
- •VOLUME 1
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •ABLATION.
- •ABSORBABLE BIOMATERIALS.
- •ACRYLIC BONE CEMENT.
- •ACTINOTHERAPY.
- •ADOPTIVE IMMUNOTHERAPY.
- •AFFINITY CHROMATOGRAPHY.
- •ALLOYS, SHAPE MEMORY
- •AMBULATORY MONITORING
- •ANALYTICAL METHODS, AUTOMATED
- •ANALYZER, OXYGEN.
- •ANESTHESIA MACHINES
- •ANESTHESIA MONITORING.
- •ANESTHESIA, COMPUTERS IN
- •ANGER CAMERA
- •ANGIOPLASTY.
- •ANORECTAL MANOMETRY
- •ANTIBODIES, MONOCLONAL.
- •APNEA DETECTION.
- •ARRHYTHMIA, TREATMENT.
- •ARRHYTHMIA ANALYSIS, AUTOMATED
- •ARTERIAL TONOMETRY.
- •ARTIFICIAL BLOOD.
- •ARTIFICIAL HEART.
- •ARTIFICIAL HEART VALVE.
- •ARTIFICIAL HIP JOINTS.
- •ARTIFICIAL LARYNX.
- •ARTIFICIAL PANCREAS.
- •ARTERIES, ELASTIC PROPERTIES OF
- •ASSISTIVE DEVICES FOR THE DISABLED.
- •ATOMIC ABSORPTION SPECTROMETRY.
- •AUDIOMETRY
- •BACTERIAL DETECTION SYSTEMS.
- •BALLOON PUMP.
- •BANKED BLOOD.
- •BAROTRAUMA.
- •BARRIER CONTRACEPTIVE DEVICES.
- •BIOCERAMICS.
- •BIOCOMPATIBILITY OF MATERIALS
- •BIOELECTRODES
- •BIOFEEDBACK
- •BIOHEAT TRANSFER
- •BIOIMPEDANCE IN CARDIOVASCULAR MEDICINE
- •BIOINFORMATICS
- •BIOLOGIC THERAPY.
- •BIOMAGNETISM
- •BIOMATERIALS, ABSORBABLE
- •BIOMATERIALS: AN OVERVIEW
- •BIOMATERIALS: BIOCERAMICS
- •BIOMATERIALS: CARBON
- •BIOMATERIALS CORROSION AND WEAR OF
- •BIOMATERIALS FOR DENTISTRY
- •BIOMATERIALS, POLYMERS
- •BIOMATERIALS, SURFACE PROPERTIES OF
- •BIOMATERIALS, TESTING AND STRUCTURAL PROPERTIES OF
- •BIOMATERIALS: TISSUE-ENGINEERING AND SCAFFOLDS
- •BIOMECHANICS OF EXERCISE FITNESS
- •BIOMECHANICS OF JOINTS.
- •BIOMECHANICS OF SCOLIOSIS.
- •BIOMECHANICS OF SKIN.
- •BIOMECHANICS OF THE HUMAN SPINE.
- •BIOMECHANICS OF TOOTH AND JAW.
- •BIOMEDICAL ENGINEERING EDUCATION
- •BIOSURFACE ENGINEERING
- •BIOSENSORS.
- •BIOTELEMETRY
- •BIRTH CONTROL.
- •BLEEDING, GASTROINTESTINAL.
- •BLADDER DYSFUNCTION, NEUROSTIMULATION OF
- •BLIND AND VISUALLY IMPAIRED, ASSISTIVE TECHNOLOGY FOR
- •BLOOD BANKING.
- •BLOOD CELL COUNTERS.
- •BLOOD COLLECTION AND PROCESSING
- •BLOOD FLOW.
- •BLOOD GAS MEASUREMENTS
- •BLOOD PRESSURE MEASUREMENT
- •BLOOD PRESSURE, AUTOMATIC CONTROL OF
- •BLOOD RHEOLOGY
- •BLOOD, ARTIFICIAL
- •BONDING, ENAMEL.
- •BONE AND TEETH, PROPERTIES OF
- •BONE CEMENT, ACRYLIC
- •BONE DENSITY MEASUREMENT
- •BORON NEUTRON CAPTURE THERAPY
- •BRACHYTHERAPY, HIGH DOSAGE RATE
- •BRACHYTHERAPY, INTRAVASCULAR
- •BRAIN ELECTRICAL ACTIVITY.
- •BURN WOUND COVERINGS.
- •BYPASS, CORONARY.
- •BYPASS, CARDIOPULMONARY.
38.Braham R. Toward an artificial eye. IEEE Spectrum May 1996;33(5):20–21.
39.Kurzweil R. The future of intelligent technology and its impact on disabilities. J Visual Impairment Blindness Oct 2003;97(10):582–585.
Reading List
Cook AM, Hussey SM. Assistive Technologies: Principles and Practice. 2nd ed., St. Louis (MO): Mosby, Inc.; 2002. A thorough text on assistive technologies that is especially suited for the rehabilitation practitioner or those in allied health.
Smith RV, Leslie JH Jr., editors. Rehabilitation Engineering. Boca Rotan (FL): CRC Press; 1990. Contains diverse articles that should be of particular interest to practitioners in the rehabilitation field although several of the articles present definitive state-of-the-art information on rehabilitation engineering.
Webster JG, Cook AM, Tompkins WJ, Vanderheiden GC, editors. Electronic Devices for Rehabilitation. New York: John Wiley & Sons Inc. Medical; 1985. Though somewhat dated, this book offers a comprehensive overview of rehabilitation engineering and describes many of the design issues that underlie various types of assistive devices. A useful introductory text for undergraduate engineering students interested in rehabilitation.
Golledge RG. Wayfinding Behavior: Cognitive Mapping and Other Spatial Processes. Baltimore: John Hopkins University Press; 1999. A good reference that covers the cognitive issues of wayfinding behavior in blind and sighted humans.
Teodorescu HNL, Jain LC, editors. Intelligent Systems and Technologies in Rehabilitation Engineering. Boca Rotan (FL): CRC Press; 2000. A compendium of technical review articles covering intelligent technologies applied to retinal prosthesis, auditory & cochlear prostheses, upper and lower limb orthoses/ prostheses, neural prostheses, pacemakers, and robotics for rehabilitation.
