
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1. Introduction
- •2. Evaluation of the Craniomaxillofacial Deformity Patient
- •3. Craniofacial Deformities: Review of Etiologies, Distribution, and Their Classification
- •4. Etiology of Skeletal Malocclusion
- •5. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Traumatic Defects
- •6. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Review of Nasal Deformities
- •7. Review of Benign Tumors of the Maxillofacial Region and Considerations for Bone Invasion
- •8. Oral Malignancies: Etiology, Distribution, and Basic Treatment Considerations
- •9. Craniomaxillofacial Bone Infections: Etiologies, Distributions, and Associated Defects
- •11. Craniomaxillofacial Bone Healing, Biomechanics, and Rigid Internal Fixation
- •12. Metal for Craniomaxillofacial Internal Fixation Implants and Its Physiological Implications
- •13. Bioresorbable Materials for Bone Fixation: Review of Biological Concepts and Mechanical Aspects
- •14. Advanced Bone Healing Concepts in Craniomaxillofacial Reconstructive and Corrective Bone Surgery
- •15. The ITI Dental Implant System
- •16. Localized Ridge Augmentation Using Guided Bone Regeneration in Deficient Implant Sites
- •17. The ITI Dental Implant System in Maxillofacial Applications
- •18. Maxillary Sinus Grafting and Osseointegration Surgery
- •19. Computerized Tomography and Its Use for Craniomaxillofacial Dental Implantology
- •20B. Atlas of Cases
- •21A. Prosthodontic Considerations in Dental Implant Restoration
- •21B. Overdenture Case Reports
- •22. AO/ASIF Mandibular Hardware
- •23. Aesthetic Considerations in Reconstructive and Corrective Craniomaxillofacial Bone Surgery
- •24. Considerations for Reconstruction of the Head and Neck Oncologic Patient
- •25. Autogenous Bone Grafts in Maxillofacial Reconstruction
- •26. Current Practice and Future Trends in Craniomaxillofacial Reconstructive and Corrective Microvascular Bone Surgery
- •27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects
- •28. Indications and Technical Considerations of Different Fibula Grafts
- •29. Soft Tissue Flaps for Coverage of Craniomaxillofacial Osseous Continuity Defects with or Without Bone Graft and Rigid Fixation
- •30. Mandibular Condyle Reconstruction with Free Costochondral Grafting
- •31. Microsurgical Reconstruction of Large Defects of the Maxilla, Midface, and Cranial Base
- •32. Condylar Prosthesis for the Replacement of the Mandibular Condyle
- •33. Problems Related to Mandibular Condylar Prosthesis
- •34. Reconstruction of Defects of the Mandibular Angle
- •35. Mandibular Body Reconstruction
- •36. Marginal Mandibulectomy
- •37. Reconstruction of Extensive Anterior Defects of the Mandible
- •38. Radiation Therapy and Considerations for Internal Fixation Devices
- •39. Management of Posttraumatic Osteomyelitis of the Mandible
- •40. Bilateral Maxillary Defects: THORP Plate Reconstruction with Removable Prosthesis
- •41. AO/ASIF Craniofacial Fixation System Hardware
- •43. Orbital Reconstruction
- •44. Nasal Reconstruction Using Bone Grafts and Rigid Internal Fixation
- •46. Orthognathic Examination
- •47. Considerations in Planning for Bimaxillary Surgery and the Implications of Rigid Internal Fixation
- •48. Reconstruction of Cleft Lip and Palate Osseous Defects and Deformities
- •49. Maxillary Osteotomies and Considerations for Rigid Internal Fixation
- •50. Mandibular Osteotomies and Considerations for Rigid Internal Fixation
- •51. Genioplasty Techniques and Considerations for Rigid Internal Fixation
- •52. Long-Term Stability of Maxillary and Mandibular Osteotomies with Rigid Internal Fixation
- •53. Le Fort II and Le Fort III Osteotomies for Midface Reconstruction and Considerations for Internal Fixation
- •54. Craniofacial Deformities: Introduction and Principles of Management
- •55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton
- •56. Calvarial Bone Graft Harvesting Techniques: Considerations for Their Use with Rigid Fixation Techniques in the Craniomaxillofacial Region
- •57. Crouzon Syndrome: Basic Dysmorphology and Staging of Reconstruction
- •58. Hemifacial Microsomia
- •59. Orbital Hypertelorism: Surgical Management
- •60. Surgical Correction of the Apert Craniofacial Deformities
- •Index
29
Soft Tissue Flaps for Coverage of Craniomaxillofacial Osseous Continuity Defects with or Without Bone Graft and Rigid Fixation
Barry L. Wenig
Mandibular continuity defects arising from trauma, infection, or tumor resection can often lead to serious and crippling disabilities. Loss of hard tissue (i.e., bone) will result in the inability to support the soft tissues of the oral cavity and oropharynx. This, in turn, will translate into significant deficiencies in the functions of swallowing, chewing, and talking as well as creating a disfiguring facial appearance.
