
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

198 Antipyretic Analgesics
Antipyretic Analgesics
The large and important family of drugs for the treatment of pain, inflammation, and fever has to be subdivided into two groups that differ in their mechanism of action and spectrum of activity, namely,
1.Antipyretic analgesics
2.Nonsteroidal anti-inflammatory drugs
(NSAIDs)
all of which have the chemical character of acids.
Antipyretic analgesics represent p-ami- nophenol or pyrazolone derivatives with clinically useful analgesic and antipyretic efficacy. Their mechanism of action is not completely understood but thought to be mediated via inhibition of prostanoid formation by variants of COX enzymes. Acetaminophen (paracetamol), phenazone, and dipyrone belong in this group.
Acetaminophen has good analgesic ef - cacy in commonplace pain, such as toothache and headaches, but is of less use in inflammatory and visceral pain. It exerts a strong antipyretic effect. The adult dosage is 0.5–1.0 g up to 4 times daily; the elimination half-life is about 2 hours. Acetaminophen is eliminated renally after conjugation to sulfuric or glucuronic acid. A small portion of the dose is converted by hepatic CYP450 to a reactive metabolite that requires detoxification by coupling to glutathione. In suicidal or accidental poisoning with acetaminophen (10 g), the depleted store of thiol groups must be replaced by administration of acetylcysteine. This measure can be life-saving. Long-term therapy with pure acetaminophen preparations does not cause renal damage, reported earlier after use of stimulant combination preparations. Fixed combinations with codeine may be used with hardly any reservation.
Dipyrone (metamizole) is a pyrazolone derivative. It producesstrong analgesia,even in pain of colic, and has an additional spasmolytic effect. The antipyretic effect is marked. The usual dosage is about 500 mg
orally. Higher doses (up to 2.5 g) are needed for biliary colic. The effect of a standard dose lasts ~ 6 hours.
Use of dipyrone is compromised by a very rare but serious adverse reaction, viz., bone marrow depression. The incidence of agranulocytosis remains controversial; probably, one case occurs in > 100 000 treatments. Hypotension may occur after intravenous injection. Dipyrone is not for routine use; however, short-term administration is recommended for appropriate individual cases.
Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
This term subsumes drugs other than COX-2 inhibitors that (a) are characterized chemically by an acidic moiety linked to an aromatic residue; and that (b) by virtue of inhibiting cyclooxygenases, are effective in suppressing inflammation, alleviating pain, and lowering fever. Cyclooxygenases (COX) localized to the endoplasmic reticulum are responsible for the formation from arachidonic acid of a group of local hormonescomprising the prostaglandins, prostacyclin, and thromboxanes. NSAIDs (except ASA) are reversible inhibitors of COX enzymes. These enzymes possess an elongated pore into which the substrate arachidonic acid is inserted and converted to an active product. NSAIDs penetrate into this pore and thus prevent access for arachidonic acid, leading to reversible blockade of the enzyme.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
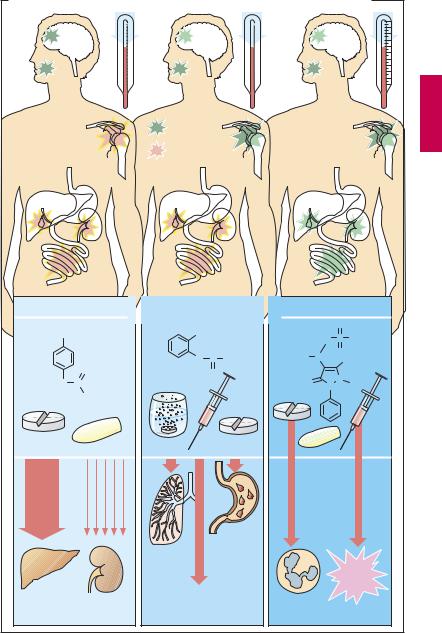
Antipyretic Analgesics vs. NSAIDs |
199 |
