
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1. Introduction
- •2. Evaluation of the Craniomaxillofacial Deformity Patient
- •3. Craniofacial Deformities: Review of Etiologies, Distribution, and Their Classification
- •4. Etiology of Skeletal Malocclusion
- •5. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Traumatic Defects
- •6. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Review of Nasal Deformities
- •7. Review of Benign Tumors of the Maxillofacial Region and Considerations for Bone Invasion
- •8. Oral Malignancies: Etiology, Distribution, and Basic Treatment Considerations
- •9. Craniomaxillofacial Bone Infections: Etiologies, Distributions, and Associated Defects
- •11. Craniomaxillofacial Bone Healing, Biomechanics, and Rigid Internal Fixation
- •12. Metal for Craniomaxillofacial Internal Fixation Implants and Its Physiological Implications
- •13. Bioresorbable Materials for Bone Fixation: Review of Biological Concepts and Mechanical Aspects
- •14. Advanced Bone Healing Concepts in Craniomaxillofacial Reconstructive and Corrective Bone Surgery
- •15. The ITI Dental Implant System
- •16. Localized Ridge Augmentation Using Guided Bone Regeneration in Deficient Implant Sites
- •17. The ITI Dental Implant System in Maxillofacial Applications
- •18. Maxillary Sinus Grafting and Osseointegration Surgery
- •19. Computerized Tomography and Its Use for Craniomaxillofacial Dental Implantology
- •20B. Atlas of Cases
- •21A. Prosthodontic Considerations in Dental Implant Restoration
- •21B. Overdenture Case Reports
- •22. AO/ASIF Mandibular Hardware
- •23. Aesthetic Considerations in Reconstructive and Corrective Craniomaxillofacial Bone Surgery
- •24. Considerations for Reconstruction of the Head and Neck Oncologic Patient
- •25. Autogenous Bone Grafts in Maxillofacial Reconstruction
- •26. Current Practice and Future Trends in Craniomaxillofacial Reconstructive and Corrective Microvascular Bone Surgery
- •27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects
- •28. Indications and Technical Considerations of Different Fibula Grafts
- •29. Soft Tissue Flaps for Coverage of Craniomaxillofacial Osseous Continuity Defects with or Without Bone Graft and Rigid Fixation
- •30. Mandibular Condyle Reconstruction with Free Costochondral Grafting
- •31. Microsurgical Reconstruction of Large Defects of the Maxilla, Midface, and Cranial Base
- •32. Condylar Prosthesis for the Replacement of the Mandibular Condyle
- •33. Problems Related to Mandibular Condylar Prosthesis
- •34. Reconstruction of Defects of the Mandibular Angle
- •35. Mandibular Body Reconstruction
- •36. Marginal Mandibulectomy
- •37. Reconstruction of Extensive Anterior Defects of the Mandible
- •38. Radiation Therapy and Considerations for Internal Fixation Devices
- •39. Management of Posttraumatic Osteomyelitis of the Mandible
- •40. Bilateral Maxillary Defects: THORP Plate Reconstruction with Removable Prosthesis
- •41. AO/ASIF Craniofacial Fixation System Hardware
- •43. Orbital Reconstruction
- •44. Nasal Reconstruction Using Bone Grafts and Rigid Internal Fixation
- •46. Orthognathic Examination
- •47. Considerations in Planning for Bimaxillary Surgery and the Implications of Rigid Internal Fixation
- •48. Reconstruction of Cleft Lip and Palate Osseous Defects and Deformities
- •49. Maxillary Osteotomies and Considerations for Rigid Internal Fixation
- •50. Mandibular Osteotomies and Considerations for Rigid Internal Fixation
- •51. Genioplasty Techniques and Considerations for Rigid Internal Fixation
- •52. Long-Term Stability of Maxillary and Mandibular Osteotomies with Rigid Internal Fixation
- •53. Le Fort II and Le Fort III Osteotomies for Midface Reconstruction and Considerations for Internal Fixation
- •54. Craniofacial Deformities: Introduction and Principles of Management
- •55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton
- •56. Calvarial Bone Graft Harvesting Techniques: Considerations for Their Use with Rigid Fixation Techniques in the Craniomaxillofacial Region