The multitude of reconstructive options that have appeared in the literature attest to the difficulties that are associated with reconstruction of these continuity defects. Regardless of the technique that is chosen, the premise behind the reconstructive effort is based on the reestablishment of continuity while maintaining a normal maxillary-mandibular relationship. Structural support obtained in this manner will result in satisfactory return of form and function.
Mandibular resection following tumor ablation clearly results in the most challenging of all continuity defects. The significant soft tissue deficit and oral contamination associated with this type of treatment as well as advancing age, malnutrition, and prior radiation therapy that often accompany this patient population makes reconstruction of these individuals extremely complicated.
Decision Making in Reconstruction
The major issue confronting the surgeon faced with a mandibular continuity defect is the timing of the reconstruction. Is it in the best interest of the patient to perform the procedure at the time of tumor resection or would a secondary reconstruction be more advantageous?
Primary reconstruction at the time of ablative surgery has several distinct advantages. The most obvious advantage is that it allows for the restoration of mandibular continuity which, in turn, enables the patient to obtain immediate functional and cosmetic results. By avoiding multiple surgical procedures, the need to dissect in a previously operated or radiated field is eliminated. The patient is not faced with a radically altered appearance or disfigurement, which could
have a potentially devastating psychological effect. The ability to tolerate an oral diet or to verbally communicate limits the self-perception of the handicap that is often associated with individuals undergoing mandibular resection.
Secondary or delayed reconstruction offers the advantage of time. Allowing a certain interval to pass affords the surgeon and patient the knowledge that local and/or regional tumor control has been obtained. This option is certainly not unreasonable in an individual with very advanced disease, who may be in poor medical condition. On the other hand, secondary reconstruction is carried out in a scarred operative field that has often been subjected to radiation therapy. The chance of obtaining a very satisfactory cosmetic and functional result under these circumstances is certainly reduced in comparison with a primary repair.
Other variables that factor into the decision-making process include the use of radiation therapy and the location of the defect. The sacrifice of bone generally indicates an advancedstage tumor. As such, radiation therapy is incorporated into the treatment plan in either a presurgical or postsurgical role. If radiation is administered in a preoperative manner, the surgeon is forced to contend with bone that is, by definition, hypoxemic. Surgical trauma may result in decreased vascularity, which will negatively impact on healing. Increased infection and fistulization can be anticipated. Delivery of radiation in an adjunctive, postoperative manner will have some impact on the mandible within the operative field. In this setting, it is imperative that vascularized tissue of some sort be transferred to the area if rigid fixation is being used. Despite this precaution, osteoradionecrosis, with resultant infection and extrusion, may ensue.
Location of defects similarly plays a role in the decisionmaking process. The anterior mandible remains the critical issue in any discussion of reconstruction. Owing to the devastating potential functional and cosmetic sequelae associated with sacrifice of the mandibular symphysis and arch, primary reconstruction in this area appears to be imperative. Reports indicate that in this region vascularized bone has a distinct advantage over any other technique.1–11 Lateral defects, how-
335
336 |
B.L. Wenig |
ever, serve as an area of greater controversy since both cosmetic and functional deficits are neither as apparent nor as debilitating. Here, questions arise as to whether reconstruction is necessary at all.