Yonaitis RB. Understanding Accessibility-A Guide to Achieving Compliance on Websites and Intranets, ISBN: 1-930616-03-1, HiSoftware, 2002. This is a free booklet in electronic form for complying with the Federal government’s ‘‘Section 508’’ of the Workplace Rehabilitation Act (amendments of 1998). The book gives a brief and clear discussion of accessibility testing and how to integrate this activity into web design and related tasks.
Blasch B, Weiner W, Welsh R. Foundations of Orientation and Mobility. 2nd ed., New York: American Foundation for the Blind; 1997. A useful book for general background regarding the issues of orientation and mobility.
IEEE Spectrum, Vol. 33(5), May 1996, carries six special reports on the development of an artificial eye. Articles in this issue examine physiology of the retina, neural network signal processing, electrode array design, and sensor technology.
Journal of Visual Impairment and Blindness, Vol. 97(10), Oct. 2003, is a special issue that focused on the impact of technology on blindness.
Speech Technology, a magazine published bimonthly by AmCom Publications, 2628 Wilhite Court, Suite 100, Lexington, KY 450503. This magazine regularly covers the development and implementation of technologies that underlie speech recognition and speech generation. For example, its March/April 2005 issue contained articles on the following topics: guide to speech standards; applications of transcription; role of speech in healthcare, embedding speech into mobile devices, technology trends, new products, and speech recognition software.
See also COMMUNICATION DEVICES; ENVIRONMENTAL CONTROL; MOBILITY AIDS; VISUAL PROSTHESES.
BLOOD COLLECTION AND PROCESSING |
455 |
BLOOD BANKING. See BLOOD COLLECTION AND
PROCESSING.
BLOOD CELL COUNTERS. See CELL COUNTERS,
BLOOD.
BLOOD COLLECTION AND PROCESSING
TERESA M. TERRY
JOSEPHINE H. COX
Walter Reed Army Institute of
Research
Rockville, Maryland
INTRODUCTION
Phlebotomy may date back to the Stone Age when crude tools were used to puncture vessels to allow excess blood to drain out of the body (1). This purging of blood, subsequently known as blood letting, was used for therapeutic rather than diagnostic purposes and was practiced through to modern times. Phlebotomy started to be practiced in a more regulated and dependable fashion after the Keidel vacuum tube for the collection of blood was manufactured by Hynson, Wescott, and Dunning. The system consisted of a sealed ampoule with or without culture medium connected to a short rubber tube with a needle at the end. After insertion onto the vein, the stem of the ampoule was crushed and the blood entered the ampoule by vacuum. Although effective, the system did not become popular until evacuated blood collection systems started to be used in the mid-twentieth century. With evacuated blood collection systems came a new interest in phlebotomy and blood drawing techniques and systems. A lot of technical improvements have been made, not only are needles smaller, sharper, and sterile, they are also less painful. The improved techniques of obtaining blood samples assure more accurate diagnostic results and less permanent damage to the patient. Today, the main purpose of phlebotomy synonymous with venipuncture is to obtain blood for diagnostic testing.
Venipuncture Standards and Recent Standard Changes
The Clinical and Laboratory Standards Institute (CLSI, formerly the National Committee for Clinical Laboratory Standards, NCCLS) develops guidelines and sets standards for all areas of the laboratory (www.CLSI.org). Phlebotomy program approval as well as certification examination questions are based on these important national standards. Another agency that affects the standards of phlebotomy is the College of American Pathologists (CAP; www.CAP.org). This national organization is an outgrowth of the American Society of Clinical Pathologists (ASCP). The membership in this specialty organization is made up of board-certified pathologists only and offers, among other services, a continuous form of laboratory inspection by pathologists. The CAP Inspection and Accreditation Program do not compete with the Joint Commission on Accreditation of Health Care Organizations
456 BLOOD COLLECTION AND PROCESSING
(JCAHO) accreditation for health care facilities, because it was designed for pathology services only.
The CLSI have published the most current research and industry regulations on standards and guidelines for clinical laboratory procedures (2,3). The most significant changes to specimen collection are (1) collectors are now advised to discard the collection device without disassembling it, this reflects the Occupational Safety and Health Administration’s (OSHA) mandate against removing needles from tube holders; (2) the standard now permits gloves to be applied just prior to site preparation instead of prior to surveying the veins; (3) collectors are advised to inquire if the patient has a latex sensitivity; (4) sharp containers should be easily accessible and positioned at the point of use; (5) there is a caution recommended against the use of ammonia inhalants on fainting patients in case the patient is asthmatic; (6) collectors must attempt to locate the median cubital vein on either arm before considering alternative veins due to the proximity of the basilica vein to the brachial artery and the median nerve; (7) forbids lateral needle relocation in an effort to access the basilica vein to avoid perforating or lacerating the brachial artery; (8) immediate release of tourniquet ‘‘if possible’’ upon venous access to prevent the effects of hemoconcentration from altering test results.
The Role of the Phlebotomist Today
Professionalism. Phlebotomists are healthcare workers and must practice professionalism and abide by state and federal requirements. A number of agencies have evolved offering the phlebotomist options for professional recognition (1). Certification is a process that indicates the completion of defined academic and training requirements and the attainment of a satisfactory score on a national examination. Agencies that certify phlebotomists and the title each awards include the following: American Society of Clinical Pathologists (ASCP): Phlebotomy Technician, PBT (ASCP); American Society for Phlebotomy Technology (ASPT): Certified Phlebotomy Technician, CPT (ASPT); National Certification Agency for Medical Laboratory Personnel (NCA): Clinical Laboratory Phlebotomist (CLP) (NCA); National Phlebotomy Association (NPA); Certified Phlebotomy Technician, CPT (NPA). Licensure is defined as a process similar to certification, but at the state or local level. A license to practice a specific trade is granted through examination to a person who can meet the requirements for education and experience in that field. Accreditation and approval of healthcare training programs provides an individual with an indication of the quality of the program or institution. The accreditation process involves external peer review of the educational program, including an on-site survey to determine if the program meets certain established qualification or educational standards referred to as ‘essentials’. The approval process is similar to accreditation; however, programs must meet educational—standards and competencies—rather than essentials, and an on-site survey is not required.
