
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1. Introduction
- •2. Evaluation of the Craniomaxillofacial Deformity Patient
- •3. Craniofacial Deformities: Review of Etiologies, Distribution, and Their Classification
- •4. Etiology of Skeletal Malocclusion
- •5. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Traumatic Defects
- •6. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Review of Nasal Deformities
- •7. Review of Benign Tumors of the Maxillofacial Region and Considerations for Bone Invasion
- •8. Oral Malignancies: Etiology, Distribution, and Basic Treatment Considerations
- •9. Craniomaxillofacial Bone Infections: Etiologies, Distributions, and Associated Defects
- •11. Craniomaxillofacial Bone Healing, Biomechanics, and Rigid Internal Fixation
- •12. Metal for Craniomaxillofacial Internal Fixation Implants and Its Physiological Implications
- •13. Bioresorbable Materials for Bone Fixation: Review of Biological Concepts and Mechanical Aspects
- •14. Advanced Bone Healing Concepts in Craniomaxillofacial Reconstructive and Corrective Bone Surgery
- •15. The ITI Dental Implant System
- •16. Localized Ridge Augmentation Using Guided Bone Regeneration in Deficient Implant Sites
- •17. The ITI Dental Implant System in Maxillofacial Applications
- •18. Maxillary Sinus Grafting and Osseointegration Surgery
- •19. Computerized Tomography and Its Use for Craniomaxillofacial Dental Implantology
- •20B. Atlas of Cases
- •21A. Prosthodontic Considerations in Dental Implant Restoration
- •21B. Overdenture Case Reports
- •22. AO/ASIF Mandibular Hardware
- •23. Aesthetic Considerations in Reconstructive and Corrective Craniomaxillofacial Bone Surgery
- •24. Considerations for Reconstruction of the Head and Neck Oncologic Patient
- •25. Autogenous Bone Grafts in Maxillofacial Reconstruction
- •26. Current Practice and Future Trends in Craniomaxillofacial Reconstructive and Corrective Microvascular Bone Surgery
- •27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects
- •28. Indications and Technical Considerations of Different Fibula Grafts
- •29. Soft Tissue Flaps for Coverage of Craniomaxillofacial Osseous Continuity Defects with or Without Bone Graft and Rigid Fixation
- •30. Mandibular Condyle Reconstruction with Free Costochondral Grafting
- •31. Microsurgical Reconstruction of Large Defects of the Maxilla, Midface, and Cranial Base
- •32. Condylar Prosthesis for the Replacement of the Mandibular Condyle
- •33. Problems Related to Mandibular Condylar Prosthesis
- •34. Reconstruction of Defects of the Mandibular Angle
- •35. Mandibular Body Reconstruction
- •36. Marginal Mandibulectomy
- •37. Reconstruction of Extensive Anterior Defects of the Mandible
- •38. Radiation Therapy and Considerations for Internal Fixation Devices
- •39. Management of Posttraumatic Osteomyelitis of the Mandible
- •40. Bilateral Maxillary Defects: THORP Plate Reconstruction with Removable Prosthesis
- •41. AO/ASIF Craniofacial Fixation System Hardware
- •43. Orbital Reconstruction
- •44. Nasal Reconstruction Using Bone Grafts and Rigid Internal Fixation
- •46. Orthognathic Examination
- •47. Considerations in Planning for Bimaxillary Surgery and the Implications of Rigid Internal Fixation
- •48. Reconstruction of Cleft Lip and Palate Osseous Defects and Deformities
- •49. Maxillary Osteotomies and Considerations for Rigid Internal Fixation
- •50. Mandibular Osteotomies and Considerations for Rigid Internal Fixation
- •51. Genioplasty Techniques and Considerations for Rigid Internal Fixation
- •52. Long-Term Stability of Maxillary and Mandibular Osteotomies with Rigid Internal Fixation
- •53. Le Fort II and Le Fort III Osteotomies for Midface Reconstruction and Considerations for Internal Fixation
- •54. Craniofacial Deformities: Introduction and Principles of Management
- •55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton
- •56. Calvarial Bone Graft Harvesting Techniques: Considerations for Their Use with Rigid Fixation Techniques in the Craniomaxillofacial Region
- •57. Crouzon Syndrome: Basic Dysmorphology and Staging of Reconstruction
- •58. Hemifacial Microsomia
- •59. Orbital Hypertelorism: Surgical Management
- •60. Surgical Correction of the Apert Craniofacial Deformities
- •Index
27
Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects
Peter Stoll, Joachim Prein, Wolfgang Bähr, and Rüdiger Wächter
Treatment of malignant tumors of the oral cavity frequently requires resection of bone that is infiltrated by the tumor. Particularly, if sections of the mandible are resected, this causes problems as far as form and function are concerned. Serious and life-threatening sequelae can occur, especially following resection of the anterior part of the mandible.1 The goal of mandibular reconstruction, however, is not only restitution of continuity and form but the reestablishment of masticatory function. The repair of soft tissue defects is highly dependent on the underlying supporting structures.