- •57. Crouzon Syndrome: Basic Dysmorphology and Staging of Reconstruction
- •58. Hemifacial Microsomia
- •59. Orbital Hypertelorism: Surgical Management
- •60. Surgical Correction of the Apert Craniofacial Deformities
- •Index
55
The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton
Michael J. Yaremchuk
The plate and screw fixation of surgically created and repositioned osteotomies or reduced fracture segments has revolutionized the practice of adult craniomaxillofacial surgery. The superior results obtained with rigid fixation techniques has prompted its use in the pediatric population.1,2 Plate and screw stabilization of reshaped and repositioned bone units and bone grafts may have certain advantages over no stabilization or interfragmentary wire stabilization. Posnick has listed the potential advantages of miniaturized plate and screw fixation techniques for pediatric craniofacial surgery.3 These advantages may include:
1.Improved three-dimensional shape control of refashioned osteotomized segments
2.Improved three-dimensional position control due to prevention of osteotomy or bone graft collapse after soft tissue closure
3.Facilitation of bone healing
4.The need for fewer drill holes when plates are used rather than interfragmentary wires
5.Decreased bone segment mobility, which may decrease resorption and infection rates and may also obviate the need for protective head gear
6.Elimination of the “sharpwire” pain that may accompany the use of interfragmentary wires under thin areas of the skin
There are certain negative aspects associated with the use of rigid fixation in the growing facial skeleton. These include metal-induced artifacts, artifacts on later computerized tomographic (CT) and magnetic resonance (MR) images, plate migration, and the potential for growth restriction. Recent data have shown that image artifact is negligible when the implant is made of titanium.4,5 The use of plates and screws should, therefore, have little impact on later diagnostic CT and MR images when the implants are manufactured from titanium. However, the tendency for plates and screws to alter their position relative to the bone–dura interface or to restrict the growth of the craniofacial skeleton has not been defined. No objective clinical data that address either of these latter issues
exist. This chapter presents experimental data examining the potential for plate and screw fixation to influence the growth of the growing craniofacial skeleton.
Animal Research
Lower Animals
Lower animal studies using cats6 and rabbits7,8 have shown a tendency for growth restriction when plates and screws were fixated onto the growing cranial vault. In the growing cat, Lin et al.6 noted a mild growth restriction when they studied the effect of frontal bone osteotomy and fixation with miniplates. In studies using rabbit models, miniplates were placed across the coronal suture7 and frontal bone8 without osteotomies being performed. A subsequent restriction of growth was also noted.
The relevance of the results of these studies is limited by the experimental methodology imposed by the scale, growth characteristics, and skeletal morphology of the animal models employed. The large scale of the plates relative to the experimental skull size makes clinical correlation unclear. Small skull size also necessitated the placement of a fixation plate across suture lines, a feature known likely to influence growth.9–11
Primates
To avoid some of the problems inherent in using lower animal models, a primate model is attractive. The rhesus monkey (Macaca mulatta) has an established history of use in craniomaxillofacial surgery research,12–14 an orbitozygomatic anatomy similar to humans,12 and growth and development of the facial skeleton resembling humans.15,16 For these reasons, a study that used infant rhesus monkeys was performed17 to examine the effect of plate and screw fixation on the growth of the craniofacial skeleton. This project was supported by the AO/ASIF (Swiss Association for the Study of Internal Fixation).
