
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

150 Antithrombotics
Fibrinolytics
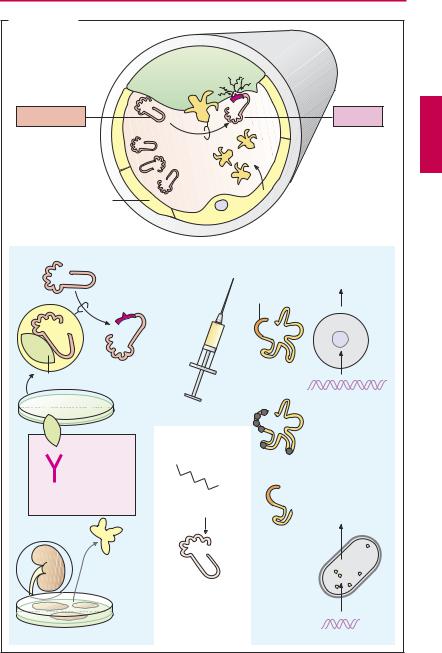
The fibrin meshwork of a blood clot can be cleaved by plasmin. As a protease, plasmin can break down not only fibrin but also fibrinogen and other proteins. Plasmin derives from an inactive precursor, plasminogen, present in blood. Under physiological conditions, specificity of action for fibrin is achieved because, among other things, activation takes place on the fibrin clot.
The tissue plasminogen activator (t-PA) is released into the blood from endothelial cells when blood flow stagnates. Next to its catalytic center, this protease possesses other functional domains, including docking sites for fibrin. During contact with fibrin, plasminogen–plasmin conversion rate is several-fold higher than in streaming blood. Plasminogen also contains a binding domain for fibrin.
Plasminogen activators available for therapeutic use are designated as fibrinolytics; they are infused intravenously in myocardial infarction, stroke, deep leg vein thrombosis, pulmonary embolism, and other thrombotic vascular occlusions. The earlier treatment is started after thrombus formation, the better is the chance of achieving patency of the occluded vessel.
The desired effect carries with it the risk of bleeding as the most important adverse effect, because, apart from the intravascular fibrin clot forming the thrombus, other fibrin coagula sealing defects in the vascular wall are dissolved as well. Moreover, use of fibrinolytics entails the risk that fibrinogen and other clotting factors circulating in blood will undergo cleavage (“systemic lytic state”).
Streptokinase is the oldest available fibrinolytic. By itself it lacks enzymatic activity; only after binding to a plasminogen molecule is a complex formed that activates plasminogen. Streptokinase is produced by streptococcal bacteria. Streptokinase antibodies may be present as a result of previous streptococcal infections and may lead to incompatibility reactions.
Urokinase is an endogenous plasminogen activator that occurs in different organs. Urokinase used therapeutically is obtained from human cultured kidney cells. Circulating antibodies are not expected. The substance is more expensive than streptokinase and also does not depend on fibrin in its action.
Alteplase is a recombinant tissue plasminogen activator (rt-PA). As a result of its production in eukaryotic Chinese hamster ovary (CHO) cells, carbohydrate residues are present as in the native substance. At the therapeutically used dosage, alteplase loses its “fibrin dependence” and thus also activates circulating plasminogen. In fresh myocardial infarctions, alteplase appears to produce better results than does streptokinase.
Tenecteplase is a variant of alteplase that has been altered by six point mutations, resulting in a significant prolongation of its plasma half-life (tenecteplase t½ = 20 minutes; alteplase t½ = 3–4 minutes). Tenecteplase is dosed according to body weight and given by intravenous bolus injection.