A decisive step in the improvement of quality of life in patients suffering from loss or partial loss of the mandible due to malignant tumors was the development of reconstruction plates to bridge the bony defects as shown in our patient (Figure 27.1). They fulfill special biomechanical and anatomic re- quirements.2–6
Temporary or permanent reconstruction of the mandible after continuity resection by using alloplastic materials has to take the following conditions into consideration:
1.Stability under function
2.Fixation of the remaining bone stumps in the anatomically correct position
3.Preservation of the possibility of primary or secondary bone grafting
4.Preservation of the possibility of adjuvant radiotherapy
Recent investigations have confirmed the clinical experience that despite the use of those metallic “foreign bodies” an adjuvant, fractionated radiotherapy is feasible (see Chapter 34).7–11 Bridging osteosynthesis by using reconstruction plates, however, represents only one step in the patient’s rehabilitation after continuity resection of the mandible. The low perioperative morbidity rate is overshadowed by a high long-term morbidity rate.11–16 In addition, if no bony reconstruction is performed the result may be poor, especially so far as func-
tion is concerned.
Pressure of the plate against the bone may interfere with the blood circulation within the bony cortex and cause demineralization (Figure 27.2). Experimental studies with over-
sized plates used for the fixation of mandibular fractures in sheep have shown this phenomenon.17 After injecting ink into the sheep’s carotid arteries at the time of sacrifice of the animal, it was clearly visible that the area underneath the plate was less well supplied (Figures 27.3 and 27.4). This finding should not be called stress protection.18,19 The consequences are loss of contact between plate and bone, eventually leading to instability of the entire system. When the plate has lost its contact with the bone surface, it exerts uncontrolled forces upon the screws during masticatory function. Primarily well-fixed screws become overloaded, and the result is loosening of the screws with further bone loss in the screw holes (Figure 27.5).
Plate fractures (Figure 27.6) as well as hardware extrusion (Figure 27.7) may also occur following bridging osteosynthesis, even if the soft tissue conditions are adequate.11
Reconstruction of the bony continuity with alloplastic material alone can only be a temporary measure for the majority of the cases. Although 70% of our patients aged 60 or more years do not want further and/or extensive surgery after bridging osteosynthesis, the surgeon has to insist and do a bony reconstruction by using free or microanastomosed vascularized bone graft in a second procedure.
Keeping this in mind, one has to consider whether bone grafting after continuity resection either by using free or microvascular grafts should be performed primarily to make a second operation unnecessary.
The choice of the graft depends on these points:
1.The size and location of the bony defect
2.The type and size of the soft tissue defect (“composite defect”)
3.The question of preoperative or postoperative radiation therapy, or both
4.The type of tumor and prognosis for the patient
5.The condition of the recipient area
6.The timing of the reconstruction
7.The donor site morbidity
8.The patient’s compliance20
9.The question of cost-effectiveness
317
318 |
|
|
|
P. Stoll et al. |
a |
|
|
|
b |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
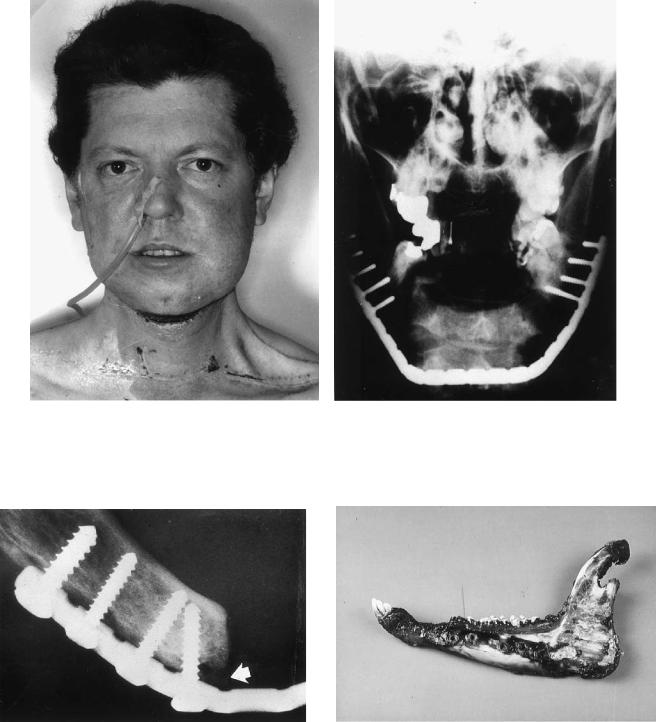
FIGURE 27.1 (a,b) A 34-year-old patient 12 days after resection of the anterior segment of the mandible and immediate alloplastic reconstruction of the chin area using an AO reconstruction plate [three
dimensionally bendable reconstruction plate (3-DBRP)]. Percutaneous radiation therapy has already started.
FIGURE 27.2 Resorption underneath a conventional AO reconstruction plate (3-DBRP) due to pressure against the bone surface (arrow).
FIGURE 27.3 Sheep mandible, left side. Red area indicates zone of disturbance of circulation. The reason was pressure caused by an oversized plate.
27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects |
319 |
FIGURE 27.4 Cut section through a sheep mandible after fracture fixation with an oversized plate. Zone of demineralization on the left side where the plate was pressed against the cortex.
Full rehabilitation, however, is achieved only after the reestablishment of masticatory function with osseointegrated dental implants (Figure 27.8) and prosthetic suprastructures. Therefore, the bone grafts should be suitable for this procedure.21 In recent years, microvascular reconstruction of the mandible has reached enormous popularity.22 Not only the
FIGURE 27.6 Clinical site of a plate fracture in a case of alloplastic repair (arrows).
number but the success rate of those reconstructions has increased dramatically. In this context, however, it has to be stressed that the free nonvascularized iliac bone graft still has its importance as a “workhorse” in the majority of the cases.
