close contact with the prosthesis. Bone resorption in the region of the screws was seen in 5 cases (Table 33.2, patients D and E; Table 33.3, patient F). Heterotopic new bone formation was found in a total of 12 joints (52%). In 1 case, this resulted in almost complete restriction of condylar movement, and reoperation was necessary.
In our department the need for alloplastic implants constitute about 20% of cases with indications for TMJ reconstruction. If the pathology is mainly confined to the joint region, costochondral transplantation has been, whenever
381
possible, the primary choice during the last 20 years. Several biological reasons speak for an autogenous transplant,8,9 and there seem to be few reasons for abolishing this concept.5 Thus, unlike some authors,10–12 we do not believe that, for example, rheumatoid arthritis, per se, constitutes an indication for the use of a prosthesis rather than an autogenous graft. Resorption of the fossa with erosion through the skull base secondary to a metallic prosthesis has been reported earlier.10 This is a serious complication, which occurred in one of our patients with RA. In nearly half of the cases some degree of resorption was noted. It must be emphasized that clinical signs revealed neither the real location of the condyle or the resorption. Therefore, radiologic follow-up examinations are essential in the continued evaluation of the joint replaced with an artificial prosthesis.
We have no experience with a glenoid fossa implant, but bone resorption probably cannot be prevented without the use of one. An artificial socket certainly allows a more even distribution of bite forces over a wide area, but it also involves disadvantages and technical problems.10,13–15 These problems differ significantly from those encountered in the acetabulum of the hip joint. We believe that a costochondral or any other autogenous graft is still a far better solution than any foreign material in cases with TMJ ankylosis. When using a costochondral graft, there is no need for an artificial fossa, and the risk of heterotopic bone formation is minimal. New bone formation was rarely seen in our series of 16 rib transplants even after a follow-up of more than 10 years.16
Heterotopic bone occurs in 70% to 75% of the patients who have undergone total hip arthroplasty.17–19 However, significant amounts of bone develop in only one fifth of cases (Brooker’s classes III and IV). The ectopic tissue limits postoperative motion and causes pain (and need for reoperation) in 2% to 4% of the patients.19 Nonsteroid antiinflammatory drugs and irradiation have been used to prevent heterotopic bone formation after hip arthroplasty. We are not aware of any corresponding studies with respect to TMJ arthroplasty. Even a fossa implant might not have prevented the formation of new bone, which in our series occurred in 52% of the joints. Grade III or IV bone formation was diagnosed in 22%, and in one patient (4%), it resulted in reoperation. These figures seem to be in accordance with the ones presented in patient materials concerning total hip arthroplasty.17–19 In tumor surgery there is often no way or reason to try to avoid an alloplastic, albeit temporary, primary reconstruction. Before the introduction of the THORP (titanium hollow-screw reconstruction plate) system by Raveh20,21 it was difficult to preserve a large enough condylar segment, with space for 4 or 5 screws, without impinging on the extent of resection or ablation. With the development of the THORP system, in which the plate-screw locking principle results in both internal and external fixation, 2 to 3 screws per segment are sufficient to secure plate stability. Even in a small condylar fragment, there is space enough for fixation, and the TMJ can be left intact.22

382 |
C. Lindqvist, A.-L. Söderholm, and D. Hallikainen |
a
b
c
d
FIGURE 33.5 (a) A 40-year-old woman with rheumatoid arthritis. Note the open bite. (b) Five months after bilateral alloarthroplasty. Panoramic radiograph shows signs of bone destruction to the right.
(c) Lateral tomogram of right joint reveals perforation to the cranial base. (d) Left joint has no signs of bone destruction.

33. Problems Related to Mandibular Condylar Prosthesis |
383 |
e
f
FIGURE 33.5 Continued. (e) Anteroposterior detailed image of right joint 10 months after arthroplasty. Perforation is now evident. (f) Situation 4 months after removal of right prosthesis and costochondral arthroplasty. MIO 22 mm. Left side is still unaffected.
TABLE 33.2 TMJ ankylosis. Patient data and follow-up.
|
|
|
|
|
Maximum |
|
|
|
|
|
|
|
Follow-up |
opening |
Position |
Resorption |
Heterotopic |
Patient |
Sex |
Age |
Diagnosis |
(months) |
(mm) |
of condyle |
of fossa |
bone formation* |
|
|
|
|
|
|
|
|
|
A |
M |
56 |
PA |
3 |
33 |
displaced |
|
Gr I |
B |
F |
49 |
tumor |
24*** |
4–5 |
correct |
|
Gr IV |
C |
M |
20 |
PA |
66 |
27 |
correct |
|
Gr II |
D |
F |
69 |
RA |
22 |
30 |
|
|
|
left |
|
|
|
|
|
displaced |
|
— |
right |
|
|
|
|
|
correct |
|
Gr II–III |
E |
F |
40 |
RA |
12 |
15 |
|
|
|
left |
|
|
|
|
|
correct |
|
— |
right |
|
|
|
*** |
|
correct |
(perforation) |
— |
F |
F |
45 |
PA |
35 |
25 |
correct |
|
Gr II |
G |
M |
37 |
PA** |
65 |
30 |
correct |
|
Gr II–IV |
H |
M |
20 |
RA |
48 |
18 |
|
|
|
left |
|
|
** |
|
|
displaced |
|
Gr III |
right |
|
|
|
|
|
correct |
|
Gr III–IV |
PA posttraumatic ankylosis RA rheumatic ankylosis
*The amount of bone increased with time in three patients **Reankylosis after costochondral arthroplasty
***Prosthesis removed