Public Relations and Legal Considerations. The Patient’s Bill of Rights was originally published in 1975 by the
American Hospital Association. The document, while not legally binding, is an accepted statement of principles that guides healthcare workers in their dealings with patients. It states that all healthcare professionals, including phlebotomists, have a primary responsibility for quality patient care, while at the same time maintaining the patient’s personal rights and dignity. Two rights especially pertinent to the phlebotomist are the right of the patient to refuse to have blood drawn and the right to have results of lab work remain confidential. Right of Privacy: ‘‘An individual’s right to be let alone, recognized in all United States jurisdictions, includes the right to be free of intrusion upon physical and mental solitude or seclusion and the right to be free of public disclosure of private facts. Every healthcare institution and worker has a duty to respect a patient’s or client’s right of privacy, which includes the privacy and confidentiality of information obtained from the patient– client for purposes of diagnosis, medical records, and public health reporting requirements. If a healthcare worker conducts tests on or publishes information about a patient–client without that person’s consent, the healthcare worker could be sued for wrongful invasion of privacy, defamation, or a variety of other actionable torts.’’ In 1996, the Health Insurance Portability and Accountability Act (HIPAA) law was signed. It is a set of rules to be followed by health plans, doctors, hospitals, and other healthcare providers. Patients must be able to access their record and correct errors and must be informed of how their personal information will be used. Other provisions involve confidentiality of patient information and documentation of privacy procedures.
SAFETY
Universal Precautions
An approach to infection control that is mandated by federal and state laws is the so-called Universal Precautions. The guidelines for Universal Precautions are outlined by OSHA (www.OSHA.gov). According to the concept of Universal Precautions, all human blood and certain human body fluids are treated as if known to be infectious for human immunodeficiency virus (HIV), hepatitis B virus (HBV), and other blood borne pathogens. For blood collections, the use of needles with a safety device or a needle integrated into a safety device and the use of gloves is now mandatory in most institutions. Biohazard material should be disposed of in an appropriately labeled biohazard container. Needles and other sharp instruments should be disposed of in rigid puncture-resistant biohazard containers.
First Aid Procedures
Most phlebotomy programs require cardio pulmonary resuscitation (CPR) certification as a prerequisite or include it as part of the course and in the event of an emergency situation: basic First Aid Procedures should be performed by the phlebotomist. These procedures are not in the scope of this article and training needs to be performed by qualified experts.

BLOOD COLLECTION AND PROCESSING |
457 |
Figure 1. Basic components of the Evacuated Blood Collection System.
BLOOD COLLECTION SYSTEM AND EQUIPMENT
Blood Collection System
The components of the Evacuated Blood Collection System are shown in Fig. 1. The system consists of the following;
Plastic evacuated collection tube: The tubes are designed to fill with a predetermined volume of blood by vacuum. The rubber stoppers are color coded according to the additive that the tube contains (see Table 1). Evacuated collection devices are supplied by many vendors worldwide. These evacuated collection devices use similar color coding systems, proprietary additives, and recommended uses. Various sizes are available.
Tube holder (single use): For use with the evacuated collection system.
Needles (also available with safety device): The gauge number indicates the bore size: the larger the gauge number, the smaller the needle bore. Needles are available for evacuated systems and for use with a syringe, single draw, or butterfly system.
Additional Materials
Tourniquet: Wipe off with alcohol and replace frequently. Nonlatex tourniquets are recommended.
Table 1. Tube Guidea
|
|
Inversions |
|
|
|
at Blood |
|
Tube Top Color |
Additive |
Collectiona |
Laboratory Use |
|
|
|
|
Gold or Red/Black |
Clot activator |
5 |
Tube for serum determinations in chemistry. |
|
Gel for serum separation |
|
Blood clotting time: 30 min |
Light Green or |
Lithium heparin |
8 |
Tube for plasma determinations in chemistry |
Green/Gray |
Gel for plasma separation |
|
|
Red |
Clot activator |
5 |
Tube for serum determination in chemistry, |
|
|
|
serology, and immunohematology testing |
Orange or |
Thrombin |
8 |
Tube for stat serum determinations in |
Gray/Yellow |
|
|
chemistry. Blood clotting occurs in |
|
|
|
< 5 min |
Royal Blue |
Clot activator |
5 |
Tube for trace-element, toxicology and |
|
K2EDTA, where |
8 |
nutritional chemistry determinations. |
|
EDTA ¼ ethylenediaminetetraacetic acid |
|
|
Green |
Sodium heparin |
8 |
Tube for plasma determination in chemistry |
|
Lithium heparin |
8 |
|
Gray |
Potassium oxalate/sodium fluoride |
8 |
Tube for glucose determination. Oxalate and |
|
Sodium fluoride/Na2EDTA |
8 |
EDTA anticoagulants will give plasma |
|
Sodium fluoride (serum tube) |
8 |
samples. Sodium fluoride is the |
|
|
|
antiglycolytic agent |
Tan |
K2EDTA |
8 |
Tube for lead determination. This tube is |
|
|
|
certified to contain < 0.01 mg mL 1 lead |
Lavender |
Spray-coated K2EDTA |
8 |
Tube for whole blood hematology |
|
|
|
determination and immunohematology |
|
|
|
testing |
White |
K2EDTA with gel |
8 |
Tube for molecular diagnostic test methods |
|
|
|
such as polymerase chain reaction (PCR) |
|
|
|
and/or DNA amplification techniques. |
Pink |
Spray-coated K2EDTA |
8 |
Tube for whole blood hematology determination |
|
|
|
and immunohematology test. Designed |
|
|
|
with special cross-match label for required |
|
|
|
patient information by the AABBb |
Light Blue |
Buffered sodium citrate (3.2%) |
3 |
Tube for coagulation determinations. |
|
Citrate, theophylline, adenosine, |
|
The CTAD for selected platelet function |
|
dipyridamole (CTAD) |
|
assays and routine coagulation determination |
aReproduced from Becton Dickinson www.bd.com/vacutainer. Evacuated collection devices made by other manufacturers use similar color coding systems and additives. Recommended inversion times and directions for use are provided by each supplier.
bAABB ¼ American Association of Blood Banks.
458 BLOOD COLLECTION AND PROCESSING
Gloves: Worn to protect the patient and the phlebotomist. Nonlatex gloves are recommended.
Antiseptics–Disinfectants: 70% isopropyl alcohol or iodine wipes (used if blood culture is to be drawn).