693
694 |
M.J. Yaremchuk |
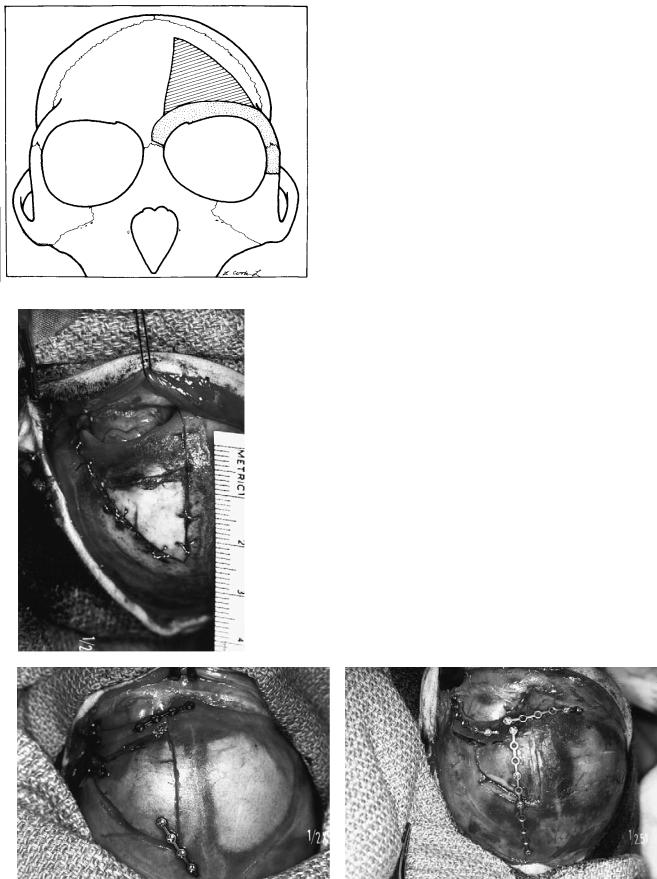
FIGURE 55.1 Design of supraorbital and frontal osteotomies. Dark gray zone indicates area of frontal osteotomy. Light gray indicates supraorbital osteotomy. (From Yaremchuk et al.,17 by permission of Plastic and Reconstructive Surgery)
a
FIGURE 55.2 Intraoperative photographs taken from above to demonstrate the three types of fixation employed. (a) Wire fixation. (b) Microplate fixation. (c) “Extensive” microplate fixation. (From Yaremchuk et al.,17 by permission of Plastic and Reconstructive Surgery)
b |
c |

55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton |
695 |
Research Using Macaca mulatta
determine the intrinsic craniofacial skeletal symmetry in this species.
Experimental Design
Twelve infant rhesus monkeys (Macaca mulatta) underwent unilateral supraorbital and frontal osteotomy through a bicoronal incision. The osteotomized segments were anatomically replaced and fixated using three different methods (Figure 55.1). The fixation methods included:
1.Interfragmentary wiring using 28-gauge stainless steel (n 4) [Figure 55.2(a)]
2.Rigid fixation using a microfixation system (n 4). Plates were positioned to avoid crossing suture lines. Two screws on either side of the osteotomy were applied to each plate [Figure 55.2(b)]
3.Extensive use of microplates (n 4). Longer plates, using multiple screws, were used. They purposely crossed the coronal and sagittal sutures so as to create a cagelike arrangement [Figure 55.2(c)]
The animals were allowed to mature to a mean age of 16.7 months (range, 11.1 to 27.3 months). Cranial growth in the rhesus monkey has been shown to be 95% completed by this time.18 During a second general anesthetic, the fixation hardware was removed, and standard craniometric measurements obtained (Figure 55.3). One week after the direct measurements were made, CT scans of the face and cranial vault were obtained from each animal. Three-dimensional images were then created using computer software so that both local and remote skull morphology could be documented. Five unoperated adult male rhesus skulls were measured and imaged to
Results
Craniometric measurements were normalized to account for variations in skull size in individual animals prior to statistically analyzing the data. All results were decided significant at p 0.05.
Differences among treatment groups were examined using the analysis of variance (ANOVA) statistical test. Of the 39 craniometric measurements made in each treatment group, only 4 were found to vary significantly with the type of fixation (Figure 55.4). In general, a greater restriction of growth was seen with increasing amounts of fixation hardware in the frontal region.
Using the paired t-test, right–left differences measured between the operated and unoperated sides with each treatment group were not large enough to be statistically significant. A trend toward smaller measurements on the operated side was observed.