384 |
C. Lindqvist, A.-L. Söderholm, and D. Hallikainen |
a
b
c
d
FIGURE 33.6 (a) A 56-year-old woman with severe rheumatoid arthritis. (b) Five years later, total destruction of both condyles. No movement in joints. (c) Bilateral TMJ arthroplasty showing that both condylar heads lie anteriorly to the articular eminence. Significant pain to the left. (d) Situation after left rearthroplasty. Condyle is now in correct position. The prebent angle of the prosthesis was straightened. Because of symptomless right joint, no reoperation has been performed.

33. Problems Related to Mandibular Condylar Prosthesis |
385 |
TABLE 33.3 Malignant tumors and trauma. Patient data and follow-up.
|
|
|
|
|
Maximum |
|
|
|
|
|
|
|
Follow-up |
opening |
Position |
Resorption |
Heterotopic |
Patient |
Sex |
Age |
Diagnosis |
(months) |
(mm) |
of condyle |
of fossa |
bone formation |
|
|
|
|
|
|
|
|
|
A |
M |
|
SCC |
|
|
|
|
|
plate 1 |
|
77 |
|
26* |
40 |
correct |
|
Gr I |
plate 2 |
|
79 |
|
40 |
42 |
displaced |
|
Gr I |
B |
M |
45 |
SCC |
18 |
27 |
correct |
|
Gr I |
C |
M |
56 |
SCC |
57 |
45 |
displaced |
|
Gr II |
D |
M |
89 |
SCC |
30 |
38 |
correct |
|
— |
E |
F |
77 |
SCC |
4** |
25 |
correct |
|
— |
F |
M |
60 |
SCC |
30 |
15 |
correct |
|
— |
G |
F |
25 |
sarcoma |
23 DFD |
20 |
correct |
|
— |
H |
F |
60 |
SCC |
7 days** |
25 |
displaced |
|
— |
I |
M |
67 |
SCC |
6 DFD |
20 |
correct |
|
— |
J |
M |
26 |
gunshot |
13*** |
52 |
displaced |
|
— |
K |
M |
48 |
gunshot |
28** |
35 |
correct |
|
— |
SCC squamous cell carcinoma DFD dead from disease *Plate fracture
**Plate removed due to infection
***Plate removed because of reconstruction with bone
a
b
FIGURE 33.7 (a) Hemimandibulectomy reconstructed with alloplast. Condyle in correct position according to panoramic tomogram. (b) Detailed image shows slight resorption dorsally. Condyle is not in exact position.

386 |
C. Lindqvist, A.-L. Söderholm, and D. Hallikainen |
a
b
c
FIGURE 33.8 (a) Panoramic tomogram and (b) CT of 81-year-old male with a large fibrosarcoma in the mandible to the left. (c) Panoramic tomogram showing reconstruction.