Approaches to Reconstruction
The recent trend toward mandibular preservation has changed the approach of many physicians. Traditionally, the mandible was sacrificed if tumor even approximated the periosteum for both oncologic and practical reasons. As a result of the work of McGregor et al.12–14 and Carter et al.,15 it appears that two patterns of spread of squamous cell carcinoma within the mandible can be identified. The first involves spread in relation to the inferior alveolar nerve, while the second relates to spread in spaces between cancellous bony trabeculae. Based on these data, it appears that the extent of bone resection required can be estimated on the basis of tumor extent on the occlusal surface of the mandible regardless of whether only the upper border is being removed or a segmental resection is being undertaken. Furthermore, an adequate margin of safety can be considered to be 5 to 10 mm of apparently normal bone on either side of the main tumor mass. These concepts have radically altered opinion on the need to resect full segments of mandible, thereby eliminating much of the disability associated with extirpation and reconstruction. However, in cases where prior radiation has been administered, rim resection appears to be unsafe because of the variable and unpredictable routes of tumor entry.
Once the decision to resect has been reached, the degree or extent of resection then factors into the reconstruction deci- sion-making process. Will it be necessary to reconstitute bone, lining, coverage, or a combination of these? Will the defect involve the symphysis and arch, body, or hemimandible?
As previously mentioned, the mandibular arch remains the most difficult region to reconstruct. Gravitational and muscular forces effectively eliminate the possibility of using any tissue other than vascularized bone as a free microvascular transfer. Although other methods have been successful in this area, the literature bears out the clear advantage enjoyed by this technique.5,11,16 Decisions regarding reconstruction of ramus and/or body mandibular defects, however, are clinically based and relate to the functional and cosmetic goals that are desired.
Bone Substitutes
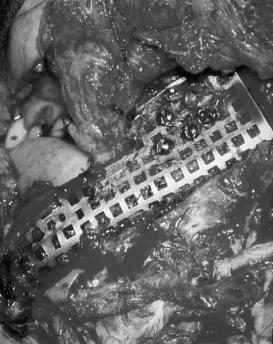
Numerous bone substitutes for mandibular defects have been tried. Irradiated17,18 or cryopreserved mandible,19 standard autologous bone grafts, particulate corticocancellous grafts with and without tray alloplasts (Figure 29.1),20–22 and combinations of these techniques all were used in an attempt to replace the bone that was removed. These were generally done as secondary procedures for fear of contamination and infection or eventual tumor recurrence. While mandibular conti-
FIGURE 29.1 Corticocancellous bone graft within a vitallium tray alloplast.
nuity may have been reconstituted, large, pedicled flaps were often added to restore soft tissue defects.
Rigid Fixation
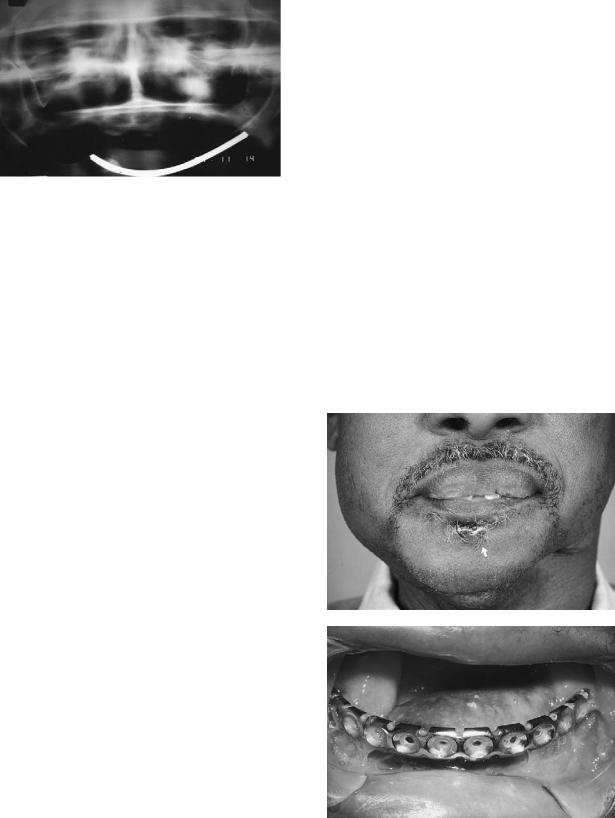
Early methods of stabilizing bony defects of the mandible have included Kirschner wires and their variations (Figure 29.2)23–25 and extraskeletal fixation.26,27 Metal impants were initially extensively described by Conley28,29 and have been employed in numerous forms and ways since that time.30
Approximately 20 years ago, Schmoker et al.31 introduced the concept of the reconstruction plate for the bridging of a mandibular defect. The major advantage offered by this plate was stability of the remaining mandibular segments following local trauma. The principles that developed from the treatment of traumatic injuries were then applied to patients undergoing mandibulectomy for malignancy.32,33 Although initially made of steel, the current versions are fabricated from vitallium, or more commonly, titanium. The ability to contour and adapt these plates intraoperatively makes them ideal as replacement materials where large discontinuity defects are created during surgery.