Reteplase is a deletion variant of t-PA that lacks both fibrin-binding domains and oligosaccharide side chains (manufactured in prokaryotic E. coli). It is eliminated more slowly than alteplase. Whereas alteplase is given by infusion, reteplase can be administered in two bolus injections spaced 30 minutes apart
Plasmin inhibitors. ε-Aminocaproic acid as well as tranexamic acid and p-aminomethyl- benzoic acid (PAMBA) are plasmin inhibitors that can be useful in bleeding complications. They exert an inhibitory effect by occupying the fibrin binding site of plasminogen or plasmin.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Fibrinolytics 151
A. Fibrinolytics
Plasminogen
Endothelium
Streptokinase
Streptococci
Antibodies from prior infections
Fevers, chills, inactivation
Urokinase
Human kidney cell culture
Fibrin
|
|
|
|
oly |
si |
|
|
|
n |
||
|
|
i |
|
s |
|
|
|
r |
|
|
|
F |
i |
b |
|
|
|
|
|
|
|
|
|
t-PA
Plasmin
t-PA: tissue plasminogen activator
Plasminogen activators
Alteplase = recombinant t-PA
Active center
CHO cells
cDNA
Plasmin inhibitor
H2N
COOH
ε -Aminocaproic acid
Blockade of plasminogen/plasmin binding site on fibrin
Tenecteplase = t-PA with 6 amino acid mutations
Reteplase = nonglycosylated variant of t-PA
E. coli
Truncated cDNA
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

152 Antithrombotics
Intra-arterial Thrombus Formation (A)
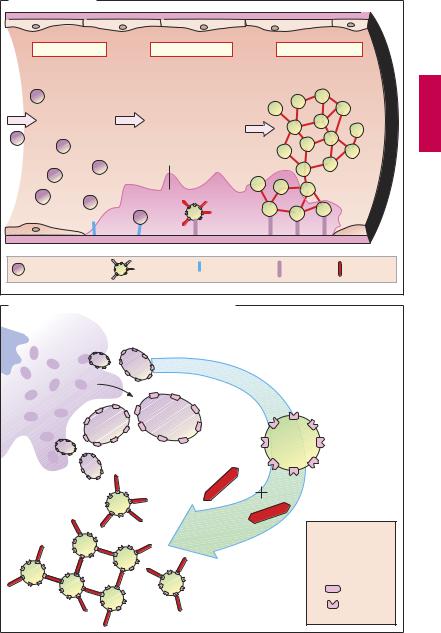
Activation of platelets, e.g., upon contact with collagen of the extracellular matrix after injury to the vascular wall, constitutes the immediate and decisive step in initiating the process of primary hemostasis, i.e., cessation of bleeding. However in the absence of vascular injury, platelets can be activated as a result of damage to the endothelial cell lining of blood vessels. Among the multiple functions of the endothelium, the production of prostacyclin and nitric oxide (NO) plays an important role because both substances inhibit the tendency of platelets to adhere to the endothelial surface. Impairment of endothelial function, e.g., due to chronic hypertension, chronic elevation of plasma LDL levels or of blood glucose, and cigarette smoking, increases the probability of adhesion between thrombocytes and endothelium. The deceleration of fast flowing platelets occurs through an interaction between the glycoprotein Ibα (GP I) in the platelet membrane and von Willebrand factor in the endothelium and basal membrane (denuded after endothelial injury). For the proper activation of the platelet, interaction with subendothelial collagen of an additional platelet glycoprotein (GP IV) is necessary. As soon as platelets are activated (see p.154), they change their shape and gain af nity for fibrinogen. Thisresultsfromaconformational change of glycoprotein IIb/IIIa in the platelet membrane. Platelets can now be linked to each other via fibrinogen bridges (A).
Platelet aggregation proceeds like an avalanche because, once activated, one platelet can activate other platelets. On the injured endothelial cell a thrombus is formed, which obstructs blood flow. Ultimately, the vascular lumen is occluded by the thrombusasthe latter is solidified by vasoconstriction promoted by the release of serotonin and thromboxane A2 from the aggregated platelets and by locally activated thrombin. Thrombin plays a twofold part in thrombus
formation: as a protease, thrombin cleaves fibrinogen and thus initiates the formation of fibrin clot (blood coagulation, p.144). The effects of thrombin on platelets and endothelial cells, however, involve a proteolytic activation of receptorscoupled to G-proteins (so-called protease-activated receptors). When these events occur in a larger, functionally important artery, myocardial infarction or stroke may be the result.
Von Willebrand factor plays a key role in thrombogenesis. Lack of this factor is the cause of thrombasthenia, the inability to staunch bleeding by platelet aggregation. A relative deficiency of von Willebrand factor can be transiently relieved by injection of the vasopressin analogue desmopressin, because this substance makes factor available from stored supplies.
Formation, Activation, and Aggregation of Platelets (B)
Platelets are fragments of multicellular megakaryocytes. They constitute the smallest formed elements of blood (diameter 1–4 µm) and, devoid of a cell nucleus, are no longer capable of protein synthesis. Platelets can be activated by various stimuli, leading to:
Change in shape
Conversion of integrin GP IIb/IIIa into its active conformation
Release of active substances such as serotonin, platelet-activating factor (PAF), ADP, and thromboxane A2. All these substances activate other platelets.
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
Intra-arterial Thrombus Formation |
153 |
A. Thrombogenesis |
|
|
|
|
|
1. Adhesion |
2. Activation |
3. Aggregation |
|||
|
Endothelial defect |
|
|
||
Platelet |
Activated |
von Willebrand |
Collagen |
Fibrinogen |
|
not activated |
platelet |
factor |
|||
|
|
||||
B. Aggregation of platelets by the integrin GPIIb/IIIa |
|
Megakaryocyte |
|
|
Contact with |
|
collagen |
Activation |
ADP |
Thrombin |
|
|
Thromboxane A2 |
|
Serotonin |
Glyco- |
|
protein |
|
IIb/IIIa |
|
Platelet |
|
|
Platelet |
Fibrinogen |
Glycoprotein |
|
IIb/IIIa |
|
Fibrinogen- |
|
binding: |
|
impossible |
Aggregation |
possible |
Luellmann, Color Atlas of Pharmacology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
