
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

302 Anticancer Drugs
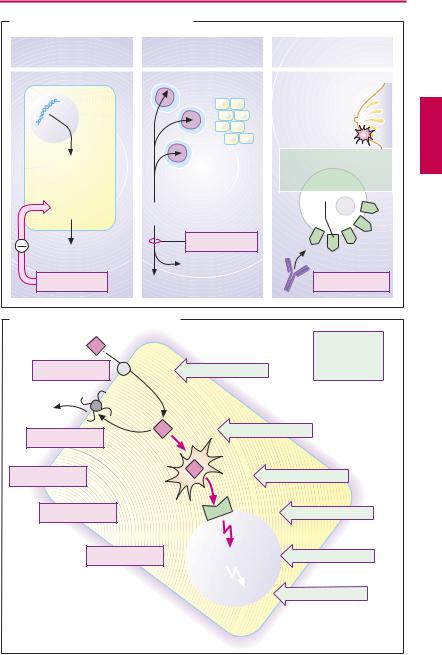
Targeting of Antineoplastic Drug Action (A)
When degenerating neoplastic cells display special metabolic properties which are different from those of normal cells, targeted pharmacotherapeutic intervention becomes possible.
Imatinib. Chronic myelogenous leukemia (CML) results from a genetic defect in the hematopoietic stem cells of the bone marrow. Nearly all CML patients possess the
Philadelphia chromosome. It results from translocation between chromosomes 9 and 22 of the c-abl protooncogene, leading to the hybrid bcr-abl fusion gene on chromosome 22. The recombinant gene encodes a tyrosine kinase mutant with unregulated (constitutive), enhanced activity that promotes cell proliferation. Imatinib is a tyrosine kinase inhibitor that specifically affects this mutant but also interacts with some other kinases. It can be used orally in Philadelphia chromo- some-positive CML.
Asparaginase cleaves the amino acid asparagine into aspartate and ammonia. Certain cells, in particular the tumor cells in acute lymphatic leukemia, require asparagine for protein synthesis and must take it up from the extracellular space, whereas many other cell types are themselves able to synthesize asparagine. Supply of the amino acid can be disrupted by administration of the asparagine-hydrolyzing enzyme. Consequently, protein synthesis and proliferation of neoplastic cells are inhibited. Asparaginase is obtained from E. coli bacteria or may be of plant origin (Erwinia chrysanthemi), when it is also named crisantaspase. Allergic reactions against the exogenous protein occur after parenteral administration.
Trastuzumab exemplifies a growing number of monoclonal antibodies that have become available for antineoplastic therapy. These are directed against cell surface proteins that are strongly expressed by cancer cells. Trastuzumab binds to HER2, the recep-
tor for epidermal growth factor. The density of this receptor is greatly increased in some types of breast cancer. When the tumor cells have bound antibody, immune cells can recognize them as elements to be eliminated. Trastuzumab is indicated in advanced cases under certain conditions. The antibody is cardiotoxic; it is likely that cardiomyocytes also express HER2.
Mechanisms of Resistance to Cytostatics (B)
Initial success can be followed by loss of effect because of the emergence of resistant tumor cells. Mechanisms of resistance are multifactorial.
Diminished cellular uptake may result from reduced synthesis of a transport protein that may be needed for membrane penetration (e.g., methotrexate).
Augmented drug extrusion: increased synthesis of the P-glycoprotein that extrudes drugs from the cell (e.g., anthracyclines, vinca alkaloids, epipodophyllotoxins, and paclitaxel) is responsible for multidrug resistance (mdr1 gene amplification).
Diminished bioactivation of a prodrug, e.g., cytarabine, which requires intracellular phosphorylation to become cytotoxic.
Change in site of action: e.g., increased synthesis of dihydrofolate reductase may occur as a compensatory response to methotrexate.