Sterile gauze pads: For application on the site from which the needle is withdrawn.
Bandages: Protects the venipuncture site after collection.
Disposal containers: Needles should never be broken, bent, or recapped. Needles should be placed in a proper disposal unit immediately after use.
Syringe: May be used in place of evacuated collection system in special circumstances.
Permanent marker or pen: To put phlebotomist initials, time, and date of collection on tube as well as any patient identification information not provided by test order label.
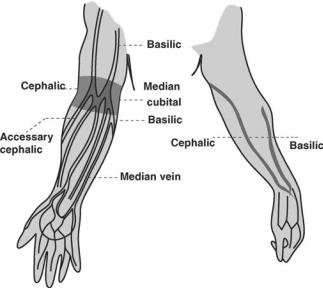
BEST SITES FOR VENIPUNCTURE
The most common sites for venipuncture are located in the antecubital (inside elbow) area of the arm (see Fig. 2). The primary vein used is the median cubical vein. The basilica and cephalic veins can be used as a second choice. Although the larger and fuller median cubital and cephalic veins of the arm are used most frequently, wrist and hand veins are also acceptable for venipuncture. Certain areas are to be avoided when choosing site such as; (1) Skin areas with extensive scars from burns and surgery (it is difficult to puncture the scar tissue and obtain a specimen); (2) the upper extremity on the side of a previous mastectomy (test results may be affected because of lymphedema); (3) site of a hematoma (may cause erroneous test results). If another site is not available, collect the specimen distal to the hematoma; (4) Intravenous therapy (IV)/blood transfusions (fluid may dilute the specimen, so
Figure 2. Venipuncture sites.
collect from the opposite arm if possible); (5) cannula/ fistula/heparin lock (hospitals have special safety and handling policies regarding these devices). In general, blood should not be drawn from an arm with a fistula or cannula without consulting the attending physician; (6) edematous extremities (tissue fluid accumulation alters test results).
ROUTINE PHLEBOTOMY PROCEDURE
Venipuncture is often referred to as ‘‘drawing blood’’. Most tests require collection of a blood specimen by venipuncture and a routine venipuncture involves the following steps Note: The following steps were written using guidelines established by the CLSI/NCCLS (3).
1. Prepare Order. The test collection process begins when the physician orders or requests a test to be performed on a patient. All laboratory testing must be requested by a physician and results reported to a physician. The form on which the test is ordered and sent to the lab is called the test requisition. The requisition may be a computer-generated form or a manual form.
2. Greet and Identify Patient. Approach the patient in a friendly, calm manner. Identify yourself to the patient by stating you name. Provide for their comfort as much as possible. The most important step in specimen collection is patient identification. When identifying a patient, ask the patient to state their name and date of birth. Outpatients can use an identification card as verification of identity. Even if the patient has been properly identified by the receptionist, the phlebotomist must verify the patient’s ID once the patient is actually called into the blood drawing area. The phlebotomist should ask for two identifiers that match the test requisition form (e.g., name and social security or name and date of birth).
3. Verify Diet Restrictions and Latex Sensitivity.
Once a patient has been identified, the phlebotomist should verify that the patient has followed any special diet instructions or restrictions. The phlebotomist should also inquire about the patients’ sensitivity to latex.
Assemble Supplies: See the section on Blood Collection System and Equipment
Position Patient
Apatientshouldbeeitherseatedorlyingdown while having blood drawn. The patient’s arm that will be used for the venipuncture should be supported firmly and extended downward in a straight line.
4. Apply Tourniquet. A tourniquet is applied to increase pressure in the veins and aid in vein selection. The tourniquet is applied 3–4 in. (7.62–10.18 cm) above the intended venipuncture site. Never leave the tourniquet in place longer than 1 min.
5. Select a Vein. Palpate and trace the path of veins in the antecubital (inside elbow) area of the arm with the index finger. Having a patient make a fist will help make the veins more prominent. Palpating will help to determine the size, depth, and direction of the vein. The main veins in the antecubital area are the median cubical, basilica, and cephalic (see the section; Best Sites for Venipucture). Select a vein that is large and well anchored.
6. Put on Gloves. Properly wash hands followed by glove application.
7. Cleanse Venipuncture Site. Clean the site using a circular motion, starting at the center of the site and moving outward in widening concentric circles. Allow the area to air dry.
8. Perform Venipuncture. Grasp patients arm
firmly to anchor the vein. Line the needle up with the vein. The needle should be inserted at a 15–308 angle BEVEL UP. When the needle enters the vein, a slight ‘‘give’’ or decrease in resistance should be felt. At this point, using a vacuum tube, slightly, with firm pressure, push the tube into the needle holder. Allow tube to fill until the vacuum is exhausted and blood ceases to flow to assure proper ratio of additive to blood. Remove the tube, using a twisting and pulling motion while bracing the holder with the thumb. If the tube contains an additive, mix it immediately by inverting it 5–10 times before putting it down.
9. Order of Draw. Blood tubes are drawn in a particular order to ensure integrity of each sample by lessening the chances of anticoagulants interference and mixing. The order of draw also provide a standardized method for all laboratories (3,4).
Blood Cultures: With sodium polyanethol sulfonate anticoagulant and other supplements for bacterial growth.
Light Blue: Citrate Tube (Note: When a citrate tube is the first specimen tube to be drawn, a discard tube should be drawn first). The discard tube should be a nonadditive or coagulation tube.
Gold or Red/Black: Gel Serum Separator Tube, no additive.
Red: Serum Tube, no additive. Green: Heparin Tube.
Light Green or Green/Gray: Gel Plasma Separator Tube with Heparin.
Lavender: EDTA Tube.
Gray: Fluoride (glucose) Tube.
10. Release the Tourniquet. Once blood begins to
flow the tourniquet may be released to prevent hemoconcentration.
11. Place the Gauze Pad. Fold clean gauze square in half or in fourths and place it directly over the needle without pressing down. Withdraw the needle in one smooth motion, and immediately apply pressure to the site with a gauze pad for 3–5 min, or until the bleeding has stopped. Failure to apply pressure
BLOOD COLLECTION AND PROCESSING |
459 |
will result in leakage of blood and hematoma formation. Do not bend the arm up, keep it extended or raised.
