
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

222 Psychopharmacologicals
Benzodiazepines
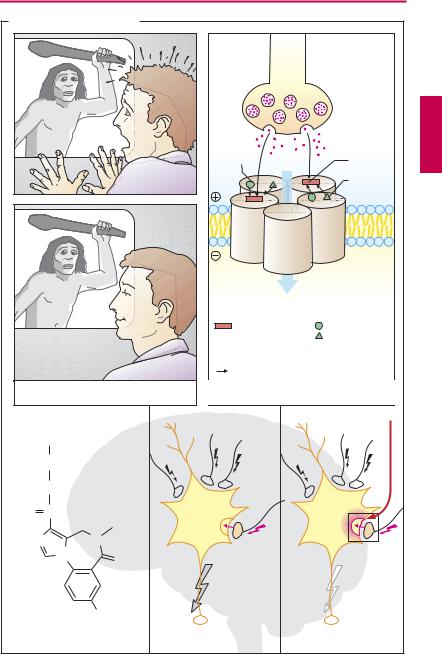
Balanced CNS activity requires inhibitory and excitatory mechanisms. Spinal and cerebral inhibitory interneurons chiefly utilize γ-aminobutyric acid (GABA) as transmitter substance, which decreases the excitability of target cells via GABAA receptors. Binding of GABA to the receptor leads to opening of a chloride (Cl–)ion channel, chloride influx, neuronal hyperpolarization, and decreased excitability. The pentameric subunit assembly making up the receptor/ion channel contains a high-af nity binding site for benzodiazepines, in addition to the GABA binding locus. Binding of benzodiazepine agonists allosterically enhances binding of GABA and its action on the channel. The prototypical benzodiazepine is diazepam. Barbiturates also possess an allosteric binding site on the Cl−-channel protein; their effect is to increase the channel mean open-time during GABA stimulation.
Benzodiazepinesexhibit a broad spectrum of activity: they exert sedating, sleep-induc- ing, anxiolytic, myorelaxant, and anticonvulsant effects and can be used for induction of anesthesia. Of special significance for the use of benzodiazepines is their wide margin of safety. At therapeutic dosages, neither central respiratory control nor cardiovascular regulation are affected. By virtue of these favorable properties, benzodiazepines have proved themselves for a variety of indications. At low dosage, they calm restless or agitated patients and allay anxiety, though without solving problems. Use of benzodiazepines as sleep remedies is widespread. Here, preference is given to substances that are completely eliminated during the night hours (tetracyclic compounds such as triazolam, brotizolam, alprazolam). For longerlasting anxiolytic therapy, compounds should be selected that are eliminated slowly and ensure a constant blood level (e.g., diazepam).
In psychosomatic reactions, benzodiazepines can exert an uncoupling effect. They
are therefore of great value in hyperacute disease states (e.g., myocardial infarction, p.320) or severe accidents. Status epilepticus is a necessary indication for parenteral administration (p.190); however, benzodiazepines can also be used for the long-term treatment of certain forms of epilepsy, if necessary in combination with other anticonvulsants. Rapidlyeliminatedbenzodiazepines are suitable for the intravenous induction of anesthesia.
Use of benzodiazepines may lead to personality changes characterized by flattening of affect. Subjects behave with indifference andfailto reactadequately. Anytasksrequiring prompt and target-directed action—not only driving a motor vehicle—should be left undone.
Benzodiazepine Antagonist
The drug flumazenil bindswith high af nity to the benzodiazepine receptor but lacksany agonist activity. Consequently, the receptor is occupied and unavailable for binding of benzodiazepine agonists. Flumazenil is a specific antidote and is used with success for reversal of benzodiazepine toxicity or to terminate benzodiazepine sedation. When patients suffering from benzodiazepine dependence are given flumazenil, withdrawal symptoms are precipitated.