Using an unpaired t-test, wire and plate fixation were compared. Of 39 measurements, 3 were significantly smaller in the plate fixation group, indicating a more marked growth restriction in the plate fixation group. When the standard and “extensive” plate fixation groups were compared, 2 of 39 measurements showed significant difference.
Several nonmetric changes were observed in the experimental animals. There was a loss of prominence of the supraorbital rim and changes in the region of the left frontotemporal craniometric point in all treatment groups. There was
FIGURE 55.3 Examples of craniometric measurements (shown unilaterally). (From Yaremchuk et al.,17 by permission of Plastic and Reconstructive Surgery)
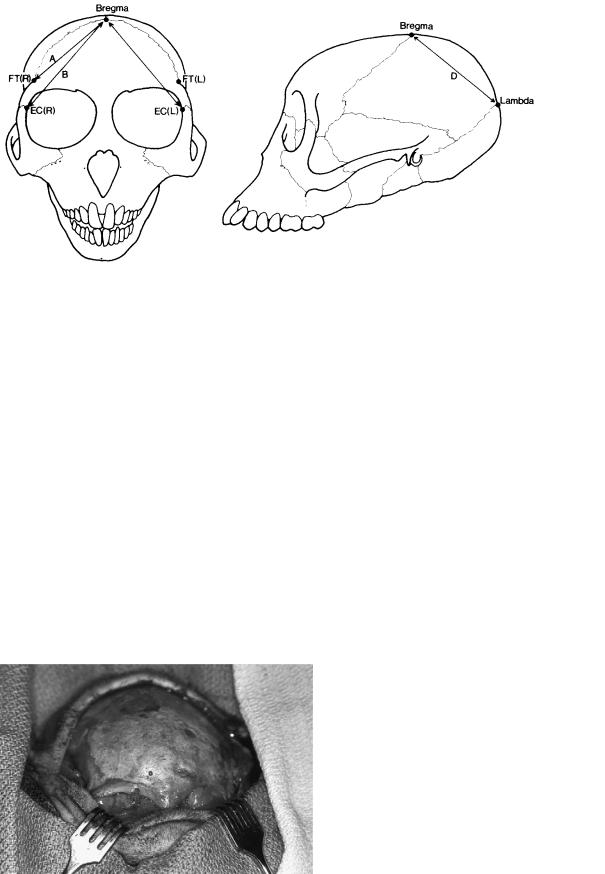
696
FIGURE 55.4 Craniometric measurements varying significantly with type of fixation (ANOVA, p 0.05). Line A: bregma-frontotempo- rale (R); line B: bregma-ectoconchion (R); line C: bregma-ectocon-
an apparent flattening of curvature with increasing fixation. These findings are presented in Figures 55.5–55.7.
Summary
This study showed that small, but measurable, visible changes occurred in a growing primate skull after osteotomy and fixation. A restriction of growth occurs in the area of operation even when suture lines are not crossed. In addition, distant, compensatory effects may occur. The magnitude of these changes appear to increase as increasing amounts of fixation hardware are used.
It is quite likely that the growth alterations seen in this study are related to osteotomy in addition to fixation effects. The
M.J. Yaremchuk
chion (L); line D: bregma-lambda. (From Yaremchuk et al.,17 by permission of Plastic and Reconstructive Surgery)
frontal and supraorbital bone flaps created in this study are, in fact, free-bone grafts. It is, therefore, not unexpected that local shape changes would occur due to resorption and remodeling effects.
Clinical Relevance
Several extrapolations can be made from this experimental data toward the indications, timing, and fixation techniques employed for clinical infant craniofacial surgery. For example, since even the least restrictive method of fixation employed (interfragmentary wire fixation) resulted in measurable and visible alterations in skull shape, the surgeon must
FIGURE 55.5 Intraoperative frontal view at time of craniometric measurements of a representative animal from wire fixation group. Note loss of prominence of supraorbital rim on left (operated) side.
