
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

278 Antibacterial Drugs
Inhibitors of Protein Synthesis
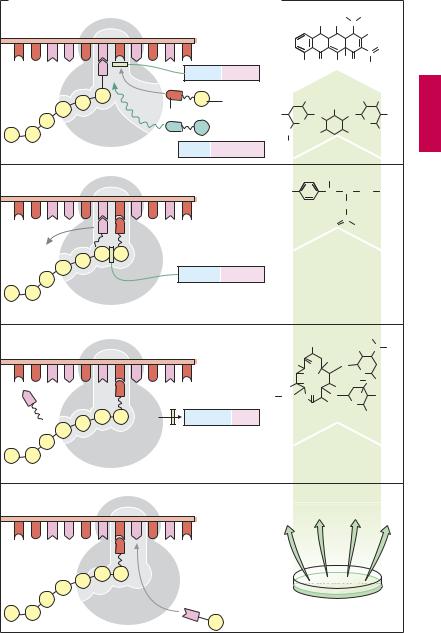
Protein synthesis means translation into a peptide chain of a genetic message first transcribed into mRNA. Amino acid (AA) assembly occurs at the ribosome. Delivery of amino acidstomRNAinvolvesdifferenttransferRNA molecules (tRNA), each of which binds a specific AA. Each tRNA bears an “anticodon” nucleobase triplet that is complementary to a particular mRNA coding unit (codon, consisting of three nucleobases).
Incorporation of an AA normally involves the following steps (A):
1.The ribosome “focuses” two codons on mRNA; one (at the left) has bound its tRNA–AA complex, the AA having already been added to the peptide chain; the other (at the right) is ready to receive the next tRNA–AA complex.
2.Afterthelatterattaches,theAAsof thetwo adjacent complexes are linked by the action of the enzyme peptide synthetase (peptidyltransferase). Concurrently, AA and tRNA of the left complex disengage.
3.The left tRNA dissociates from mRNA. The ribosome can advance along the mRNA strand and focus on the next codon.
4.Consequently, the right tRNA–AA complex
shifts to the left, allowing the next complex to be bound at the right.
These individual steps are susceptible to inhibition by antibiotics of different groups. The examples shown originate primarily from Streptomyces species, some of the aminoglycosides also being derived from Micromonospora bacteria.1
1a. Tetracyclines inhibit the binding of tRNA–AA complexes. Their action is bacteriostatic and affects a broad spectrum of pathogens.
_
1 A note on spelling: the termination -mycin denotes origin from Streptomyces species; -mi- cin (e.g., gentamicin) denotes origin from Micromonospora species.
1b. Aminoglycosides induce the binding of “wrong” tRNA–AA complexes, resulting in synthesis of false proteins. Aminoglycosides are bactericidal. Their activity spectrum encompasses mainly Gram-negative organisms. Streptomycin and kanamycin are used predominantly in the treatment of tuberculosis.
2.Chloramphenicol inhibits peptide synthetase. It has bacteriostatic activity against a broad spectrum of pathogens. The chemically simple molecule is now produced synthetically.
3.Macrolides suppresses advancement of the ribosome. Their action is predominantly bacteriostatic and is directed mainly against Gram-positive organisms. Intracellular germs such as chlamydias and mycoplasms are also affected. Macrolides are effective orally. The prototype of this group is erythromycin. Among other uses, it is suitable as a substitute in allergy or resistance to penicillin. Clarithromycin, roxithromycin and azithromycin are erythromycin derivatives with similar activity; however, their elimination is slower and, therefore, permits reduction in dosage and less frequent administration. Gastrointestinal disturbances may occur. Because of inhibition of CYP isozymes (CYP3A4) the risk of drug interactions is present. Telithromycin is a semisynthetic macrolide with a modified structure (“ketolide”). It has a different pattern of resistance that is attributed to interaction with an additional ribosomal binding site.
