
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

270 Antibacterial Drugs
Inhibitors of Cell Wall Synthesis
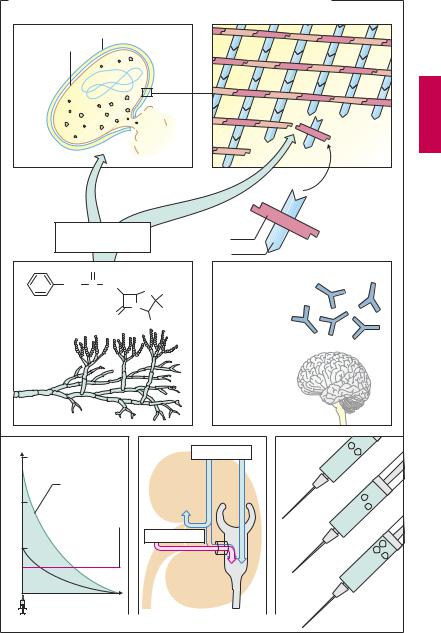
In most bacteria, a cell wall surrounds the cell like a rigid shell that protects against noxious outside influences and prevents rupture of the plasma membrane from a high internal osmotic pressure. The structural stability of the cell wall is due mainly to the murein (peptidoglycan) lattice. This consists of basic building blocks linked together to form a large macromolecule. Each basic unit contains the two linked aminosugars N-acetylglucosamine and N-acetyl- muramic acid; the latter bears a peptide chain. The building blocks are synthesized in the bacterium, transported outward through the cell membrane, and assembled as illustrated schematically. The enzyme transpeptidase cross-links the peptide chains of adjacent aminosugar chains.
Inhibitors of cell wall synthesis are suitable antibacterial agents because animal, including human, cells lack a cell wall. These agents exert a bactericidal action on growing or multiplying germs. Members of this class include β-lactam antibiotics such as the penicillins and cephalosporins, in addition to bacitracin and vancomycin.
Penicillins (A). The parent substance of this group is penicillin G (benzylpenicillin). It is obtained from cultures of mold fungi, originally from Penicillium notatum. Penicillin G contains the basic structure common to all penicillins, 6-aminopenicillanic acid (6- APA; p.273) comprising a thiazolidine and a 4-membered β-lactam ring. 6-APA itself lacks antibacterial activity. Penicillins disrupt cell wall synthesis by inhibiting transpeptidase. When bacteria are in their growth and replication phase, penicillins are bactericidal; as a result of cell wall defects, the bacteria swell and burst.
Penicillins are generally well tolerated; with penicillin G, the daily dose can range from approx. 0.6 g i.m. (= 106 international units, 1 Mega IU [MIU]) to 60 g by infusion. The most important adverse effects are due
to hypersensitivity (incidence up to 5%), with manifestations ranging from skin eruptions to anaphylactic shock (in less than 0.05% of patients). Known penicillin allergy is a contraindication for these drugs. Because of an increased risk of sensitization, penicillins must not be used locally. Neurotoxic effects, mostly convulsions due to GABA antagonism, may occur if the brain is exposed to extremely high concentrations, e.g., after rapid i.v. injection of a large dose or intrathecal injection.
Penicillin G undergoes rapid renal elimination mainly in unchanged form (plasma t½ ~ 0.5 hours). The duration of the effect can be prolonged by:
1.Use of higher doses, enabling plasma levels to remain above the minimally effective antibacterial concentration.
2.Combination with probenecid. Renal elimination of penicillin occurs chiefly via the anion (acid)-secretory system of the proximal tubule (–COOH of 6-APA). The acid probenecid (p.326) competes for this route and thus retards elimination of penicillin.