Flumazenil is eliminated relatively rapidly with a t½ of ~ 1 hour. Therefore, the required dose of 0.2–1.0 mg i.v. must be repeated a corresponding number of times when toxicity is due to long-acting benzodiazepines.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
|
Benzodiazepines |
223 |
|
A. Action of benzodiazepines |
|
|
|
|
GABAergic |
||
|
neuron |
|
|
Benzo- |
|
GABA |
|
diazepine |
Cl- |
binding |
|
binding site |
site |
||
|
|||
α |
β |
Barbiturate |
|
binding site |
|||
β |
α |
|
|
|
γ |
|
|
Anxiolyis |
|
|
|
|
|
Plasma- |
|
|
|
lemma |
|
|
Cl- |
|
|
GABA |
Binding site for |
|
binding |
Benzodiazepines |
|
site |
Barbiturates |
|
|
|
|
Positive allosteric modulators of GABA effect increase |
|
|
in Cl- conductance hyperpolarization |
|
|
decreased excitability |
|
Plus anticonvulsant effect, |
GABAA receptor |
|
sedation, muscle relaxation |
with allosteric binding sites |
|
|
|
|
|
Benzodiazepines |
|
CH3 |
|
|
|
|
CH2 |
|
|
|
|
O |
|
|
|
O |
C |
CH3 |
|
|
|
|
|
|
|
N |
|
N |
|
|
|
N |
O |
|
|
|
|
|
|
|
|
|
F |
|
|
Flumazenil |
|
Normal |
Enhanced |
|
Benzodiazepine antagonist |
GABAergic inhibition |
GABAergic inhibition |
||
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

224 Psychopharmacologicals
Pharmacokinetics of Benzodiazepines
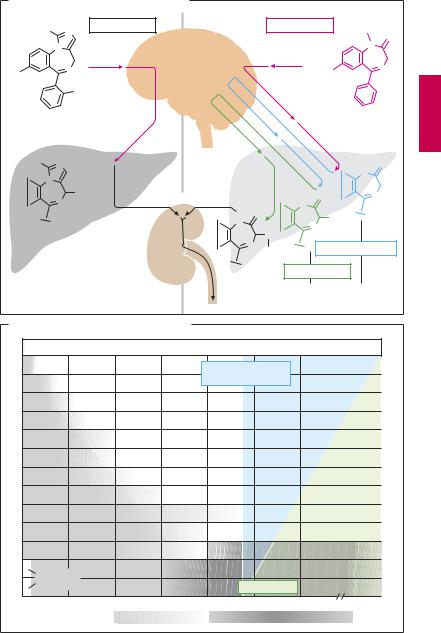
A typical metabolic pathway for benzodiazepines, as exemplified by the drug diazepam, is shown in panel (A): first the methyl group on the nitrogen atom at position 1 is removed, with a concomitant or subsequent hydroxylation of the carbon at position 3. The resulting product is the drug oxazepam. These intermediary metabolites are biologically active. Only after the hydroxyl group (position 3) has been conjugated to glucuronic acid is the substance rendered inactive and, as a hydrophilic molecule, readily excreted renally. The metabolic degradation of desmethyldiazepam (nordiazepam) is the slowest step (t½ = 30–100 hours). This sequence of metabolites encompasses other benzodiazepines that may be considered precursors of desmethyldiazepam, e.g., prazepam and chlordiazepoxide (the first benzodiazepine = Librium ). A similar metabolite pattern is seen in benzodiazepines in which an −NO2 group replaces the chlorine atom on the phenyl ring and in which the phenyl substituent on carbon 5 carries a fluorine atom (e.g., flurazepam). With the exception of oxazepam, all these substances are long-acting. Oxazepam represents those benzodiazepines that are inactivated in a single metabolic step; nevertheless, its half-life is still as long as 8 ± 2 hours. Substances with a short half-life result only from introduction of an additional nitrogen-con- taining ring (see A) bearing a methyl group that can be rapidly hydroxylated. Midazolam, brotizolam, and triazolam are members of such tetracyclic benzodiazepines; the latter two are used as hypnotics, whereas midazolam given intravenously is employed for anesthesia induction.