A. Comparison of antipyretic analgesics with a nonsteroidal anti-inflammatory drug |
||||||||
Tooth- |
|
Head- |
Fever |
|
|
|
|
|
ache |
|
ache |
|
|
|
|
|
|
Inflammatory |
Effective |
|
|
|
|
|
||
|
|
|
|
|
|
|||
pain |
|
|
Less effective |
|
|
|
|
|
|
|
|
|
|
|
|
||
Pain of colic |
|
|
|
|
|
|
||
Acetaminophen |
Acetylsalicylic acid |
Dipyrone |
||||||
|
|
|
COOH |
|
|
O |
|
|
|
OH |
|
|
H2C |
S |
OH |
||
|
|
|
|
|
|
|||
|
|
|
O |
C CH3 |
H3C |
N |
O |
CH3 |
|
|
|
|
|||||
|
|
O |
|
O |
|
|
N |
|
HN |
C |
|
O |
|
|
|||
|
|
N |
CH3 |
|||||
|
|
|
||||||
|
|
CH3 |
|
|
|
|
|
|
|
only |
|
|
|
|
|
|
|
Acute |
with |
|
|
|
|
|
|
|
|
Chronic |
|
|
very |
|
|
|
|
massive |
|
|
|
|
|
|
||
|
abuse |
|
|
rarely |
|
|
|
|
over- |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
dose |
|
|
|
|
|
|
|
|
>10g |
|
|
|
|
|
|
|
|
|
|
|
|
Irritation |
|
|
|
|
|
|
|
Broncho- |
of |
|
|
|
|
|
|
|
constriction |
gastro- |
|
|
|
Risk of |
|
|
|
|
intestinal |
|
|
|
ana- |
|
|
|
|
mucosa |
|
|
phylactoid |
|
|
|
|
Impaired |
|
|
|
|
shock |
|
|
|
|
|
|
|
|
|
Hepato- |
|
Nephro- |
hemostasis with |
Agranulo- |
|
|
||
toxicity |
|
toxicity |
risk of bleeding |
|
cytosis |
|
|
|
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

200 Nonsteroidal Anti-inflammatory Drugs
Cyclooxygenase (COX) Inhibitors
Two principal types of COX can be distinguished:
COX-1 is constitutive, that is, always present and active; it contributes to the physiological function of organs. Inhibition inevitably produces unwanted effects, such as mucosal injury, renal damage, hemodynamic changes, and disturbances of uterine function.
COX-2 is induced by inflammatory processes and produces prostaglandins that sensitize nociceptors, evoke fever, and promote inflammation by causing vasodilation and an increase in vascular permeability. However, in some organs, COX-2 is also expressed constitutively (kidney, vascular endothelium, uterus, and CNS).
Nonselective COX inhibitors derive from salicylic acid. The majority are carbonic acids, such as ibuprofen, naproxene, diclofenac, indometacin, and many more; or enolic acids, such as azapropazone and meloxicam. All these drugs inhibit both COX enzymes.
The molecules of COX-1 and COX-2 reveal a pharmacologically important difference: the enzymatic pore width of COX-2 exceeds that of COX-1. The nonselective COX inhibitors can also enter the narrower pores and thus inhibit both cyclooxygenases.
Efforts have succeeded to develop inhibitors that readily enter the slightly wider pores of COX-2 but not the COX-1 pores, giving rise to the specific COX-2 inhibitors
(denoted coxibs). These drugs consist of a hetero-aromatic ring bearing two phenyl ring substituents, one of which contains a −SO2 group (see formulas in A). The advantage of coxibs lies in their lesser propensity to cause mucosal injury. This effect has been reported in large clinical trials (but subsequently queried). However, a number of adverse reactions are now known that are consistent with COX-2 also serving constitutive functions. Furthermore, one needs to consider that COX-1 functions may be more or less
affected at suf ciently large dosages. Selectivity factors (COX-1/COX-2 ratio) determined biochemically in vitro range from 30 to 400.
Coxibs available at present include: celecoxib (daily dosage 200–400 mg), valdecoxib (10–20 mg) (no longer available in the US) and its prodrug parecoxib (40 mg i.v.!).