55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton |
697 |
a |
b |
c |
d |
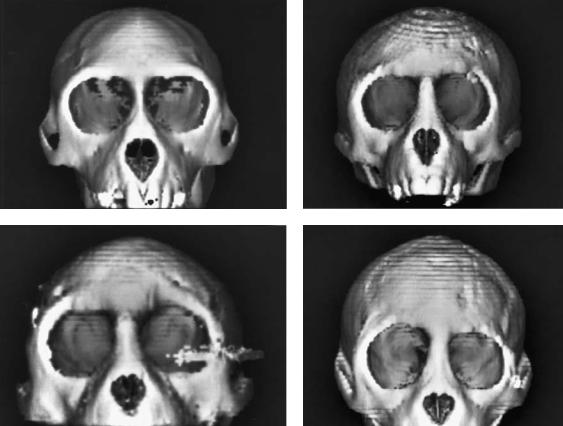
FIGURE 55.6 Representative frontal three-dimensional CT images of rhesus skulls. (a) Unoperated. (b) Wire fixation. (c) Microplate fixation (artifact is a result of a screw that was not removed before
imaging). (d) “Extensive” microplate fixation. (From Yaremchuk et al.,17 by permission of Plastic and Reconstructive Surgery)
698 |
M.J. Yaremchuk |
a |
b |
c |
d |
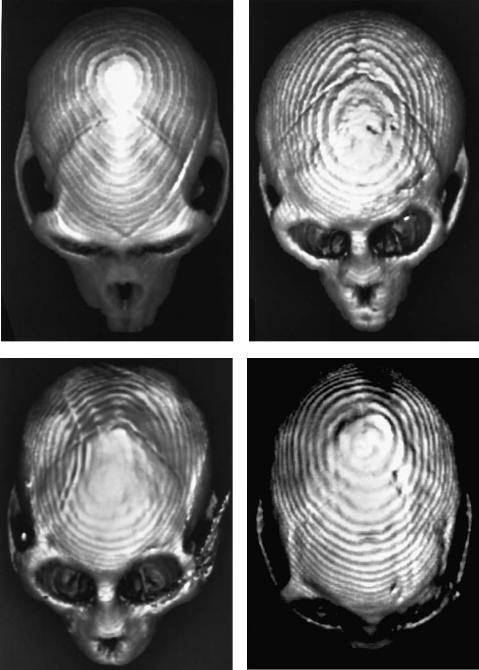
FIGURE 55.7 Representative overhead three-dimensional CT images of rhesus skulls. (a) Unoperated. (b) Wire fixation. (c) Microplate fixation (artifact is a result of screw that was not removed before
imaging). (d) “Extensive” microplate fixation. (From Yaremchuk et al.,17 by permission of Plastic and Reconstructive Surgery)
55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton |
699 |
be confident that the surgical correction and accompanying growth alterations must offer significant improvement, ultimately, over no treatment. Perhaps certain children with less severe deformities should be operated on later, when skull growth is more complete, so that the postoperative growth alterations would be concomitantly less significant. As the skull shape anomalies become more severe, the surgeon must balance the potential iatrogenic growth alterations accompanying surgery with the improved shape and position control and stability made available by the use of rigid fixation techniques. When initial deformities are more severe, one must realize that a statistically significant growth alteration accompanying surgery and stabilization may not be clinically significant relative to that initial deformity.
Other researchers have shown that the release of immobilized sutures9 and removal of fixation hardware19 in the growing infants skull allows “catch up” growth. Hence, removal of fixation hardware soon after healing at osteotomy sites may limit subsequent fixation-related alterations and should be considered in certain clinical infant surgery settings.
Recently, resorbable fixation systems have become available for clinical use. Early reports have been favorable.20 Their efficacy will be determined by the characteristics of the resorbable systems and the clinical situation. Important variables related to the fixation system include strength, time to resorption, and case of application. Timing of surgery related to remaining skull growth, bone gap distance and soft tissue deforming forces resulting from skeletal rearrangement will be relevant factors dictated by the clinical situation. Further laboratory and controlled clinical study will allow better understanding and appropriate use of resorbable fixation systems in the growing craniofacial skeleton.