The most common donor sites for microvascular bone grafts are iliac crest, scapula, fibula, and radius.23 We now use mainly grafts taken from the fibula or the scapula. As far as the quality of the bone, the amount of soft tissues, and the length of the vascular pedicle are concerned, each flap has specific characteristics.
Problems
The pros and cons of different bone grafts and their indications have been widely discussed (see Chapter 25),22,24–32 but little attention has been given to the various fixation techniques available.32–36
|
FIGURE 27.7 Lateral extrusion of a reconstruction plate 12 months |
FIGURE 27.5 Loosening of screws and osteolysis (arrows). |
after surgery. |
320
a
b
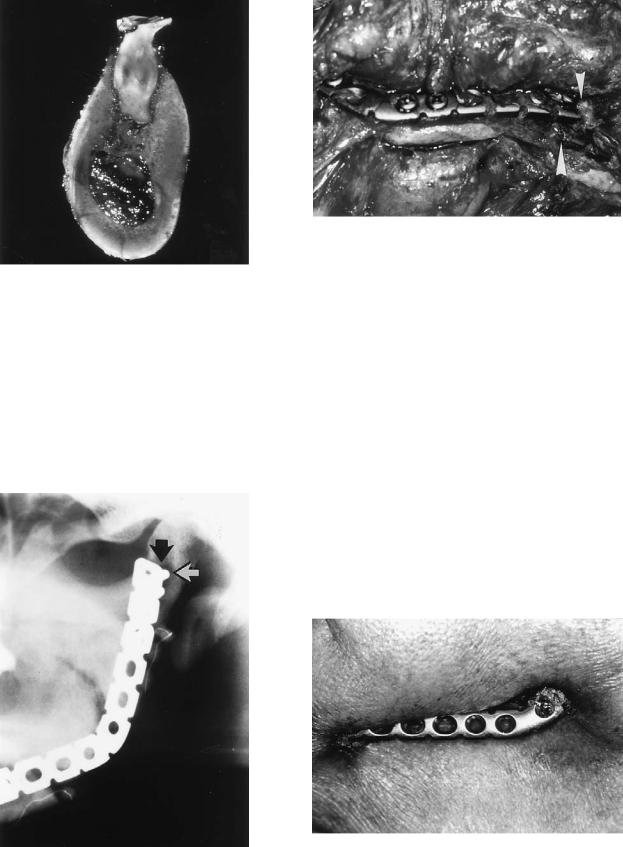
FIGURE 27.8 (a,b) Clinical and radiographic situation after insertion of dental implants (Bonefit®, ITI Strauman, Waldenberg, Switzerland) in the case of a patient with a squamous cell carcinoma as well in the original mandibular bone as in the fibula bone graft. The intraoral soft tissue defect was covered by a skin paddle.
For understanding the possibilities of graft fixation, the knowledge of their anatomy and pathophysiology is mandatory.
Fresh autogenous avascular grafts contain all the components of living tissue. A certain percentage of osteoblasts is initially nourished by diffusion until vascularization is completed. In the first days after grafting these osteoblasts proliferate and start building up a woven bone. Primary osteogenesis is achieved by the surviving bone cells and not by the surrounding soft tissue (osteoblast theory). The breakdown of the graft’s bone matrix by osteoclasts runs parallel to the composition of new woven bone. The leftover mucopolysaccharides induce undifferentiated mesenchymal cells of the ingrowing surrounding tissue to become osteoblasts (induction theory).
Therefore, the basic prerequisites of a secure integration of a free avascular bone graft are these:
1.Good vascularization of the surrounding soft tissue
2.Mechanical stability for the transplant
3.Close contact between surface of the bone transplant and the surrounding soft tissue4
Avascular bone grafts for the replacement of mandibular bony substance show a high failure rate when they are inserted in
P. Stoll et al.
an unstable surrounding environment. Creeping substitution through neovascularization is not possible if the bone graft is not adequately stabilized.
It takes 8 to 12 weeks for the bony transformation at the contact areas to occur. After primary integration of the transplant it is supposed that further remodeling depends on the functional load. One has to take into account the expected loss of bone volume of approximately 25% of the original free graft, for which the surgeon needs to overcompensate.37 Cancellous bone has a higher osteogenic potency than compact bone, but as a free iliac crest graft it does not withstand the mechanical stress when bridging mandibular defects.
In contrast to free avascular grafts, there is no progressive transformation in microanastomosed grafts, and little bone resorption may occur.24,34 The bone repair at the contact area between vascularized graft and mandible resembles the wellknown phenomenon of fracture healing, where even primary bone healing can take place. Under the conditions of adequate stability, screws for the fixation of metal plates are osseointegrated totally and are not likely to come loose due to remodeling processes as in avascular grafts.
In this context it has to be stressed that the grafts have to be inserted atraumatically. Compression osteosynthesis between graft and bone remnant is not an issue, but adequate stability has to be achieved to avoid movement between the microvascular graft and the bone stump.
Vascularized bone grafts (e.g., iliac crest or fibula) do survive under unstable conditions as long as their vascular pedicle is intact, but malunion, nonunion, or even displacement of the bone graft can greatly limit a patient’s masticatory rehabilitation and overall postoperative outcome.