The technique employed for placement has been fairly standardized. Before resection, the mandible is exposed and the anticipated sites of osteotomy are delineated. Using a template, the contour of the bone is marked, and the plate is then adapted to the form of the template. Drilling is then carried out followed by measurement of the holes. If a non–self- tapping screw system is used, the holes are then tapped, while tapping becomes uneccessary in systems using self-tapping
29. Soft Tissue Flaps with or Without Bone Graft and Rigid Fixation
FIGURE 29.2 Kirschner wire used to reconstruct mandibular defect.
screws. The plate is then fixed to the bone with the screws and removed at which point the osteotomies are carried out. Following completion of the resection, the plate is fixed in position using the appropriate screws and mandibular continuity is reestablished maintaining the contour and rigidity of the fragments.
When contouring is performed prior to resection the potential for prognathism exists because the plate is contoured to the outer mandibular cortex. This is particularly true in anterior defects and much less of a problem in lateral ones. Alternatively, the plate may be contoured and applied after resection. In patients who are dentulous, intermaxillary fixation may be used to maintain normal occlusion of the residual dentition and removed at the end of the procedure. In the edentulous patient, a splint may be fabricated in advance to hold the upper and lower jaws in position until the plate can be applied.26 Similarly, screws can be individually drilled in the upper and lower jaws and wired together to simulate occlusion until the plate is fixed in position. The fixation device can then be removed.
The Titanium Hollow-Screw Reconstruction Plate (THORP) is based on the osseointegration of titanium screws and the rigid fixation of the head of the screws to the plate.34,35 The system combines the advantages of an external fixation device and those of internal osteosynthesis. Unlike standard reconstruction plates, THORP stability comes primarily from osseointegration of the hollow screws. Although the steps used to place the plate are similar to those used with standard reconstruction plates, the holes that are drilled and the screws that are placed are wider. Following neutral placement of the hollow screws, a conical expansion bolt is inserted into the free end of the hollow screw. The purpose of this bolt is to expand the flanges on the hollow screw so that it compresses the bone screw to the plate to achieve plate stability.
337
mandibulectomy was not combined with extensive soft tissue resection, as in the case of benign lesions (e.g., ameloblastoma). In instances where extensive resection of the oral or oropharyngeal mucosa was necessary, success has been less consistent.11,36,37 These findings imply that under these conditions rigid fixation alone is insufficient and that soft tissue coverage is essential if a successful reconstruction is to be achieved. If the issue were simply a matter of stability, a high failure rate even in the absence of soft tissue defects would be expected, yet this has not proven to be the case. Additionally, information garnered from the vast orthopedic literature supports the idea that prolonged rigid fixation of long bones requires coverage with healthy tissue to reduce the risk of exposure and increase the probability of healing. With intraoral exposure, additional factors of contamination, such as constant exposure to saliva and oral bacteria, further complicate matters.
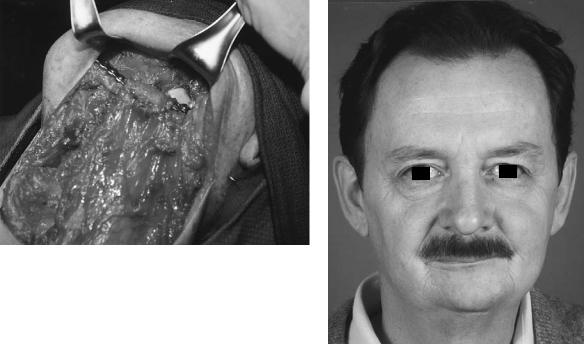
If no flap is employed in the closure of an oral or oropharyngeal defect and only rigid fixation is used following the removal of a significant volume of soft tissue, a primary closure of the wound may be tenuous. Under these circumstances, in the presence of a metal foreign body, any sutureline breakdown will predictably lead to plate exposure (Figure 29.3). This, in turn, may result in screw loosening, infection, and the ultimate extrusion or rejection of the plate.
a
b
Soft Tissue
The principles originally developed for trauma were successfully applied to individuals undergoing mandibulectomy for tumors. This technique proved to be successful when the
FIGURE 29.3 (a) External plate exposure following jaw resection and reconstruction without the use of a flap. (b) Intraoral plate exposure resulting from excessive tension on the suture line despite the use of a pectoralis major flap.