References
1.Muehlbauer W, Anderl H, Ramaatschi P, et al. Radical treatment of craniofacial anomalies in infancy and the use of miniplates in craniofacial surgery. Clin Plast Surg. 1987;14:101– 111.
2.Posnick J. The role of plate and screw fixation in the treatment of craniofacial malformations. In: Yaremchuk MJ, Gruss JG, Manson PN, eds. Rigid Fixation of the Craniomaxillofacial Skeleton. Stoneham, MA: Butterworth-Heinemann; 1992:512– 526.
3.Posnick J. Discussion of “The effects of rigid fixation on craniofacial growth in rhesus monkeys.” By: Yaremchuk MJ, Fiala
TGS, Barker F, et al. Plast Reconstr Surg. 1994;93(1):11–12.
4.Fiala TGS, Novelline RA, Yaremchuk MJ. Comparison of CT imaging artifacts from craniomaxillofacial internal fixation devices. Plast Reconstr Surg. 1993;92:1227–1232.
5.Fiala TGS, Paige KT, Davis TL, et al. Comparison of artifact from craniomaxillofacial internal fixation devices: magnetic resonance imaging. Plast Reconstr Surg. 1994;93:725–731.
6.Lin KY, Bartlett SP, Yaremchuk MJ, et al. An experimental study on the effect of rigid fixation on the developing craniofacial skeleton. Plast Reconstr Surg. 1991;87:229–235.
7.Resnick JI, Kinney BM, Kawamoto JHK. The effect of rigid internal fixation on cranial growth. Ann Plast Surg. 1990;25:372– 374.
8.Wong L, Dufresne CR, Richtsmeier JT, et al. The effect of rigid fixation on growth of the neocranium. Plast Reconstr Surg. 1991;88:395–403.
9.Persing J, Babler W, Winn R, et al. Age as a critical factor in the success of surgical correction of craniosynostosis. J Neurosurg. 1981;54:601–606.
10.Persing JA, Babler WJ, Jane JA, et al. Experimental unilateral coronal synostosis in rabbits. Plast Reconstr Surg. 1986;77:369– 377.
11.Persson KM, Roy WA, Persing JA, et al. Craniofacial growth following experimental craniosynostosis and craniectomy in rabbits. J Neurosurg. 1979;50:187–197.
12.McCarthy JG, Coccaro PJ, Keller A. Craniofacial suture manipulation in the newborn rhesus monkey. In: Caronni EP, ed. Craniofacial Surgery. Boston: Little, Brown; 1985:3–11.
13.Gans BJ, Sarnat BG. Sutural facial growth of the Macaca rhesus monkey: a gross and serial roentgenographic study by means of metallic implants. Am J Anat. 1945;60:344–356.
14.Elgoyhen JC, Riolo ML, Graber LW, et al. Craniofacial growth in juvenile Macaca mulatta: a cephalometric study. Am J Phys Anthropol. 1978;36:369–376.
15.Duterloo HS, Enlow DH. A comparative study of cranial growth in Homo and Macaca. Am J Anat. 1970;127:357–368.
16.Cheek DB. The fetus. In: Cheek DB, ed. Fetal and Postnatal Cellular Growth. New York: John Wiley and Sons; 1975:3–22.
17.Yaremchuk MJ, Fiala TGS, Barker F, et al. The effects of rigid fixation on craniofacial growth of rhesus monkeys. Plast Reconstr Surg. 1994;93(1):1–10.
18.Bhatia AK. Craniometric growth in the rhesus monkey, Macaca mulatta. J Indian Anthrop Soc. 1978;13:81–95.
19.Wong L, Woodberry K, Richtsmeier JT, et al. Modifying the effects of rigid fixation on craniofacial growth. Presented at Plastic Surgery Research Council, Toronto, Ontario, April 1992.
20.Eppley BL, Sodov AM, Havlik RJ. Resorbable plate fixation in pediatric craniofacial surgery. Plast Reconstr Surg 1997;100: 1–7.