While the use of miniplates or microplates is propagated to prevent restriction of blood supply of vascularized grafts,34,38,39 on the other hand, stable fixation of the grafts without the possibility of micromovement is empha-
sized.15,32,33,35,40,41
In our view miniplates or microplates are too weak to stabilize microvascularized bone grafts adequately. Although their survival is definitely dependent on the vascular supply and not on the amount of stability as in free grafts, we have seen dislocations of grafts because of insufficient stabilization with miniplates.42
In general, it can be stated that vital bone grafts transplanted with microvascular techniques can be fixed with either a reconstruction plate or several universal fracture plates or sometimes with miniplates. In contrast to this, it must be said that free avascular grafts must always be fixed with load-bearing reconstruction plates.
This fixation technique is also most successful in microvascular defect reconstruction.43 Particularly in cases with secondary microvascular bone grafting, when a reconstruction plate is already in place, the plate can be used as a safe pattern for adaptation of the graft in the desired shape.
Boyd and Mulholland36 revised different fixation techniques in vascularized bone grafts. They found a 75% failure rate by using several 4- to 6-hole dynamic compression plates
27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects |
321 |
for fixation of iliac crest grafts, whereas the success rate was |
a |
100% when using reconstruction plates for bridging os- |
|
teosynthesis. This is logical because dynamic compression |
|
plates may exert too much compression at the wrong place, |
|
e.g., within the graft. |
|
Methods
In our unit, bony defects up to a length of 6 cm are usually reconstructed by using corticocancellous grafts taken from the iliac crest. Cases exhibiting a compromised recipient site due to previously performed radiation therapy or for whom further radiation therapy is planned are excluded from transplantation of avascular grafts, although the bony defect may be relatively small. Nevertheless, the use of free corticocancellous hip bone is still a valuable help in the majority of minor defects, as stated before.28,44 On the other hand, larger defects, particularly after irradiation, require microvascular repair.45
In the case of defects that require only the replacement of bone without soft tissues, we prefer the fibula34 as the graft of choice. Its architecture is, unlike iliac crest or scapula, similar to that of the mandible. Defects up to a length of 25 cm can be repaired. The graft can be easily adjusted to the shape of the mandible by using the intersection technique. It is associated with very low postoperative donor site morbidity, and last but not least, it allows insertion of dental implants due to its mandibular-like width.22,46 The main disadvan- tage—the limited height—can be overcome by using the “double-barrel” technique.47
Since the skin paddle of the fibula is relatively thin and sometimes exhibits a limited reliability,48 we use the fibula osteocutaneous flap or the supramalleolar composite graft49 only in cases with small soft-tissue defects.
In cases with large soft tissue defects, scapula bone and parascapular flaps are more appropriate. The scapula, however, seems to be unfavorable as far as length and diameter are concerned. Frequently, especially in females,31 secondary insertion of dental implants is not possible. In addition, time in surgery is extended because a simultaneous two-team approach is not possible. On the other hand, like fibula grafts, scapula grafts present a low postoperative donor site morbidity rate.50,51
It is important to understand the appropriate possibilities for the fixation of different grafts. In our experience, adequate internal fixation by using reconstruction plates combined with autogenous bone grafts seems to be most satisfactory. Corticocancellous iliac crest bone as well as microanastomosed fibula or scapula grafts can easily be adjusted to the given curvature of the plate.
Bridging osteosynthesis guarantees stability during the healing phase (Figure 27.9). Generally, nonvascularized corticocancellous iliac crest grafts should not be fixed with screws to the plate. During the remodeling phase, the screws may come loose and act as a foreign body because the bone
b
FIGURE 27.9 (a,b) Clinical and radiographic situation after immediate bone repair using a free nonvascularized iliac crest bone graft in a case of an ameloblastoma.
is not vital and is subsequently replaced by newly formed woven and lamellar bone. Infection and loss of bone can occur.
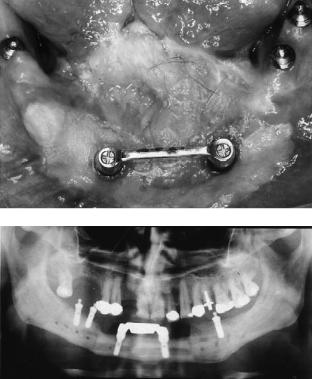
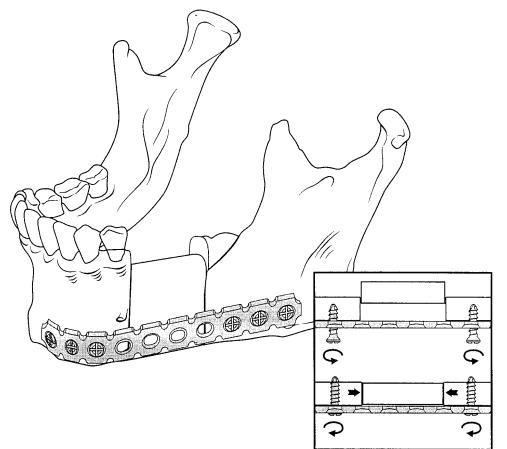
In those cases fixation of the bone grafts to the remnants is achieved, for example, by using the AO-3-Dimensionally Bendable Reconstruction Plate system (3-DBRP), which can provide compression between the graft and the bone stumps (Figure 27.10).