338
Vascularized, pedicled soft tissue flaps and microvascular free-tissue transfer have dramatically altered concepts relating to soft tissue reconstruction. The pectoralis major muscle as well as the latissimus dorsi and other bulky, pedicled flaps have been successfully employed in mandibular reconstruction (Figures 29.4 and 25.5).38–43 Although extremely effective in lateral defects,44 this technique is not without complications. Success rates vary in the literature yet, with the average approximating the 75% range.38,45 Complications such as plate exposure, extrusion, flap breakdown, wound dehiscence, and others range from 23% to 65%.38 Despite these statistics, this technique is effective in restoring immediate mandibular continuity and function. This is particularly important since the vast majority of these patients experience a recurrence of their disease, suggesting that an effective reconstruction with a minimum amount of difficulty may be in the best interest of the patient.
As microvascular techniques have advanced, many options have become available for free-tissue transfer. Differences exist with each flap regarding such things as the maneuverability and bulkiness of the soft tissue, the availability of a sensory nerve for reinnervation, the length of the vascular pedicle, the level of difficulty in harvesting and insetting, and donor-site morbidity. Although the flap can be customized, no one ideal flap exists.
When selecting a free-tissue donor flap to repair a defect involving resection of the mandible, the surgeon must take into account the size of the defect and the nature of the tissue that needs to be replaced. The rectus abdominis donor site
a
B.L. Wenig
has been well documented.33,46–48 It offers the ability to reconstruct very complex three-dimensional head and neck deformities where soft tissue is required. The myocutaneous flap has been used for coverage of very large composite defects, particularly when sufficient skin for reconstruction of the mucosal and cutaneous defects is not available.
The rectus abdominis free flap is often chosen because of its long reliable vascular pedicle, versatile skin paddle, and favorable donor site, which offers the ability to elevate the flap simultaneously with the resection of the head and neck tumor. Additionally, with proper orientation of the skin paddle, mobilization of the contralateral recipient vessels and dissection of the flap vessels to their origin, anastomoses can be effectively and safely accomplished on the contralateral neck vessels, obviating the need for vein grafts. While the flap offers sufficient muscle to envelop the rigid fixation device, it has the drawback of being quite bulky and not easy to fit and contour into a relatively small defect. Without some type of neck dissection that includes removal of the sternocleidomastoid muscle, the flap is difficult to inset and undue pressure may be placed on the pedicle in an attempt to “squeeze” it into the proper position.
Less commonly, combination flaps such as the serratus anterior muscle (SAM) together with the latissimus dorsi have been described to repair composite oromandibular defects.49 These composite flaps offer both lining and external coverage yet are often difficult to elevate and very time consuming.
The radial forearm flap (Figure 29.6)50–52 and the lateral
b
FIGURE 29.4 (a) Pectoralis major flap used to reline and cover defect. THORP plate employed to reconstruct the mandible. (b) Five-year result following surgery and postoperative radiation therapy.

29. Soft Tissue Flaps with or Without Bone Graft and Rigid Fixation |
339 |
a
b
c
FIGURE 29.5 (a) Secondary defect of lateral mandible and soft tissue. (b) THORP plate used to span defect and hold stumps in position.
(c) Pectoralis major flap to cover plate and fill in soft tissue.
arm free flap (Figure 29.7)53–55 offer excellent alternatives to the bulkier rectus abdominis or attached pectoralis major flaps. By positioning the radial forearm or lateral arm flaps intraorally, the oral contents can be separated from the reconstruction plate and the chance of plate loosening or exposure can be decreased. The flap sits up high within the oral cavity and offers a thin, pliable mucosal substitute. The difficulty associated with either of these flaps results when a large resection is performed requiring more coverage and bulk than can be supplied by either of these fasciocutaneous flaps.