12. Remove and Dispose of the Needle. Needle should be disposed of immediately by placing it and the tube holder in the proper biohazard sharps container. Dispose of all other contaminated materials in proper biohazard containers.
13. Bandage the Arm. Examine the patients arm to assure that bleeding has stopped. If bleeding has stopped, apply an adhesive bandage over the site.
14. Label Blood Collection Tubes. Specimen tube labels should contain the following information: patient’s full name, patient’s ID number, date, time, and initials of the phlebotomist must be on each label of each tube.
15. Send Blood Collection Tube to be Processed.
Specimens should be transported to the laboratory processing department in a timely fashion. Some tests may be compromised if blood cells are not separated from serum or plasma within a limited time.
SPECIMEN PROCESSING
Processing of blood is required in order to separate out the components for screening, diagnostic testing, or for therapeutic use. This section will concentrate primarily on processing of blood for screening purposes and diagnostic testing. An overview of the main blood processing procedures, specimen storage, and common uses for each of the components is provided in Table 2. Because there are many different blood components and many different end uses for these components, the list is not comprehensive and the reader should refer to other specialized literature for further details. The OSHA regulations require laboratory technicians to wear protective equipment (e.g., gloves, labcoat, and protective face gear) when processing specimens. Many laboratories mandate that such procedures are carried out in biosafety cabinets.
Whole Blood Processing
Because whole blood contains all but the active clotting components, it has the ability to rapidly deteriorate and all blood components are subject to chemical, biological, and physical changes. For this reason, whole blood has to be carefully handled and any testing using whole blood has to be performed as soon as possible after collection to ensure maximum stability. Whole blood is typically used for the complete blood count (CBC). The test is used as a broad screening test to check for such disorders as anemia, infection, and many other diseases (www.labtestsonline.org). The CBC panel typically includes measurement of the following: white blood, platelet and red blood cell count, white blood cell differential and evaluation of the red cell compartment by analysis of hemoglobin and hematocrit, red cell distribution width and mean corpuscular volume, and mean corpuscular hemoglobin. The CBC assays are now routinely performed with automated

460 |
BLOOD COLLECTION AND PROCESSING |
|
|
|
|
Table 2. Blood Processing Procedures and Specimen Storage |
|
|
|||
|
|
|
|
|
|
Component |
Processing |
Short-Term Storage |
Long-Term Storage |
Uses |
|
|
|
|
|
|
|
Red blood cells |
Gravity and/or centrifigation |
1 month at 4 8C |
Frozen up to 10 years |
Transfusion |
|
Plasma |
|
Gravity and/or centrifigation |
Use immediately |
Frozen up to 7 years |
Serology, diagnostics, |
|
|
|
|
|
immune monitoring |
|
|
|
|
|
source of biologics |
Serum |
|
Clotting and centrifugation |
Use immediately |
Frozen up to 7 years |
Serology, diagnostics, |
|
|
|
|
|
immune monitoring |
|
|
|
|
|
source of biologics |
Platelets |
|
Plasma is centrifuged to |
Five days at room |
Cannot be |
Transfusion |
|
|
enrich for platelet fraction |
temperature |
cryopreserved |
|
Granulocytes |
Centrifigation and separation |
Use within 24 h |
Cryopreserved in |
Transfusion |
|
|
|
from red blood cells |
|
liquid nitrogena |
|
Peripheral blood |
Ficoll–hypaque separation |
Use immediately |
Cryopreserved in |
Immune monitoring, |
|
mononuclear cells |
|
|
liquid nitrogena |
specialized expansion |
|
|
|
|
|
|
and reinfuison |
Albumin, immune globulin, |
Specialized processing, |
Not applicable |
Variable |
Multiple therapeutic |
|
specific immune globulins, |
fractionation and |
|
|
uses |
|
and clotting factor |
separation |
|
|
|
|
concentrates |
|
|
|
|
|
aAlthough it has been shown that cells can be stored indefinitely in liquid nitrogen, the functionality of the cells would have to be assessed and storage lengths determined for each type of use proposed.
analyzers in which capped evacuated collection devices are mixed and pierced through the rubber cap. Whole blood drawn in EDTA (lavender) tubes are usually used, although citrate (blue top) vacutainers will also work (although the result must be corrected because of dilution). Blood is sampled and diluted, and moves through a tube thin enough that cells pass by one at a time. Characteristics about the cell are measured using lasers or electrical impedance. The blood is separated into a number of different channels for different tests.
The CBC technology has expanded in scope to encompass a whole new field of diagnostics, namely, analytical cytometry. Analytical cytometry is a laser-based technology that permits rapid and precise multiparameter analysis of individual cells and particles from within a heterogeneous population of blood or tissues. Analytical cytometry is now routinely used for diagnosis of different pathological states. This technique can be used to examine cell deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) content, cell-cycle distribution, cellular apoptosis, tumor ploidy, cell function measurements (i.e., oxidative metabolism, phagocytosis), cellular biochemistry (i.e., intracellular pH, calcium mobilization, membrane potential, microviscosity, glutathione content), and fluorescence image analysis of individual blood cells. Since the blood is truly a window on what happens in the body, it is possible to use blood samples for a wide array of diagnostic and research purposes.
A second important use for whole blood is in the setting of HIV infection and treatment as a way to monitor CD4 T cell counts and percentages. These single or multiplatform tests use fresh whole blood with EDTA as anticoagulant (< 18 h after collection) samples are run with or without lysis of red cells and fixation of the lymphocytes. There are several different companies that make specialized equipment for enumeration of CD4 cell counts, the basic principle of which is to use a fluores-
cently tagged anti CD4 cell surface marker. The results for the CD4 or other subset are expressed as a percentage of total gated lymphocytes. In order to determine the absolute CD4 cell counts, the percent CD4 must be multiplied by an absolute lymphocyte count derived from a hematology analyzer or by an integrated volumetric analysis method (5–7).