Lincosamides. Clindamycin has antibacterial activity similar to that of erythromycin. It exerts a bacteriostatic effect mainly on Gram-positive aerobic, as well as on anaerobic pathogens. Clindamycin is a semisynthetic chloro analogue of lincomycin, which derives from a Streptomyces species. Taken orally, clindamycin is better absorbed than lincomycin, has greater antibacterial ef cacy and is thus preferred. Both penetrate well into bone tissue.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Inhibitors of Protein Synthesis |
279 |
A. Protein synthesis and modes of action of antibacterial drugs |
|
|
|
|
|
|
|
|||
|
|
|
|
|
H3C |
|
CH3 |
|
||
|
|
|
|
H3C |
HO |
|
N |
|
|
|
|
|
mRNA |
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
O |
||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
O |
|
|
C |
|
|
|
|
|
OH O |
HO |
O |
|
|
|
|
Ribosome |
|
Tetracyclines |
|
H |
|
NH2 |
|
|||
|
|
Doxycycline |
|
|
||||||
|
|
|
|
|
||||||
|
|
Amino acid |
|
NH2 |
OH |
|
HO |
NH2 |
||
|
tRNA |
|
|
|
|
|
|
|
|
|
|
Insertion of |
HO |
O |
|
|
O |
|
|
OH |
|
|
|
|
|
|
O |
|||||
|
|
incorrect |
|
O |
|
|
|
|
|
|
|
|
amino acid |
H2C |
H2N |
|
|
NH2 |
CH2OH |
||
Peptide chain |
Aminoglycosides |
NH2 |
Tobramycin |
|
|
|
||||
|
|
|
|
|
|
|||||
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
O2N |
|
CH |
CH |
CH2 |
OH |
||
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
O |
C |
CHCl2 |
|
||
|
|
|
|
|
|
|
||||
|
|
|
|
Chloramphenicol |
|
|||||
|
Chloramphenicol |
|
|
|
|
|
|
|
|
|
|
Peptide |
|
|
|
|
|
|
|
|
|
|
synthetase |
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
H3C |
|
|
|
|
|
|
|
|
HO |
|
N |
CH3 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
H3C |
O |
CH3 O |
|
|
|
|
|
|
|
|
|
OH OH |
|
H3C |
O |
|
||
|
|
|
H3C |
OH |
|
|
O CH3 |
|||
|
|
|
O |
CH3 |
|
|
|
|
||
|
|
|
H3C H2C |
O |
|
H3C OH |
||||
|
|
|
O |
|
||||||
|
|
|
|
|
CH3 |
|
O |
|
|
|
|
|
Macrolides |
|
|
|
|
|
|
CH3 |
|
|
|
|
|
Erythromycin |
|
|
||||
|
|
|
Streptomyces species |
|
||||||
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

280 Antibacterial Drugs
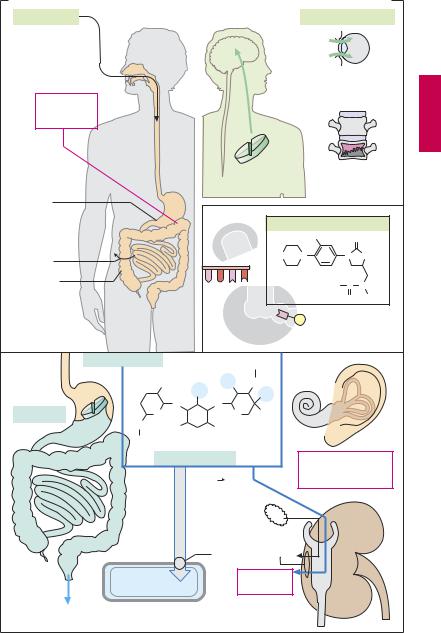
4. Oxazolidinones, such as linezolide, are a newly discovered drug group (p.281). They inhibit initiation of synthesis of a new peptide strand at the point where ribosome, mRNA, and the “start-tRNA–AA” complex aggregate. Oxazolidinones exert a bacteriostatic effect on Gram-positive bacteria. Since bone marrow depression has been reported, hematological monitoring is necessary.
Tetracyclines are absorbed from the gastrointestinal tract to differing degrees, depending on the substance, absorption being nearly complete for doxycycline and minocycline. Intravenous injection is rarely needed (rolitetracycline is available only for i.v. administration). The most common unwanted effect is gastrointestinal upset (nausea, vomiting, diarrhea, etc.) due to (1) a direct mucosal irritant action of these substances and
(2) damage to the natural bacterial gut flora (broad-spectrum antibiotics) allowing colonization by pathogenic organisms, including Candida fungi. Concurrent ingestion of antacids or milk would, however, be inappropriate because tetracyclines form insoluble complexes with plurivalent cations (e.g., Ca2+, Mg2+, Al3+, Fe2+/3+) resulting in their inactivation; that is, absorbability, antibacterial activity, and local irritant action are abolished. The ability to chelate Ca2+ accounts for the propensity of tetracyclines to accumulate in growing teeth and bones. As a result, there occurs an irreversible yellowbrown discoloration of teeth and a reversible inhibition of bone growth. Because of these adverse effects, tetracycline should not be given after the second month of pregnancy and not prescribed to children aged 8 years and under. Other adverse effects are increased photosensitivity of the skin and hepatic damage, mainly after i.v. administration.