Bone Grafts
Autogenous free, nonvascularized bone grafts have been used to reconstruct mandibular defects since 1900.56 Although rib, tibia, and clavicle all have been reported as donor sites, it appears that the best results are associated with grafts taken from the iliac bone. Corticocancellous autogenous bone from the
ilium provides viable cellular and osteoconductive capacity.57 Blocks of this bone or particulate cancellous bone and marrow in an allogeneic bone tray are considered more acceptable than alloplastic replacements. This technique is, however, associated with a high morbidity due to complications such as infection (Figure 29.8), necrosis, or functional impairment. High donor-site morbidity, bone resorption, poor contour, lack of tissue bulk, and unpredictable results1 all raise serious doubt as to the efficacy of this approach.
Several factors must be taken into consideration when bone grafting is contemplated. If significant bone stress shielding results from the rigid internal fixation device or if the period of healing is prolonged, then the graft may undergo undue bone resorption. Furthermore, the grafted bone must come into contact with the mucous membrane on its inner surface and the skin on its outer. These surfaces contain bacteria that can infect and destroy a graft. Additionally, the grafted bone must contain enough cortex to help it withstand the forces of

340 |
B.L. Wenig |
a |
b |
c
FIGURE 29.6 (a) T4N0M0 SCC of the right retromolar trigone. (b) Soft tissue and bone defect following resection. (c) Radial forearm flap inset over mandibular reconstruction plate. Five-year result.
a |
|
|
|
b |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FIGURE 29.7 (a) Lateral arm flap inset over mandibular reconstruction plate in a patient with recurrence following radiation therapy. (b) Five-year result.

29. Soft Tissue Flaps with or Without Bone Graft and Rigid Fixation
FIGURE 29.8 Exposure and infection following bone graft and alloplastic tray.
jaw function and provide a barrier to soft tissue ingrowth, which limits bone regeneration. The graft must also contain sufficient cancellous bone, with its nutrient-rich cellular components, to assist in rapid graft incorporation.56
With the deleterious effects of radiation therapy, which is commonly used following surgical extirpation, it seems reasonable to conclude that primary grafting is at best a risky adventure. Bone grafting appears to be most successful in the patient who has had surgery and has received postoperative radiation therapy and who requires a secondary reconstruction. Here, the factors noted earlier play a much smaller role. Primary internal stabilization of the remaining segments using mandibular reconstruction plates followed by delayed, secondary reconstruction appears to be the most widely accepted treatment option.32,35,58–60
Conclusions
Mandibular continuity defects continue to challenge the technical skills of surgeons involved in the care of these patients. The goals remain to reestablish bony and soft tissue contour, to provide proper occlusion, to allow sufficient mobility of the oral and oropharyngeal tissues, and to create an optimal situation to allow for dental rehabilitation.
As described here, any of several alternatives may be employed in the repair of such a defect. The correct choice will depend on the skill, experience, and judgment of the physician. The method chosen in any particular case should endeavor to achieve the stated goals with the least morbidity and the greatest chance for success.
References
1.Kuriloff DB, Sullivan MJ. Mandibular reconstruction using vascularized bone grafts. Otolaryngol Clin N Am. 1991;24:1391– 1418.
2.Boyd JB, Morris S, Rosen IB, et al. The through-and-through
341
oromandibular defect: rationale for aggressive reconstruction.
Plast Reconstr Surg. 1994;93:44–53.
3.Urken ML, Vickery C, Weinberg H, et al. The internal obliqueiliac crest osseomyocutaneous microvascular free flap in head and neck reconstruction. J Reconstr Microsurg. 1989;5:203– 214.
4.Moscoso JF, Keller J, Genden E, et al. Vascularized bone flaps in oromandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1994;120:36–43.
5.Kroll SS, Schusterman MA, Reece GP. Immediate vascularized bone reconstruction of anterior mandibular defects with free iliac crest. Laryngoscope. 1991;101:791–794.
6.Buchbinder D, Urken ML, Vickery C, et al. Bone contouring and fixation in functional, primary microvascular mandibular reconstruction. Head Neck. 1991;13:191–199.
7.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71–79.
8.Baker SR, Sullivan MJ. Osteocutaneous free scapular flap for one-stage mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1988;114:267–277.
9.Hoffman HT, Harrison N, Sullivan MJ, et al. Mandible reconstruction with vascularized bone grafts. Arch Otolaryngol Head Neck Surg. 1991;117:917–925.
10.Urken ML, Weinberg H, Vickery C, et al. Oromandibular reconstruction using microvascular composite free flaps. Arch Otolaryngol Head Neck Surg. 1991;117:733–744.