33. Problems Related to Mandibular Condylar Prosthesis |
387 |
d |
e |
FIGURE 33.8 Continued. (d,e) Patient 2 weeks postoperatively.
Large enough safety margins might, however, require condylar exarticulation in radical cancer surgery.22,23 Especially if the condyle is arthritic, deformed, and poorly functioning, it is probably not worth saving. In such situations, reconstruction of both the condyle and remaining mandible with a plate including the condylar head seems to give good functional and cosmetic results.24 This is of major importance for the patient with malignant disease (Figure 33.8). A long reconstruction implies considerable stress, and plate fractures have been reported. We had only one such case, and the exchange of the plate was uneventful. Overall, postoperative joint function was far better in the tumor than in the ankylosis patients, and less bone resorption and heterotopic bone formation were seen. One reason for this is probably that the tumor patients did not have any significant joint pathology, and it was possible to leave an intact disc in place in most cases. Although the artificial condyle was found not to be in an exact position in several cases, this did not seem to affect joint function, and most patients were free from pain. Strangely enough, the patients with absolutely correct condyle position displayed more often than the others a resorptive process in the glenoid fossa.
In conclusion, tumor and some trauma patients can benefit from a plate that includes a condylar prosthesis for reconstruction of a large mandibular segmental defect including the condyle. If, however, there is primary joint pathology, as in posttraumatic or rheumatoid ankylosis, artificial implants that
do not include a fossa imply a significant risk and do not, at least in our hands, give a satisfactory result. Significant progress has been made during recent years in developing a functioning alloplastic TMJ prothesis for patients with joint ankylosis.10–12 At the present time, 2.4 mm titanium condylar plates are available for use (see Chapter 22). The special anatomic and functional conditions in the region of the temporomandibular articulation seem to indicate that autogenous materials should still be preferred.
References
1.Smith AE, Robinson M. A new surgical procedure in bilateral reconstruction of condyles utilizing iliac bone grafts and creation of new joints by means of nonelectrolytic metal: a preliminary report. Plast Reconstr Surg. 1952;9:393–409.
2.Ware WH, Taylor RC. Cartilaginous growth centers transplanted to replace mandibular condyles in monkeys. J Oral Surg. 1966;24:33–43.
3.Ware WH, Taylor RC. Growth centre transplantation to replace damaged mandibular condyles. J Am Dent Assoc. 1966;73:128.
4.Matukas VJ, Szymela VF, Schmidt JF. Surgical treatment of bony ankylosis in a child using a composite cartilage-bone iliac crest graft. J Oral Surg. 1980;38:903–905.
5.Kaban LB, Perrott DH, Fisher K. A protocol for management of temporomandibular joint ankylosis. J Oral Maxillofac Surg. 1990;48:1145–1151.
6.Brooker AF, Bowerman JW, Robinson RA. Ectopic ossification
388
following total hip replacement. J Bone Joint Surg (Am). 1973; 55:1629–1632.
7.Lindqvist C, Söderholm A-L, Hallikainen D, et al. Erosion and heterotopic bone formation after alloplastic TMJ reconstruction.
J Oral Maxillofac Surg. 1992;50:942–949.
8.Poswillo D. Experimental reconstruction of the mandibular joint. Int J Oral Surg. 1974;3:400–411.
9.Ware WH, Brown SL. Growth centre transplantation to replace mandibular condyles. J Maxillofac Surg. 1981;9:50–58.
10.Kent JN, Misiek DJ, Akin RK, et al. Temporomandibular joint condylar prosthesis: a ten year report. J Oral Maxillofac Surg. 1983;41:245–254.
11.Kent JN, Block MS, Homsy CA, et al. Experience with a polymer glenoid fossa prosthesis for partial total temporomandibular joint reconstruction. J Oral Maxillofac Surg. 1986;44:520– 533.
12.Kent JN, Carlton DM, Zide MF. Rheumatoid disease and related arthroplasties. Oral Surg Oral Med Oral Pathol. 1986;61: 423–439.
13.Chassagne JF, Flot F, Stricker M, et al. A complete intermediate temporomandibular joint prosthesis. Evaluation after 6 years.
Rev Stomatol Chir Maxillofac. 1990;91:423–429.
14.House LR, Morgan DH, Hall WP. Clinical evaluation of TMJ arthroplasties with insertion of articular eminence prosthesis on ninety patients (an eight year study). Laryngoscope. 1977;87: 1182–1187.
15.Rooney TP, Haug RH, Toor AH, et al. Rapid condylar degeneration after glenoid fossa prothesis insertion: report of three cases. J Oral Surg Maxillofac Surg. 1988;46:240–246.
C.Lindqvist, A.-L. Söderholm, and D. Hallikainen
16.Lindqvist C, Jokinen J, Paukku P, et al. Adaptation of autogenous costochondral grafts used for temporomandibular joint reconstruction. A long term clinical and radiological follow-up.
J Oral Maxillofac Surg. 1988;46:465–470.
17.Ahrengart L, Lindgren U. Functional significance of heterotopic bone formation after total hip arthroplasty. J Arthroplasty. 1989; 4:125–131.
18.Pedersen NW, Kristensen SS, Schmidt SA, et al. Factors associated with heterotopic bone formation following total hip replacement. Arch Orthop Trauma Surg. 1989;108:92–95.
19.Warren SB. Heterotopic ossification after total hip replacement. Orthop Rev. 1990;19:603–611.
20.Raveh J, Stich F, Sutter F, et al. New concepts in the reconstruction of mandibular defects following tumor reconstruction.
J Oral Maxillofac Surg. 1983;41:3–16.
21.Raveh J, Sutter F, Hellem S. Surgical procedures for reconstruction of the lower jaw using the titanium-coated hollowscrew reconstruction plate system: bridging of defects. Otolaryngol Clin North Am. 1987;20:535–558.
22.Söderholm A-L, Lindqvist C, Sankila R, et al. Evaluation of various treatments for carcinoma of the mandibular region. Br J Oral Maxillofac Surg.1991;29:223–229.
23.Söderholm A-L, Lindqvist C, Hietanen J, et al. Bone scanning for evaluating mandibular bone extension of oral squamous cell carcinoma. J Oral Maxillofac Surg. 1990;48:252–257.
24.Lindqvist C, Söderholm A-L, Laine P, et al. Rigid reconstruction plates for immediate reconstruction following mandibular resection for malignant tumors. J Oral Maxillofac Surg. 1992; 50:1158–1163.