3.Intramuscular administration in depot form. In its anionic form (–COO–) penicillin G forms poorly water-soluble salts with substances containing a positively charged amino group (procaine; clemizole, an antihistaminic; benzathine, dicationic). Depending on the substance, release of penicillin from the depot occurs over a variable interval.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Inhibitors of Cell Wall Synthesis |
271 |
A.Penicillin G: structure and origin; mode of action of penicillins; methods for prolonging duration of action
Cell wall
Cell membrane
Bacterium |
|
|
|
|
Cross-linked by |
|
|
|
|
|
transpeptidase |
Inhibition of |
|
|
Amino acid |
|
|
|
|
chain |
|
||
cell wall synthesis |
|
|
Cell wall |
||
|
|
Sugar |
|||
|
|
|
|
building block |
|
|
O |
|
|
Human |
|
C 2 |
C NH |
|
|
|
Antibody |
S |
CH3 |
|
|
||
H |
|
|
|
||
|
|
|
|
|
|
|
N |
|
CH3 |
Penicillin |
|
Penicillin G |
O |
|
allergy |
|
|
COOH |
|
||||
|
|
|
|
||
|
Neurotoxicity |
Fungus |
at very |
Penicillium notatum |
high dosage |
Plasma concentration
Penicillin
3 x Dose
Minimal bactericidal concentration
Probenecid
Anion secretory system
Time
Increasing the dose |
Combination with probenecid |
|
|
Procaine |
+ |
- |
|
|
|
|
|
|
|
|
|
|
|||
|
|
Penicillin |
|
|
|
|
||
|
|
Clemizole |
+ |
|
|
|||
|
~1 |
- |
|
|
||||
|
Penicillin |
|
|
|||||
|
|
|
|
|
|
|
||
(d) |
|
|
|
|
|
|
+ |
|
actionof |
~2 |
|
|
|
|
+ |
||
Benzathine |
- |
|||||||
|
|
|
||||||
Duration |
|
|
|
Penicillins |
|
|||
|
~7–28 |
|
|
|||||
|
|
|
2 |
|
|
|
|
|
Depot preparations
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

272 Antibacterial Drugs
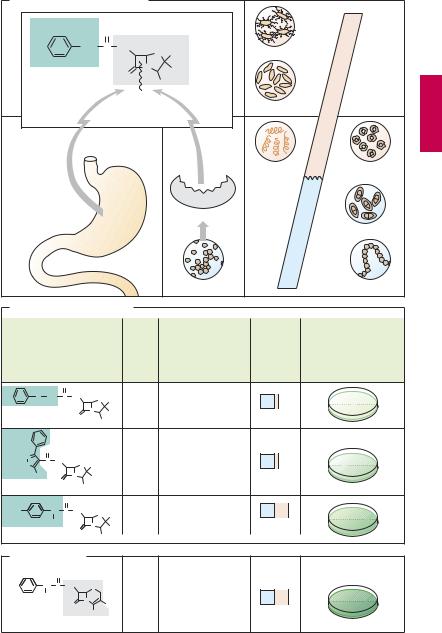
Although very well tolerated, penicillin G has disadvantages (A) that limit its therapeutic usefulness: (1) it is inactivated by gastric acid, which cleaves the β-lactam ring, necessitating parenteral administration. (2) The β-lactam ring can also be opened by bacterial enzymes (β-lactamases); in particular, penicillinase, which can be produced by staphylococcal strains, renders them resistant to penicillin G. (3) The antibacterial spectrum is narrow; although it encompasses many Gram-positive bacteria, Gramnegative cocci, and spirochetes, many Gramnegative pathogens are unaffected.
Derivatives with a different substituent on 6-APA possess advantages (B):
1.Acid resistance permits oral administration, provided that enteral absorption is possible. All derivatives shown in (B) can be given orally. Penicillin V (phenoxymethylpenicillin) exhibits antibacterial properties similar to those of penicillin G.
2.Owing to their penicillinase resistance, isoxazolylpenicillins (oxacillin, dicloxacillin, floxacillin) are suitable for the (oral) treatment of infections caused by penicillin- ase-producing staphylococci.
3.Extended-activity spectrum: The aminopenicillin amoxicillin is active against many Gram-negative organisms, e.g., colibacteria or Salmonella typhi. It can be protected from destruction by penicillinase by combination with inhibitors of
penicillinase (clavulanic acid, sulbactam, tazobactam).
The structurally close congener ampicillin (no 4-hydroxy group) has a similar activity spectrum. However, because it is poorly absorbed (<50%) and therefore causes more extensive damage to the gut microbial flora (side effect: diarrhea), it should be given only by injection.
A still broader spectrum (including pseudomonad bacteria) is shown by carboxypenicillins (carbenicillin, ticarcillin) and acylaminopenicillins (mezclocillin, azlocillin, piperacillin). These substances are neither acidstable nor penicillinase-resistant.