Coxibs are not drugs for routine use and should only be employed in a targeted manner, in particular when antiarthritis treatment with nonselective NSAIDs has led to gastrointestinal mucosal damage (bleeding, gastritis, ulcerations). Contraindications must be heeded, in particular, advanced congestive heart failure, hepatic and renal diseases, inflammatory bowel diseases, and asthma. Concern over an increased risk of stroke and myocardial infarction in vulnerable patient populations has led to withdrawal of rofecoxib (Sept. 2004), raising strong suspicion that this represents a coxib class effect.
Acetylsalicylic acid (ASA) merits a separate comment. Acetylation of salicylic acid significantlyreducesitsabilitytoinduce mucosal injury. After absorption of ASA, the acetyl moiety is cleaved with a t½ of 15–20 minutes, salicylic acid then being present in vivo. For anti-inflammatory therapy, the required dosage of ASA lies above 3 g daily. For
treatment |
of |
ordinary pain, a dose of |
~ 500 mg |
is |
needed. At low dosage |
(100–200 mg |
daily), following absorption |
|
into the portal circulation, ASA causes a long-lasting blockade of COX-1-mediated thromboxane synthesis in platelets because of an irreversible acetylation of the enzyme. Since platelets represent anuclear cell fragments, they are unable to synthesize new COX molecules.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Nonsteroidal Anti-inflammatory Drugs |
201 |
A. Nonsteroidal anti-inflammatory drugs (NSAIDs)
0.3 – 6.0 g |
Nonselective COX inhibitors |
|
|
COO – |
|
|
COO– |
|
|
0.05 – 0.15 g |
|
200 – 400 mg |
|
|
|
|
CH2 |
|
|
|
|
|
OH |
|
H |
|
|
|
|
||
|
|
|
|
|
|
|
|||
Acetyl- |
Salicylic acid |
Cl |
N |
Cl |
|
|
|
||
salicylic |
COO – |
|
|
|
|
|
|||
acid |
|
|
|
|
|
|
|
||
O |
O |
C |
CH3 |
Diclofenac |
|
CH3 |
|
|
|
H2N |
|
|
|
|
|
||||
S |
|
O |
|
|
|
|
CH |
COOH |
|
O |
|
|
|
CH3 |
|
||||
N |
|
|
|
|
|
|
|
||
N |
CF3 |
|
|
|
|
|
|
|
|
Celecoxib |
|
|
H3C |
CH CH2 |
|
|
|
||
H3C |
|
|
|
|
|
Ibuprofen |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
Rofecoxib* |
Naproxen |
CH3 |
|
|
||||
|
|
|
|
||||||
H3C |
|
|
|
|
|
CH |
COO– |
|
|
S |
|
|
|
|
|
|
|
|
|
O |
|
|
|
H3C |
O |
|
|
|
|
|
|
|
O |
|
|
|
|
||
|
|
|
|
|
|
|
|
0.6 – 2.4 g |
|
|
|
|
|
|
|
|
|
|
|
12.5 – 25 mg |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COX-2 inhibitors |
|
|
|
|
|
0.5 – 1.0 g |
daily doses |
||
|
B. Adverse effects of nonsteroidal anti-inflammatory drugs |
|
|
|
|
|
|||||||
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Arachidonic acid |
|
|
|
|
|||
|
Cyclooxygenases |
|
|
|
|
|
|
|
|
|
|
Lipoxygenases |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Nonselective |
|
|
COX -2 |
|
|
|
|
|
|
|||
|
COX inhibitors |
|
|
inhibitors |
|
|
|
|
|
|
|||
|
Prostaglandins decreased |
Leukotrienes increased |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gastric mucosal |
|
|
Lower |
|
|
|
(depending on |
|
||||
|
damage with |
|
|
incidence of |
|
|
|
supply of arachidonic |
|
||||
|
ulcer formation, |
|
|
gastropathy |
|
|
|
acid) |
|
||||
|
bleeding, and |
|
|
|
|
|
|
|
|
|
|
|
|
|
perforation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nephropathy, decreased excretion of NaCl and |
|
|
Bronchoconstriction, |
|
||||||||
|
H2O, edemas, increased blood pressure, impaired |
|
|
bronchial asthma, |
|
||||||||
|
wound healing, diarrhea, disturbed uterine motility |
|
|
proinflammatory effect |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