Chloramphenicol. The broad-spectrum antibiotic chloramphenicol is completely absorbed after oral ingestion. It undergoes even distribution in the body and readily crosses diffusion barriers such as the blood–
brain barrier. Despite these advantageous properties, use of chloramphenicol is only rarely indicated (e.g., in CNS infections) because of the danger of bone marrow damage.
Two types of bone marrow depression can occur: (1) a dose-dependent, toxic, reversible form manifested during therapy, and (2) a frequently fatal form that may occur after a latency of weeks and is not dose-dependent. Owing to high tissue penetrability, the danger of bone marrow depression must be taken into account even after local use (e.g., eye drops).
Aminoglycoside antibiotics consist of glyco- side-linked amino sugars (cf. gentamicin C1a, a constituent of the gentamicin mixture). They contain numerous hydroxyl groups and amino groups that can bind protons. Hence, these compounds are highly polar, poorly membrane permeable and not absorbed enterally. Neomycin and paromomycin are given orally in order to eradicate intestinal bacteria (prior to bowel surgery or for reducing NH3 formation by gut bacteria in hepatic coma). Aminoglycosides for the treatment of serious infections must be injected (e.g., gentamicin, tobramycin, amikacin, netilmicin, sisomycin). In addition, local inlays of a gentamicin-releasing carrier can be used in infections of bone or soft tissues. Aminoglycosides gain access to the bacterial interior via bacterial transport systems. In the kidney, they enter the cells of the proximal tubules via an uptake system for oligopeptides. Tubular cells are susceptible to damage (nephrotoxicity, mostly reversible). In the inner ear, sensory cells of the vestibular apparatus and Corti’s organ may be injured (ototoxicity, in part irreversible).
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Inhibitors of Protein Synthesis |
281 |
A. Aspects of the therapeutic use of tetracyclines, chloramphenicol, and aminoglycosides |
|||||||||
Tetracyclines |
|
|
|
|
|
|
|
Chloramphenicol |
|
|
|
|
|
|
|
|
|
Advantage: |
|
|
|
|
|
|
|
|
|
good penetration |
|
|
|
|
|
|
|
|
|
through barriers |
|
Inactivation by |
|
|
|
|
|
|
|
|
|
chelation of |
|
|
|
|
|
|
|
|
|
Ca2+, Al3+ etc. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disadvantage: |
|
|
|
|
|
|
|
|
|
bone marrow |
|
|
|
|
|
|
|
|
|
toxicity |
|
Irritation of |
|
|
|
|
|
|
|
|
|
mucous |
|
|
|
|
|
|
|
|
|
membranes |
|
|
|
|
|
|
|
Linezolide |
|
|
|
|
|
|
|
|
|
|
|
|
|
formation |
|
|
|
|
|
F |
O |
Absorption |
complex |
|
|
|
O |
N |
N |
O |
|
Antibacterial |
|
|
|
|
|
|
H3C C N |
||
effect on |
|
|
|
|
|
|
|||
gut flora |
|
|
|
|
|
|
|
O |
H |
|
Aminoglycoside |
|
|
|
CH3 |
|
|
|
|
|
|
H+ |
|
|
|
|
|
|
|
|
|
|
|
H+NH |
|
|
|
|
|
|
|
NH2 |
HO |
|
|
|
|
|
|
e.g., |
|
|
OH |
|
OH |
|
|
|
|
|
O |
O |
|
|
|
|
|
||
|
|
|
|
|
|
|
|||
neomycin |
|
|
CH3 |
|
|
|
|
||
|
O |
|
O |
|
|
|
|
||
|
H2C |
H2N |
NH2 |
|
|
|
|
|
|
|
HNH+ 2 |
H+ |
H+ |
|
|
|
|
|
|
|
Gentamicin C1a |
|
|
|
|
|
|
||
|
|
|
|
|
|
Cochlear and |
|||
|
|
|
|
|
|
|
|
vestibular |
|
|
High hydrophilicity |
|
|
|
|
ototoxicity |
|||
|
no passive diffusion |
|
H+ |
|
|
|
|
||
|
through membranes, |
|
H+ |
|
|
|
|||
|
therefore parenteral |
|
H+ |
|
|
|
|||
|
use |
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
|
|
||
|
|
|
|
oligopeptides |
|
|
|
||
|
|
|
Transport system |
|
|
|
|||
|
|
|
|
|
Nephro- |
|
|
|
|
|
Bacterium |
|
|
|
toxicity |
|
|
|
|
No absorption |
|
|
|
|
|
|
|
|
|
“bowel sterilization” |
|
|
|
|
|
|
|
|
|
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