Another common use for whole blood is for the detection of secretion of cytokines from antigen or mitogen stimulated lymphocytes. This assay can provide information on a patients T cell response to pathogens [e.g., cytomegalo virus (CMV) and Epstein Ban virus (EBV), HIV, and tuberculosis (TB)]. The technique can also be used to monitor vaccine induced responses or responses to immunotherapy. Whole blood is drawn into heparin, 0.5–1 mL of blood is stimulated with antigen of interest and costimulatory antibodies in the presence of Brefeldin-A. The latter inhibits transport of proteins from the Golgi so that secreted cytokines accumulate inside the cell. The samples
are |
incubated |
at |
37 8C for |
6 h, after which they can |
be |
placed at |
4 8C |
overnight |
or processed immediately. |
The samples are treated with EDTA to reduce clumping, red cells are lysed, and the sample is fixed by addition of paraformaldehyde. At this stage, the samples can be stored frozen for up to 4 months prior to detection of cell surface markers and intracellular cytokines by flow cytometry (8).
Serum Processing
Because of the ease of performing serum separation and the fact that so many tests rely on the use of serum, the technique has become routine in clinical and diagnostic laboratories. Specimens are drawn into tubes that contain no additives or anticoagulants (Table 1). Two commonly used tubes are the red serum collection tubes or commercially available serum separation tubes. Serum is obtained
by drawing the blood into a red top or the serum separator tube, allowing it to clot, and centrifuging to separate the serum. The time allowed for clotting depends on the ambient temperature and the patient sample. The typical recommendation is to allow the tube to clot for 20–30 min in a vertical position. A maximum of 1 h should suffice for all samples except those from patients with clotting disorders. Once the clot has formed, the sample is centrifuged for a recommended time of 10 min at 3000 revolutions per minute (rpm). The serum is transferred into a plastic transport tube or for storage purposes into a cryovial. Many tests collected in the serum separator tubes do not require transferring the supernatant serum unless the serum is to be stored frozen. Specimens transported by mail or stored > 4 h should be separated from the clot and placed into a transport tube. Polypropylene plastic test tubes or cryovials are more resistant to breakage than most glass or plastic containers, especially when specimens are frozen. Caution needs to be observed with serum separator tubes for some tests since the analyte of interest may absorb to the gel barrier. Erroneous results may be obtained if the serum or plasma is hemolyzed, lipemic, or icteric. As eloquently described by Terry Kotrla, phlebotomist at Austin Community College these conditions cause specimen problems. (www.austin.cc.tx.us/ kotrla/ PHBLab15SpecimenProcessingSum03.pdf).
1. Hemolysis is a red or reddish color in the serum or plasma that will appear as a result of red blood cells rupturing and releasing the hemoglobin molecules. Hemolysis is usually due to a traumatic venipuncture (i.e., vein collapses due to excessive pressure exerted with a syringe, ‘‘ digging’’ for veins, or negative pressure damages innately fragile cells. Gross hemolysis (serum or plasma is bright red) affects most lab tests performed and the specimen should be recollected. Slight hemolysis (serum or plasma is lightly red) affects some tests, especially serum potassium and LDH (lactate dehydrogenase). Red blood cells contain large amounts of both of these substances and hemolysis will falsely elevate their measurements to a great extent. In addition to hemolysis caused during blood draw procedures, blood collection tubes (for serum and or whole blood) that are not transported correctly or in a timely fashion to the processing laboratory may be subject to hemolysis. Extremes of heat and cold in particular can cause red blood cells to lyze and sheering stresses caused by shaking of the specimens during transport may cause lysis. Finally, incorrect centrifugation temperatures and speeds may cause hemolysis of red blood cells.
2. Icterus. Serum or plasma can be bright yellow or even brownish due to either liver disease or damage or excessive red cell breakdown inside the body. Icterus can, like hemolysis, affect many lab tests, but unfortunately, recollection is not an option since the coloration of the serum or plasma is due to the patient’s disease state.
BLOOD COLLECTION AND PROCESSING |
461 |
3. Lipemia. Occasionally, serum or plasma may appear milky. Slight milkiness may be caused when the specimen is drawn from a nonfasting patient who has eaten a heavy meal. A thick milky appearance occurs in rare cases of hereditary lipemia.
Both for serum and plasma there are documented guidelines for specimen handling dependent on which analyte, is being examined. The kinds of tests that can be done on blood samples is ever expanding and includes allergy evaluations, cytogenetics, cytopathology, histopathology, molecular diagnostics, tests for analytes, viruses, bacteria, parasites, and fungi. Incorrect preparation, shipment, and storage of specimens may lead to erroneous results. The guidelines for preparing samples can be obtained from the CLSI (9). Diagnostic testing laboratories (e.g., Quest diagnostics) provide comprehensive lists of the preferred specimen type, transport temperature, and rejection criteria (www.questest.com).
Plasma Processing
Specimens are drawn into tubes that contain anticoagulant (Table 1.). The plasma is obtained by drawing a whole blood specimen with subsequent centrifugation to separate the plasma. Plasma can be obtained from standard blood tubes containing the appropriate anticoagulant or from commercially available plasma separation tubes. The plasma separation tubes combine spray-dried anticoagulants and a polyester material that separates most of the erythrocytes and granulocytes, and some of the lymphocytes and monocytes away from the supernatant. The result is a convenient, safe, single-tube system for the collection of whole blood and the separation of plasma. Samples can be collected, processed, and transported in situ thereby reducing the possibility of exposure to bloodborne pathogens at the collection and sample processing sites. One drawback is that plasma prepared in a plasma separation tube may contain a higher concentration of platelets than that found in whole blood. For plasma processing, after drawing the blood, the tube for plasma separation must be inverted five to six times to ensure adequate mixing and prevent coagulation. The recommended centrifugation time is at least 10 min at 3000 rpm. Depending on the tests required, plasma specimens may be used immediately, shipped at ambient or cooled temperatures, or may require freezing. The plasma is transferred into a plastic transport tube or for storage purposes into a cryovial. Some tests require platelet poor plasma, in which case the plasma is centrifuged at least two times.
Processing and Collection of Peripheral Blood Mononuclear Cells (PBMC) from Whole Blood
Peripheral blood mononuclear cells are a convenient source of white blood cells, T cells comprise 70% of the white cell compartment and are the work-horses of the immune system. These T cells play a crucial role in protection from or amelioration of many human diseases and can keep tumors in check. The most readily accessible source of T

462 BLOOD COLLECTION AND PROCESSING
cells is the peripheral blood. Thus collection, processing, cryopreservation, storage, and manipulation of human PBMC are all key steps for assessment of vaccine and disease induced immune responses. The assessment of T cell function in assays may be affected by procedures beginning with the blood draw through cell separation, cryopreservation, storage, and thawing of the cells prior to the assays. Additionally, the time of blood collection to actual processing for lymphocyte separation is critical. Procedures for PBMC collection and separation are shown in Table 3 along with potential advantages and disadvantages.