Since 1984, we have used the AO-Titanium Hollow Screw Reconstruction Plate system (THORP). With this system one cannot exert compression, but because its anchoring device between the screwhead and plate acts as an “internal fixator,” it is possible to avoid bone resorption underneath the plate and secondary instability of the entire osteosynthesis.5,52–55 By using this system, screw fixation of an avascular graft may be possible since the screwhead does not move inside the screw hole. Nevertheless, we intend not to interfere with the bone’s remodeling and prefer adaptation of the graft to the plate by using resorbable sutures.
Statistical evaluation of our patient sample, however, has shown that since we have abandoned fixation of avascular grafts to the plate by using screws, the infection rate could be dramatically reduced (screw fixation, N 97 32%; without screw fixation, N 82 4%).
Today we generally do not use nonvascularized bone grafts in an irradiated bed or when postoperative external radiation
322 |
P. Stoll et al. |
FIGURE 27.10 Schematic drawing of the fixation of a free bone graft for the replacement of a defect in the lateral mandible. The inset shows loose screws (above) at the time of placement of the graft. By tightening of the screws (below) the graft is fixed via compression.
therapy is planned. This may contribute to the better results. On the other hand, microanastomosed bone grafts can be fixed to reconstruction plates with metal screws. It should be emphasized though that those screws serve only to hold the graft in position between the rigidly fixed mandibular
segments.
Since microvascular grafts consist of living tissue and behave like an edentulous mandible, osseointegration of the screws can be expected. Compression of the bone grafts between the bone remnants is not necessary for fixation but can carefully be exerted. Impairment of the blood supply of the graft has to be avoided. Gaps between the bone graft and the remnant, if any, are filled with bone dust and/or bone slices or cancellous bone from the iliac crest.
In our hands blood supply of microvascular grafts is not impaired when using functionally stable AO-reconstruction- plates (3-DBRP or THORP). On the contrary, this procedure seems to protect the anastomosis and promote uneventful healing. Loosening of plate and screws, pseudoarthrosis, and infection, which can occur from using functionally unstable fixation devices like miniplates, are unusual in our sample. Sometimes, however, the use of reconstruction plates is not possible, especially in cases with composite grafts. Here, sev-
eral smaller plates like universal fracture plates may avoid impairment of the blood supply of the skin paddle.
Conclusion
Various types of bone graft fixation are used in oral and maxillofacial surgery. It is important to understand that adequate stability favors the incorporation of the transplant. Generally, alloplastic restitution of the mandibular continuity is performed by using a reconstruction plate.
This plate preserves the distance between the bone stumps. Bone grafts can be adjusted and fixed to the plate either primarily or secondarily. The plate acts like a template for the shaping of the bone graft because it follows the original mandibular arch.
Two main types of grafts or flaps are available for autogenous reconstruction of mandibular defects. In general, either an avascular free-bone graft or a bone graft that is reanastomosed with microvascular technique and therefore vital is used.
While free avascular grafts must always be stabilized with the help of complete bridging osteosynthesis, there may be
27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects |
323 |
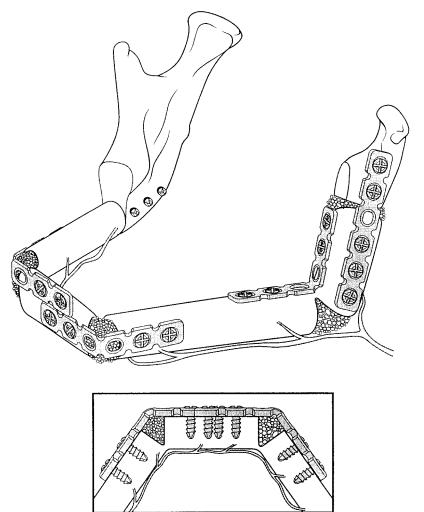
an option for fixation of microvascular grafts by using smaller plates. Particularly in cases with large soft tissue defects where the repair has to be performed by using composite grafts, a reconstruction plate may hinder the vascular supply of the soft tissue compartment of the graft. In the majority of the cases, however, the application of a reconstruction plate is a comfortable measure to insert a bone graft. Nevertheless, a microvascular graft with several intersections, which are necessary to achieve a natural curvature, may be further stabilized by using smaller plates, preferably universal fracture plates (Figures 27.11 and 27.12).
In the case of secondary bone repair, a primarily applied reconstruction plate preserves the distance between the bone stumps during the postoperative follow-up period and facilitates the placement of a graft.
The AO-THORP system offers a long-term reliable fixation that will not fail due to micromovement or bone remodeling. The locking-screw plate design makes it possible to achieve a stable reconstruction by using only three or four
screws per bone stump. The new 2.4 mm Unilock reconstruction plates with special locking screws have been designed to be similar in function to the AO-THORP system to prevent screw loosening after graft healing has occurred and may be used for nonvascular and vascularized grafts (see Chapter 41 for 2.4 Unilock module specifications). These newer plates are less thick and may be used in situations where the AO-THORP system and AO3-DBRP are considered for use, with caution concerning the size of the graft and defect.
Conventional reconstruction plate systems as the AO-3- DBRP, where the plate is pressed against the bony surface during tightening of the screws, may become loose with time due to bone resorption underneath the plate. Therefore, they are less suitable for long-term alloplastic repair alone. This kind of reconstruction device is used preferably in combination with primary bone repair (Figure 27.13). Then, bony restitution takes place before the plate loses its stability. In addition, a bone graft can be compressed between the stumps and fixed by using compression.