11.Wenig BL, Keller AJ. Microvascular free tissue transfer with rigid internal fixation for reconstruction of the mandible following tumor resection. Otolaryngol Clin N Am. 1987;20:621– 633.
12.McGregor AD, MacDonald DG. Patterns of spread of squamous cell carcinoma within the mandible. Head Neck. 1989;11:457– 461.
13.McGregor IA, MacDonald DG. Spread of squamous cell carcinoma to the non-irradiated edentulous mandible—a preliminary report. Head Neck. 1987;9:157–161.
14.McGregor AD, MacDonald DG. Routes of entry of squamous cell carcinoma into the mandible. Head Neck. 1989;11:457–461.
15.Carter RL, Pittam MR. Squamous cell carcinomas of the head and neck: some patterns of spread. J R Soc Med. 1980;73: 420–427.
16.Shockley WW, Weissler MC. Reconstructive alternatives following segmental mandibulectomy. Am J Otolaryngol. 1992;13: 156–167.
17.Hamaker RC. Irradiated autogenous mandibular grafts in primary reconstruction. Laryngoscope. 1981;91:1031–1051.
18.Hamaker RC, Singer MI. Irradiated mandibular autografts update. Arch Otolaryngol Head Neck Surg. 1986;112:277–279.
19.Cummings CW, Leipzig B. Replacement of tumor involved mandible by cryosurgically devitalized autograft. Arch Otolaryngol Head Neck Surg. 1980;106:252–254.
20.Lawson W, Biller HF. Mandibular reconstruction: bone graft techniques. Otolaryngol Head Neck Surg. 1982;90:589–594.
21.Maisel RH, Hilger PA, Adams GL. Reconstruction of the mandible. Laryngoscope. 1983;93:1122–1126.
22.Lowlicht RA, Delacure MD, Sasaki CT. Allogenic (Homograft) reconstruction of the mandible. Laryngoscope. 1990;100:837– 843.
23.Lee KY, Lore JM, Perry CJ. Use of the Kirschner wire for mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1988;114:68–72.
342
24.Gaisford JC, Hanna BC, Gutman D. Management of the mandibular fragments following resection. Plast Reconstr Surg. 1968;28:192–206.
25.Reyneke JP, Wilcock VE. Immediate mandibular reconstruction after resection using a modified Kirschner wire splint. J Oral Surg. 1979;37:415–418.
26.Reece GP, Martin JW, Lemon JC, et al. Mandible fragment fixation during reconstruction: the splint and plate technique. Ann Plast Surg. 1993;31:128–133.
27.Fleming ID, Morris JH. Use of acrylic external splint after mandible resection. Am J Surg. 1969;118:708–715.
28.Conley JJ. The use of vitallium prosthesis and implants in the reconstruction of the mandibular arch. Plast Reconstr Surg. 1951;8:150–162.
29.Conley JJ. A technique of immediate bone grafting in the treatment of benign and malignant tumors of the mandible and review of 17 cases. Cancer. 1953;6:568–577.
30.Söderholm A-L, Lindqvist C, Laine P, et al. Primary reconstruction of the mandible in cancer surgery. Int J Oral Maxillofac Surg. 1988;17:194–197.
31.Schmoker R, Spiessl B, Mathys R. A total mandibular plate to bridge large defects of the mandible. In: New Concepts in Maxillofacial Bone Surgery. New York: Springer-Verlag, 1976: 156–166.
32.Kellman RM, Gullane PJ. Use of the AO mandibular reconstruction plate for bridging mandibular defects. Otolaryngol Clin N Am. 1987;20:519–533.
33.Wenig BL, Keller AJ, Shikowitz MJ, et al. Anatomic reconstruction and functional rehabilitation of oromandibular defects with rigid internal fixation. Laryngoscope. 1988;98:2154–2159.
34.Vuillemin T, Raveh J, Sutter F. Mandibular reconstruction with the titanium hollow screw reconstruction (THORP) system: evaluation of 62 cases. Plast Reconstr Surg. 1988;82:804–814.
35.Hellem S, Olofsson J. Titanium-coated hollow screw and reconstruction plate system (THORP) in mandibular reconstruction. J Craniomaxillofac Surg. 1988;16:173–183.
36.Gullane PJ, Holmes H. Mandibular reconstruction: new concepts. Arch Otolaryngol Head Neck Surg. 1986;112:714–719.