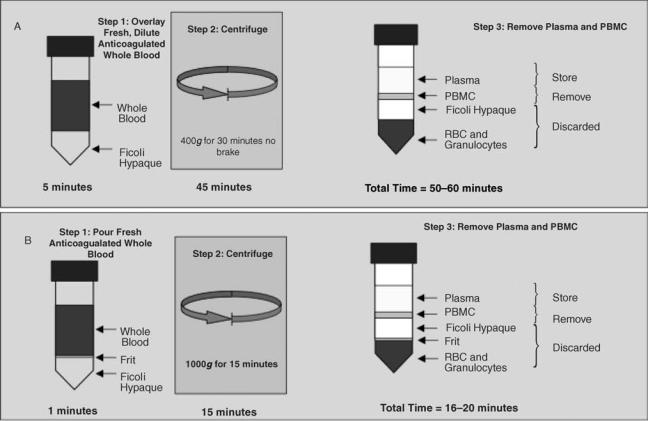
When conducting cellular immunology assays, the integrity of the PBMC, especially the cellular membranes, is critical for success. A correct cellular separation process yields a pure, highly viable population of mononuclear cells consisting of lymphocytes and monocytes, minimal red blood cell and platelet contamination, and optimum functional capacity. The standard method for separation of PBMC is the use of Ficoll-hypaque gradients as originally described by Boyum in 1968 (10). A high degree of technical expertise is required to execute the procedure from accurate centrifuge rpm and careful removal of the cellular interface to avoid red cell contamination. Within the last 10 years, simplified separation ficoll procedures have largely replaced the standard ficoll method, two such procedures are outlined below (Adapted from Ref. 11) and in Fig. 3. The simplicity of these methods, superior technical reliability, reduced interperson variability, faster turnaround, and higher cell yields makes these the methods of choice.
The Cell Preparation Tube (CPT) method is described below and in greater detail in literature provided by Becton Dickinson (http://www.bd.com/vacutainer/products/molecular/citrate/procedures.asp). Vacutainer cell preparation tubes (VACUTAINER CPT tubes, Becton Dickinson) provide a convenient, single-tube system for the collection and separation of mononuclear cells from whole blood. The CPT tube is convenient to use and results in high viability of the cells after transportation. The blood specimens in the tubes can be transported at ambient temperature, as the gel
forms a stable barrier between the anticoagulated blood and ficoll after a single centrifuge step. Cell separation is performed at the processing–storage laboratory using a single centrifugation step. This reduces the risk of sample contamination and eliminates the need for additional tubes and processing reagents. In many instances, and in particular when biosafety level 2 (BL2) cabinets are not available on site, the CPT method is useful because the centrifugation step can be done on site and the remaining processing steps can be performed after shipment to a central laboratory within the shortest time possible, optimally within 8 h. The central laboratory can complete cell processing in a BL2 cabinet and set up functional assays or cryopreserve the samples as needed.
Centrifuge speed is critical for PBMC processing. The centrifugal force is dependent on the radius of the rotation of the rotor, the speed at which it rotates, and the design of the rotor itself. Centrifugation procedures are given as xg measures, since rpm and other parameters will vary with the particular instrument and rotor used. The rpms may be calculated using the following formula where r ¼ radius of rotor g ¼ gravity; g ¼ 1.12 r (rpm/1000)2. This conversion can be read-off a nomogram chart available readily online or in centrifuge maintenance manuals. Typically laboratory centrifuges can be programmed to provide the correct rpm.
Protocol 1. Separation of PBMC Using CPT Tubes
1. Materials and Reagents: Vacutainer CPT tubes (Becton Dickinson); Sterile Phosphate Buffered Saline (PBS) without Caþ and Mgþ, supplemented with antibiotics (Penicillin and Streptomycin); Sterile RPMI media containing 2% fetal bovine serum (FBS) and supplemented with antibiotics.
The CPT tubes are sensitive to excessive temperature fluctuations, resulting in deterioration of the gel and impacting successful cellular separation. This problem is particularly serious in tropical countries where ambient storage temperatures may be > 25 8C. Following PBMC separation, one
Table 3. Stages and Variables in the Separation of PBMC from Whole Blood
Procedure/Technology |
Alternatives |
Advantages |
Disadvantages |
|
|
|
|
PBMC collection |
Heparin |
Greater cellular stability than EDTA |
Impacts DNA isolation. Plasma from |
|
|
|
whole blood cannot be used for PCR |
|
|
|
based assaysa |
|
EDTA |
|
Time dependent negative impact on |
|
|
|
T cell responses |
|
Sodium Citrate |
Greater cellular stability than EDTA |
|
PBMC separation |
Standard Ficoll |
|
Technically challenging |
|
|
|
Time consuming |
|
CPT |
Rapid |
Subject to temperature fluctuations |
|
|
Technically easy and less inter-person |
manifested by gel deterioration and |
|
|
variability |
contamination in PBMC fraction. |
|
|
Blood is drawn into same tube |
|
|
|
that is used for separation |
|
|
Accuspin/Leucosep |
Rapid |
|
|
|
Technically easy and less inter-person |
|
|
|
variability |
|
aThe inhibitory effects of heparin on DNA isolation can be removed by incubation of plasma or other specimens with silicon glass beads or by heparinase treatment prior to DNA extraction.
BLOOD COLLECTION AND PROCESSING |
463 |
Figure 3. Gradient separation of peripheral blood mononuclear lymphocytes (PBMC). (a) The standard ficoll gradient method. (b) The Accuspin or Leucosep method. RBC ¼ red blood cells, g ¼ gravity. (Graphic courtesy of Greg Khoury and Clive Gray, National Institute for Communicable Diseases, Johannesburg, South Africa.)
may macroscopically observe the presence of gel spheres in the cellular layer, which are very difficult to distinguish from the actual PBMC. This has been observed after storage at temperatures > 25 8C. Where possible, the tubes should be stored at no > 25 8C. Once the tubes have blood drawn into them, an attempt should be made to keep them at temperatures of between 18 and 25 8C. Blood filled CPT should under no circumstances be stored on ice or next to an ice pack. It is recommended that they are separated from any ice-packs by bubble wrap or other type of insulation within a cooler so that the temperature fluctuations are kept to a minimum.