Another possible way of obtaining compounds with an intermediate duration of action is to replace the chlorine atom in diazepam with an NO2 residue (rapidly reduced to an amine group with immediate acetylation) or with a bromine atom (which
causes ring cleavage in the organism). In these cases, biological inactivation again consists of a one-step reaction.
Dependence potential. Prolonged regular use of benzodiazepines can lead to physical dependence. With the long-acting substances marketed initially, this problem was less obvious in comparison with other depen- dence-producing drugs, because of the delayed appearance of withdrawal symptoms (the decisive criterion for dependence). Symptoms manifested during withdrawal include restlessness, irritability, nervousness, anxiety, insomnia, and, occasionally or in susceptible patients, convulsions. These symptoms are hardly distinguishable from those considered indications for the use of benzodiazepines. Benzodiazepine withdrawal reactions are more likely to occur after abrupt cessation of prolonged or excessive dosage and are more pronounced in shorter-acting substances, but may also be evident after discontinuance of therapeutic dosages administered for no longer than 1–2 weeks.
Administration of a benzodiazepine antagonist would abruptly provoke abstinence signs. Benzodiazepines exhibit cross-de- pendence with ethanol and can thus be used in the management of delirium tremens. There are indications that substances with intermediate elimination half-lives have the highest abuse potential. Withdrawal of benzodiazepines should be done gradually and cautiously. A long-acting substance such as diazepam is the drug of choice for controlled tapering of withdrawal.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Pharmacokinetics of Benzodiazepines |
225 |
A. Biotransformation of benzodiazepines
H3C |
Midazolam |
|
|
Diazepam |
H3C |
|
|
||||
N |
|
|
|
|
|
|
|
N1 |
O |
||
|
|
|
|
|
|
|
|
|
|
||
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
Cl |
N |
|
|
|
|
|
|
Cl |
5 |
N |
|
|
F |
|
|
|
|
|
|
|
|
|
|
HOH2C |
N |
|
|
|
|
|
|
|
H |
O |
|
N |
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
as glucuronide |
|
|
H |
O |
|
N |
|
|
|
N |
|
|
|
|
|
|||||
|
|
|
|
H |
O |
N |
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
||
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
Inactive |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
N |
|
Nordiazepam |
|
||||
|
|
|
|
|
|
Gluc |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oxazepam |
|
|
|
|
|
|
|
|
|
|
|
Active metabolites |
|
|||
B. Rate of elimination of benzodiazepines |
|
|
|
|
|
|
|
|
|||
|
|
i.v. induction of anesthesia |
|
|
Midazolam |
|
|
|
|||
|
|
|
|
Hypnagogic effect |
Triazolam |
|
|
|
|||
|
|
|
|
|
|
|
Brotizolam |
|
|
|
|
|
|
|
|
|
|
|
Oxazepam |
|
|
|
|
|
|
|
|
|
|
|
Lormetazepam |
|
|
||
|
|
|
|
|
|
|
Bromazepam |
|
|
||
|
|
|
|
|
|
|
Flunitrazepam |
|
|
||
|
|
|
|
|
|
|
Lorazepam |
|
|
|
|
|
|
|
|
|
|
|
Camazepam |
|
|
||
|
|
|
|
|
|
|
Nitrazepam |
|
|
||
|
|
|
|
|
|
|
Clonazepam |
|
|
||
|
|
|
|
|
|
|
Diazepam |
|
|
|
|
Precursor |
|
|
|
|
|
Temazepam |
|
|
|||
|
|
|
|
Anxiolysis |
Prazepam |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
0 |
10 |
20 |
30 |
40 |
|
50 |
60 |
|
>60 hours |
|
|
Range of plasma t1/2 |
|
Applied drug |
|
Active metabolite |
|
|
|
|
|||
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