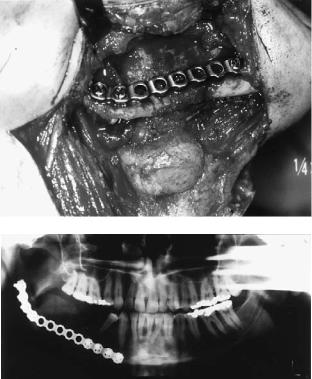
FIGURE 27.11 Schematic drawing of the reconstruction of the mandibular body, left angle and ramus with a fibula. Fixation was performed with several universal fracture plates. The bone gaps at the osteotomy site were filled with cancellous bone.
324
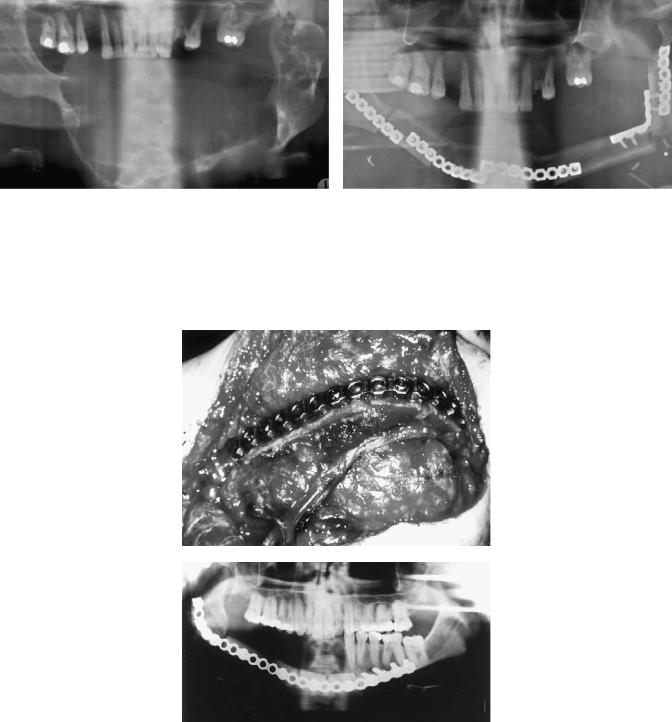
a
FIGURE 27.12 (a,b) Radiographic situation with an extensive ameloblastoma within the mandible preoperatively and postoperatively after resection and reconstruction of the defect with a mi-
a
b
P. Stoll et al.
b
crovascular fibula graft fixed with universal fracture plates as shown schematically in Figure 27.11.
FIGURE 27.13 (a,b) Clinical and radiographic situation of a vascularized fibula bone repair after extensive resection of an osteosarcoma.
27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects |
325 |
Generally it can be said that primary bone repair by using |
18. |
Predieri M, Gautier E, Sutter F, Tepic S, Perren SM. Vermei- |
|
free or vascularized bone grafts is easier to perform. Secondary |
|
dung der Porose unter Osteosyntheseplatten. Acta Med Austraca. |
|
repair after the formation of scars and soft tissue shrinkage has |
|
1990;17:49. |
|
taken place is more difficult. This is also due to the deficiency |
19. |
Schiller K, Wolf I, Kessler SB. Die Bedeutung der Knochen- |
|
of the soft tissue layer and compromised vascular supply (es- |
|
grösse für das Ausmass der plattenbedingten Zirkulationsschä- |
|
|
den. Acta Med Austrica. 1990;17:444–447. |
||
pecially after radiation), which may limit the desired treatment. |
|
||
20. |
Wächter R, Lauer G, Fabinger A, Stoll P. Zur Compliance von |
||
|
|
|
Tumorpatienten mit dentalen Implantaten. In: Jahrbuch der |
References |
|
Gesellschaft für Orale Implantologie. Berlin: Quintessenz; |
|
|
|
|
1994;299. |
1. |
Stoll P. Lebensqualität nach radikaler Tumorchirurgie im Mund- |
21. |
Wächter R, Stoll P, Bähr W, Lauer G. Osseointegration of ITI- |
|
Kiefer-Gesichtsbereich. Aktuel Inf Arzt. 1992;6:2–3. |
|
dental implants (Bonefit) in non-vascularized and vascularized |
2. |
Luhr HH. Ein Plattensystem zur Unterkieferrekonstruktion ein- |
|
mandibular bone grafts. Proceedings of the 1st World Congress |
|
schliesslich des Gelenkersatzes. Dtsch Zahnärztl Z. 1976;31: |
|
of Osseointegration, Venice, Sept. 29–Oct. 2, 1994. |
|
747. |
22. |
Urken ML. Composite free flaps in oromandibular reconstruc- |
3. |
Ewers R, Joos U. Temporäre Defektüberbrückung bei Un- |
|
tion. Review of the literature. Arch Otolaryngol Head Neck |
|
terkieferresektionen mit osteosynthese-methoden. Dtsch Zahn- |
|
Surg. 1991;117:724–732. |
|
ärztl Z. 1977;32:332–333. |
23. |
Frodel JL, Funk GF, Capper DJ, Fridrich KL, Blumer JR, Haller |
4. |
Spiessl B. Die Unterkieferresektionsplatte der AO. Ihre An- |
|
JR, et al. Osseointegrated implants: a comparative study of bone |
|
wendung bei Unterkieferdefekten in der Tumorchirurgie. Un- |
|
thickness in four vascularized bone flaps. Plast Reconstr Surg. |
|
fallheilkunde. 1978;81:389–405. |
|
1993;92:449–455. |
5. |
Raveh J, Stich H, Schawalder P, Sutter F, Straumann F. Kon- |
24. |
Riediger D. Restoration of masticatory function by microsurgi- |
|
servative und chirurgische Massnahmen zur Herstellung der |
|
cally revascularized iliac crest bone grafts using enosseous im- |
|
Kiefergelenksfunktion und neue Möglichkeitn und Methoden |
|
plants. Plast Reconstr Surg. 1988;81:861–877. |
|
zur Defektüberbrückung am Unterkiefer. Schweiz Monatsschr |
25. |
Kärcher H, Penkner K. Ergebnisse der freien und gefässgestiel- |
|
Zahnheilk. 1980;90:932–948. |
|
ten Knochenrekonstruktion nach Unterkieferkontinuitätsdefek- |
6. |
Schmoker R. Die funktionelle Unterkieferrekonstruktion. Berlin: |
|
ten. Dtsch Z Mund-Kiefer-Geischtschir. 1991;15:285–291. |
|
Springer; 1986. |
26. |