37.Papel ID, Price JC, Kashima HK, et al. Compression plates in the treatment of advanced anterior floor of mouth carcinoma. Laryngoscope. 1986;96:722–725.
38.Disher MJ, Esclamado RM, Sullivan MJ. Indications for the AO plate with a myocutaneous flap instead of revascularized tissue transfer for mandibular reconstruction. Laryngoscope. 1993; 103:1004–1007.
39.Murphy JB, Weisman RA, Kent K. The use of stabilization plates in the immediate repair of defects following mandibular resection. Oral Surg Oral Med Oral Pathol. 1989;68:380–384.
40.Klotch DW, Gump J, Kuhn L. Reconstruction of mandibular defects in irradiated patients. Am J Surg. 1990;160:396–398.
41.Lehtimaki K, Pukander J. Primary mandibular reconstruction after ablative cancer surgery. Acta Otolaryngol. 1992;Suppl. 492: 160–163.
42.Lindqvist C, Söderholm AL, Laine P, et al. Rigid reconstruction plates for immediate reconstruction following mandibular
B.L. Wenig
resection for malignant tumors. J Oral Maxillofac Surg. 1992; 50:1158–1163.
43.Margarino G, Scala M, Gipponi M, et al. Mandible reconstruction with metallic endoprosthesis following Commando’s operation for advanced head and neck cancer. Personal experience. Eur J Surg Oncol. 1993;19:320–326.
44.Schusterman MA, Reece GP, Kroll SS, et al. Use of the AO plate for immediate mandibular reconstruction in cancer patients. Plast Reconstr Surg. 1991;88:588–593.
45.Shockley WW, Weissler MC, Pillsbury HC. Immediate mandibular replacement using reconstruction plates. Arch Otolaryngol Head Neck Surg. 1991;117:745–750.
46.Meland NB, Fisher J, Irons GB, et al. Experience with 80 rectus abdominis free tissue transfers. Plast Reconstr Surg. 1989; 83:481–487.
47.Jones NF, Sekhar LN, Schramm VL. Free rectus abdominis muscle flap reconstruction of the middle and posterior cranial base.
Plast Reconstr Surg. 1986;78:471–477.
48.Taylor GI, Corlett R, Boyd JB. The versatile deep inferior epigastric inferior rectus abdominis flap. Br J Plast Surg. 1984; 37:330–350.
49.Ioannides C, Fossion E, Boeckx W. Serratus anterior muscle in composite head and neck flaps. Head Neck. 1992;14:177– 182.
50.Davidson J, Gullane PJ, Freeman J, et al. A comparison of the results following oromandibular reconstruction using a radial forearm flap with either radial bone or a reconstruction plate.
Plast Reconstr Surg. 1991;88:201–208.
51.Soutar DS, Scheker LR, Tanner NS, et al. The radial forearm flap: a versatile method for intraoral reconstruction. Br J Plast Surg. 1983;36:1–8.
52.Kawashima T, Harii K, Ono I, et al. Intraoral and oropharyngeal reconstruction using a de-epithelialized forearm flap. Head Neck. 2989;11:358–363.
53.Wenig BL. The lateral arm free flap for head and neck reconstruction. Otolaryngol Head Neck Surg. 1993;109:116–119.
54.Matloub HS, Larson DL, Kuhn JC, et al. Lateral arm free flap in oral cavity reconstruction: a functional evaluation. Head Neck. 1989;11:205–211.
55.Kuek LBK, Chuan TL. The extended lateral arm flap: a new modification. J Reconstr Microsurg. 1991;7:167–173.
56.Tidstrom KD, Keller EE. Reconstruction of mandibular discontinuity with autogenous iliac bone graft. J Oral Maxillofac Surg. 1990;48:336–346.
57.Kim MR, Donoff RB. Critical analysis of mandibular reconstruction using AO reconstruction plates. J Oral Maxillofac Surg. 1992;50:1152–1157.
58.Kudo K, Fujioka Y. Review of bone grafting for reconstruction of discontinuity defects of the mandible. J Oral Surg. 1978; 36:791–795.
59.Kruger E, Krumholz K. Results of bone grafting after rigid fixation. J Oral Maxillofac Surg. 1984;42:491–494.
60.Vuillemin T, Raveh J, Sutter F. Mandibular reconstruction with the THORP prosthesis after hemimandibulectomy. J Craniomaxillofac Surg. 1989;17:78–87.