2. Method: (a) Specimens should be transported to the laboratory as soon as possible after collection. The manufacturer recommends the initial centrifugation to separate the lymphocytes be within 2 h. The samples may then be mixed by inversion and the processing completed preferably within 8 h after centrifugation. If there is a significant time delay, the specimens should be put into a cooler box and transported at room temperature (18–25 8C). (b) Spin tubes at room temperature (18–25 8C) in a horizontal rotor (swinging bucket head) for a mini-
mum of 20 min and maximum of 30 min at 400 g. The brake is left off to assure that the PBMC layer is not jarred or disturbed while the centrifuge rotors are being mechanically halted. (c) Remove the tubes from the centrifuge and pipete the entire contents of the tube above the gel into a 50 mL tube. This tube will now contain both PBMC and undiluted plasma. An additional centrifugation step will allow removal of undiluted plasma if desired. Wash each CPT tube with 5 mL of PBS/1% Penicillin/Streptomycin (Pen/ Strep). This wash step will remove cells from the top of the gel plug. Combine with cells removed from tube. This wash increases yield of cells by as much as 30–40%. (d) Spin down this tube at 300 g for 15–20 min at room temperature with the brake on. (e) The PBMC pellet is resuspended in RPMI, 2% FBS and washed one more time to remove contaminating platelets. The PBMC are counted and cryopreserved or used as required.
3. Separation of PBMC Using Accuspin or Leucosep Tubes. More recently, the Leucosep and Accuspin tube have become available. Further information on the Leucosep is available at www.gbo.com and for the Accuspin at www.sigmaaldrich.com The principle of these tubes is the
464 BLOOD COLLECTION AND PROCESSING
same. The tube is separated into two chambers by a porous barrier made of highly transparent polypropylene (the frit). This biologically inert barrier allows elimination of the laborious overlaying of the sample material over Ficoll. The barrier allows separation of the sample material added to the top from the separation medium (ficoll added to the bottom). Figure 3 shows a comparison of the standard ficoll method and the Accuspin or Leucosep method. The tubes are available in two sizes and may be purchased with or without Ficoll. There is an advantage of buying the tubes without Ficoll because they can be stored at room temperature rather than refrigerated. This may be an important problem if cold space is limiting or cold chain is difficult. The expiration date of the Ficoll will not affect the tube expiration. The following procedure describes the separation procedure for Leucosep tubes that are not prefilled with Ficoll-hypaque. The Accuspin procedure is virtually identical. Note that whole blood can be diluted 1:2 with balanced salt solution. While this dilution step is not necessary, it can improve the separation of PBMC and enhance PBMC yield. The procedure is carried out using aseptic technique.
Protocol 2: Separation of PBMC Using Accuspin or Leucosep Tubes
1. Warm-up the separation medium (Ficoll-hypaque) to room temperature protected from light.
2. Fill the Leucosep tube with separation medium: 3 mL for the 14 mL tube and 15 mL for the 40 mL tube.
3. Close the tubes and centrifuge at 1000 g for 30 s at room temperature.
4. Pour the whole blood or diluted blood into the tube: 3–8 mL for the 14 mL tube and 15–30 mL for the 50 mL tube.
5. Centrifuge for 10 min at 1000 g or 15 min at 800 g in a swinging bucket rotor, with the centrifuge brake off. The brake is left off to assure that the PBMC layer is not jarred or disturbed while the centrifuge rotors are being mechanically halted.
6. After centrifugation, the sequence of layers from top to bottom should be plasma and platelets; enriched PBMC fraction; Separation medium; porous barrier; Separation medium; Pellet (erythrocytes and granulocytes).
7. Plasma can be collected to within 5–10 mm of the enriched PBMC fraction and further processed or stored for additional assays.
8. Harvest the enriched PBMC and wash with 10 mL of PBS containing 1% Pen/Strep and centrifuge at 250 g for 10 min.
9. The PBMC pellet is resuspended in RPMI, 2% FBS and washed one more time to remove contaminating platelets. The PBMC are counted and cryopreserved or used as required.
1. Specimen Rejection Criteria
Incomplete or inaccurate specimen identification. Inadequate volume of blood in additive tubes (i.e.,
partially filed coagulation tube) can lead to inappropriate dilution of addition and blood.
Hemolysis (i.e., potassium determinations) Specimen collected in the wrong tube (i.e., end pro-
duct is serum and test requires plasma). Improper handling (i.e., specimen was centrifuged
and test requires whole blood).
Insufficient specimen or quantity not sufficient (QNS). For PBMC, the rejection criteria are not usually evaluated at the time of draw due to the complexity of the tests performed. However, a minimum of 95% viability would be expected after PBMC separation unless the specimens have been subjected to heat or other adverse conditions (see note below).
The optimal time frame between collection of blood sample to processing, separation and cryopreservation of PBMC should be < 8 h or on the same day as collection. It is not always feasible to process, separate and cryopreserve PBMC within 8 h when samples are being shipped to distant processing centers. Under these conditions, PBMC left too long in the presence of anticoagulants or at noncompatible temperatures, adversely affect PBMC function and causes changes which affect the PBMC separation process (11).
There have been significant revisions to the procedures for the handling and processing of blood specimens; specimens for potassium analysis should not be recentrifuged because centrifugation may cause results to be falsely increased; the guidelines recommend that serum or plasma exposed to cells in a bloodcollection tube prior to centrifugation should not exceed 2 h; storage recommendations for serum– plasma may be kept at room temperature up to 8 h, but for assays not completed within 8 h, refrigeration is recommended (2–8 8C), if the assay is not completed within 48 h serum–plasma should be frozen at or below 20 8C.
2. Disclaimer. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
BIBLIOGRAPHY
Cited References
1.McCall RE, Tankersley CM. Phlebotomy Essentials. Philadelphia: J.B. Lippincott; 1993.
2.Ernst DJ, Szamosi DI. 2005. Medical Laboratory Observer Clinical Laboratory, Specimen-collection standards complete major revisions, Available at www.mlo-online.com, Accessed 2005 Feb.
3.Arkin CF, et al. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture; CLSI (NCCLS) Approved