Kuriloff DB, Sullivan MJ. Mandibular reconstruction using vas- |
7. |
Stoll P, Wächter R, Hodapp N, Schilli W. Radiation and osteo- |
|
cularized bone grafts. Otolaryngol Clin North Am. 1991;24: |
|
synthesis? Dosimetry on an irradiation phantom. J Cranio- |
|
1391–1418. |
|
maxillofac Surg. 1990;18:361–366. |
27. |
Divaris M, Goudot P, Princ G, Lalo J, Vaillant JM. Recon- |
8. |
Klotch DW, Gump J, Kuhn L. Reconstruction of mandibular de- |
|
struction mandibulaire par lambeaux libres osseux microanas- |
|
fects in irradiated patients. Am J Surg. 1990;160:396. |
|
tomoses. Nos indications actuelles. Ann Chir Plast Esthetique. |
9. |
Gullane PJ. Primary mandibular reconstruction analysis of 64 |
|
1992;37:297–308. |
|
cases and evaluations of interface radiation dosimetry on bridg- |
28. |
Lindqvist C. Mandibular reconstruction with free bone grafts. |
|
ing plates. Laryngoscope. 1991;101:1–24. |
|
Curr Opin Dent. 1992;2:25–37, Review. |
10. |
Maurer J, Kirschner H, Halling F, Duhmke E. Klinische und |
29. |
Mayot D, Perrin C, Lindas P, Dron K. Reconstruction de la sym- |
|
strahlenphysikalische Untersuchungen über den Einfluss von |
|
physe mandibulaire par transferts osseux vascularises libres il- |
|
Unterkieferrekonstruktionsplatten (MRS) auf die Dosisver- |
|
iaques et scarpulaire. Ann Otolaryngol Chir Cervico-faciale. |
|
teilung von ultraharten Photonen. Strahlenther Onkol. 1993; |
|
1992;109:123. |
|
169:279–284. |
30. |
Ferri J, Piot B, Farah A, Gaillard A, Mercier J. Notre experi- |
11. |
Stoll P, Wächter R. Tumorbestrahlung und Überbückungsosteo- |
|
ence des lambeaux libres vascularises osseux dans le recon- |
|
synthese? Dtsch Z Mund-Kiefer-Gesichtschir. 1993;15:224–229. |
|
structions mandibulaires. Le lambeau brachial extreme, le lam- |
12. |
Komisar A, Warman S, Danziger E. A critical analysis of im- |
|
beau fibulaire, le lambeau parascapulaire. Rev Stomatol Chir |
|
mediate and delayed mandibular reconstruction using AO plates. |
|
Maxillofac. 1993;94:74. |
|
Arch Otolaryngol Head Neck Surg. 1989;115:830–833. |
31. |
Bekiscz O, Adant J, Denoel C, Lahaye T. Mandibular recon- |
13. |
Davidson J, Birt BD, Gruss J. AO plate mandibular reconstruc- |
|
struction. An anatomical study of bone thickness in three donor |
|
tion: a complication critique. J Otolaryngol. 1991;20:104–107. |
|
sites. 12th Congress of the European Association for Cranio- |
14. |
Freitag V, Hell B, Fischer H. Experience with AO reconstruc- |
|
Maxillofacial Surgery, The Hague, 5–10 September 1994. |
|
tion plates after partial mandibular resection involving its con- |
32. |
Komisar A, Shapiro BM, Danziger E, Szporn M, Cobelli N. The |
|
tinuity. J Craniomaxillofac Surg. 1991;19:191–198. |
|
use of osteosynthesis in immediate and delayed mandibular re- |
15. |
Schusterman MA, Reece GP, Kroll SS, Weldon MA. Use of the |
|
construction. Laryngoscope. 1985;95:1363–1366. |
|
AO plate for immediate mandibular reconstruction in cancer pa- |
33. |
Gullane PJ, Holmes H. Mandibular reconstruction. New con- |
|
tients. Plast Reconstr Surg. 1991;88:588. |
|
cepts. Arch Otolaryngol Head Neck Surg. 1986;112:714–719. |
16. |
Kim MR, Donoff RB. Critical analysis of mandibular recon- |
34. |
Hidalgo DA. Titanium miniplate fixation in free-flap-mandible |
|
struction using AO-reconstruction plates. J Oral Maxillofac |
|
reconstruction. Ann Plast Surg. 1989;23:498–507. |
|
Surg. 1992;50:1152–1157. |
35. |
Buchbinder D, Urken ML, Vickery C, Weinberg H, Biller HF. |
17. |
Rahn B, Prein J. Unpublished experiment. AO Research Insti- |
|
Bone contouring and fixation in functional, primary microvascu- |
|
tut, Clavadelerstrasse, CH-7270 Davos/Switzerland, 1973. |
|
lar mandibular reconstruction. Head Neck. 1991;13:191–199. |
326
36.Boyd JB, Mulholland RS. Fixation of the vascularized bone graft in mandibular reconstruction. Plast Reconstr Surg. 1993;91:274.