Cephalosporins (C). These β-lactam antibiotics are also fungal products and have bactericidal activity due to inhibition of transpeptidase. Their shared basic structure is 7- aminocephalosporanic acid, as exemplified by cefalexin (gray rectangle). Cephalosporins are acid-stable, but many are poorly absorbed. Because they must be given parenterally, most—including those with high activity—are used only in clinical settings. A few, e.g., cefalexin, are suitable for oral use. Cephalosporins are penicillinase-resistant but cephalosporinase-forming organisms do exist. However, some derivatives are also resistant to this β-lactamase. Cephalosporins are broad-spectrum antibacterials. Newer derivatives (e.g., cefotaxime, cefmenoxin, ceftriaxone, ceftazidime) are also effective against pathogens resistant to various other antibacterials. Cephalosporins are mostly well tolerated. All can cause allergic reactions, some also renal injury, alcohol intolerance, and bleeding (vitamin K antagonism).
Other inhibitors of cell wall synthesis. Bacitracin and vancomycin interfere with the transport of peptidoglycans through the cytoplasmic membrane and are only active against Gram-positive bacteria. Bacitracin is a polypeptide mixture; it is markedly nephrotoxic and is used only topically. Vancomycin is a glycopeptide and the drug of choice for the (oral) treatment of bowel inflammations occurring as a complication of antibiotic therapy (pseudomembranous enterocolitis caused by Clostridium dif cile). It is not absorbed. Infections with Gram-posi- tive cocci that are resistant against better tolerated drugs can also be treated with vancomycin given systemically. This entails an increased risk of ototoxicity (hearing loss, tinnitus) or vestibular toxicity (vertigo, ataxia, and nystagmus).
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Inhibitors of Cell Wall Synthesis |
273 |
A. Disadvantages of penicillin G |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
6-Aminopenicillanic acid |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
C |
NH |
S |
CH3 |
Salmonella typhi |
|
|
|
|
active |
||||
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
O |
N |
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COOH |
|
|
|
|
|
|
|
|
Not |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
negative |
|||
Penicillin G |
|
|
|
|
|
|
E. coli |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Gram |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Acid sensitivity |
|
|
|
|
Penicillinase |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
sensitivity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Treponema |
|
|
|
|
|
|
Gonococci |
|
|
|
|
|
|
|
|
|
pallidum |
|
|
|
|
|
|
||
|
|
|
|
H+Cl- |
|
Penicillinase |
actionspectrum |
|
|
|
positive |
|
|
|
|
|
|
|
|
|
|
|
|
Active |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
Gram |
- |
|
|
|
Pneumococci |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
Narrow- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Staphylococci |
|
|
|
|
|
|
|
Streptococci |
|
B. Derivatives of penicillin G |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration needed |
|||
|
|
|
|
|
|
Acid |
Penicillinase |
Spectrum |
|
|
|
to inhibit penicillin G- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
sensitive bacteria |
|||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
CH2 C |
NH |
S |
CH3 |
Resis- |
Sensitive |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Penicillin V |
O |
N |
CH3 |
tant |
|
|
Narrow |
|
|
|
|
|
|
|
||
|
|
COOH |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
N |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C NH |
|
|
|
|
Resis- |
Resistant |
|
|
|
|
|
|
|
|
|
|
O |
S |
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
tant |
|
|
|
|
|
|
|
|
|
|
|||
CH3 |
|
N |
CH3 |
|
|
|
|
Narrow |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Oxacillin O |
COOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
CH C |
NH |
S |
CH3 |
Resis- |
Sensitive |
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
tant |
|
|
|
|
|
|
|
|
|
|
|||
|
|
N |
CH3 |
|
|
Broad |
|
|
|
|
|
|
|
|||
Amoxicillin |
O |
|
|
|
|
|
|
|
|
|
|
|||||
COOH |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
C. Cephalosporin |
|
|
|
|
|
O |
|
|
|
|
|
CH C |
NH |
S |
|
Resistant, |
|
|
|
|
|
||
NH2 |
|
|
Resis- |
|
|
N |
|
but sensitive to |
|
||
Cefalexin |
O |
CH3 |
tant |
cephalosporinase |
Broad |
|
COOH |
||||
|
|
|
|||
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