37.Hausamen JE, Neukam FW. Transplantation von Knochen. Eur Arch Otorhinolaryngol I Suppl. 1992:163–177.
38.Hidalgo DA. Aesthetic improvements in free-flap-mandible reconstruction. Plast Reconstr Surg. 1991;88:574–585.
39.Barnard NA, Vaughan ED. Osteosynthetic titanium miniplate fixation of composite radial forearm flaps in mandibular reconstruction. J Craniomaxillofac Surg. 1991;19:243–248.
40.Wenig BL, Keller AJ. Microvascular free-tissue transfer with rigid internal fixation for reconstruction of the mandible following tumor resection. Otolaryngol Clin North Am. 1987;20:621–633.
41.Wenig BL, Keller AJ, Shikowitz MJ, Stern JR, Casino AJ, Pollack JM, et al. Anatomic reconstruction and functional rehabilitation of oromandibular defects with rigid internal fixation. Laryngoscope. 1988;98:154–159.
42.Prein J, Ettlin D, Hammer B. Vorund Nachteile unterschiedlicher Fixationstechniken für mikrovaskuläre Transplantate bei der Unterkieferrekonstruktion. IV. International Symposium on Microsurgery in Reconstructive and Plastic Surgery, Microsurgery ‘95. Jena 1995; Publication 1998.
43.Stoll P, Bähr W, Wächter R. Die Wertigkeit des Fibulatransplantates bei der Rekonstruktion von Unterkieferdefekten. Proceedings of the IV International Symposium on Microsurgery in Reconstructive and Plastic Surgery. Jena 1995; Publication 1998.
44.Tidstrom KD, Keller EE. Reconstruction of mandibular discontinuity with autogenous iliac bone graft: report of 34 consecutive patients. J Oral Maxillofac Surg. 1990;48:336–346.
45.Fossion E, Boeckx W, Jacobs D, Ioannides C, Vrielinck L. La reconstruction microchirurgicale de la mandibule irradiée par le lambeau circonflexe iliaque profond. Ann Chir Plast Esthetique. 1992;37:246–251.
P. Stoll et al.
46.Huryn JM, Zlotolow JM, Piro JD, Lenchewski E. Osseointegrated implants in microvascular fibula free flap reconstructed mandibles. J Prosthet Dent. 1993;70:443–446.
47.Bähr W, Stoll P, Wächter R. “Fibuladoppeltransplantat” als gefässgestielter Unterkieferersatz. Dtsch Z Mund-Kiefer- Gesichtschir. 1994;18:219–223.
48.Schusterman MA, Reece GP, Miller MJ, Harris S. The osteocutaneous free fibula flap: is the skin paddle reliable? Plast Reconstr Surg. 1992;90:787–793.
49.Wolff KD, Stellmach R. The osteoseptocutaneous or purely septocutaneous peroneal flap with a supramalleolar skin paddle. Int J Oral Maxillofac Surg. 1995;24:38–43.
50.Sullivan M, Baker S, Crompton R, Smith-Wheelock M. Free scapular osteocutaneous flap for mandibular reconstruction.
Arch Otolaryngol Head Neck Surg. 1989;115:1334–1340.
51.Thomassin JM, Bardot J, Inedjian JM. Le lambeau osteocutane scapulaire dans reconstruction mandibulaire. Rev Stomatol Chir Maxillofac. 1990;91, Suppl. 1:15.
52.Raveh J, Stich H, Sutter F, Greiner R. Use of titanium-coated hollow screw and reconstruction system in bridging of lower jaw defects. J Oral Maxillofac Surg. 1984;42:281–294.
53.Sutter F, Raveh J. Titanium-coated hollow screw and reconstruction plate system for bridging of lower jaw defects: biomechanical aspects. Int J Oral Maxillofac Surg. 1988;17:267– 274.
54.Stoll P, Wächter R, Bähr W. Bridging lower jaw defects with
AO plates: comparison of THORP and 3-DBRP systems.
J Craniomaxillofac Surg. 1992;20:87–90.
55.Wächter R, Stoll P. Komplikationen nach primärer Unterkieferrekonstruktion mit THORP-Platten. In: Ästhetische und plas- tisch-rekonstruktive Gesichtschirurgie. Neumann H-J, Hrsg. Reinbek: Einhorn-Presse-Verlag; 1993;259.