Damage repair: DNA repair enzymes may become more ef cient in repairing defects caused by cisplatin. Inhibition of apoptosis due to activation of antiapoptotic cellular mechanisms.
|
Targeting of Antineoplastic Drugs |
303 |
||
A. Targeting of antineoplastic drug action |
|
|
||
Chronic myelogenous |
Acute lymphatic leukemia |
Breast carcinoma |
|
|
leukemia |
|
|
|
|
|
Normal cells |
in 1/4 of cases: |
|
|
Philadelphia |
|
|
||
|
Overexpression |
|
||
chromosome |
|
|
||
|
of HER2 |
|
||
|
|
|
||
Tyrosine |
Endogenous |
H Human |
|
|
synthesis |
E Epidermal growth factor |
|||
kinase |
||||
of L-asparagine |
|
|||
mutant with |
|
|||
|
R Receptor |
|
||
constitutively |
|
|
||
|
|
|
||
enhanced |
Uptake of |
|
|
|
activity |
|
|
||
|
L-asparagine |
|
|
|
Cell proliferation |
Asparaginase |
|
|
|
|
|
|
||
|
NH3 |
|
|
|
Imatinib |
L-Aspartate |
Trastuzumab |
|
|
B. Mechanisms of cytostatic resistance |
|
|
||
Cytostatic drug |
|
Mutation |
|
|
|
|
and selection |
|
|
|
|
of resistant |
|
|
Uptake |
Decrease |
cells |
|
|
Efflux pumping |
|
Increase |
|
|
|
|
|
||
Bioactivation |
|
Decrease |
|
|
Site of action |
|
Change |
|
|
Effect |
Damage |
Repair |
|
|
|
Apoptosis |
Inhibition |
|
|

304 Immune Modulators
Inhibition of Immune Responses
Both the prevention of transplant rejection and the treatment of autoimmune disorders call for a suppression of immune responses. However, immune suppression also entails weakened defenses against infectious pathogens and a long-term increase in the risk of neoplasms.
A specific immune response begins with the binding of antigen by lymphocytes carrying specific receptors with the appropriate antigen-binding site. B-lymphocytes “recognize” antigen surface structures by means of membrane receptors that resemble the antibodies formed subsequently. T-lymphocytes (and naive B cells) require the antigen to be presented on the surface of macrophages or other cells in conjunction with the major histocompatibility complex (MHC); the latter permits recognition of antigenic structures by means of the T-cell receptor. T-help- er (TH) cells carry adjacent CD3 and CD4 complexes, cytotoxic T cells a CD8 complex. The CD proteins assist in docking to the MHC. Besides recognition of antigen, stimulation by cytokines plays an essential part in the activation of lymphocytes. Interleukin-1 is formed by macrophages, and various interleukins (IL), including IL-2, are made by T- helper cells. As antigen-specific lymphocytes proliferate, immune defenses are set into motion.
I. Interference with antigen recognition.
Muromonab CD3 is a monoclonal antibody directed against mouse CD3 that blocks antigen recognition by T-lymphocytes (use in graft rejection).
Glatirameracetate consists of peptides of varying lengths, polymerized in random sequence from the amino acids glutamine, lysine, alanine, and tyrosine. It can be used in the treatment of multiple sclerosis besides β- interferon. This disease is caused by a T-lym- phocyte-mediated autoaggression directed against oligodendrocytes that form myelin sheaths of CNS axons. The culprit antigen
appears to be myelin basic protein. Glatiramer resembles the latter; by blocking antigen receptors, it interferes with antigen recognition by lymphocytes.
II. Inhibition of cytokine production and action. Glucocorticoids modulate the expression of numerous genes; thus, the production of IL-1 and IL-2 is inhibited, which explains the suppression of T-cell-depend- ent immune responses. In addition, glucocorticoids interfere with inflammatory cytokines and signaling molecules at various other sites. Glucocorticoids are used in organ transplantations, autoimmune diseases, and allergic disorders. Systemic use carries the risk of iatrogenic Cushing syndrome (p.244).
Ciclosporin and related substances inhibit the production of cytokines, in particular interleukin-2. In contrast to glucocorticoids, the plethora of accompanying metabolic effects is absent (see p.306 for more details).
Daclizumab and basiliximab are monoclonal antibodies against the receptor for IL- 2. They consist of murine Fab fragments and a human Fc-segment. They are used to suppress transplant rejection reactions.
Anakinra is a recombinant form of an endogenous antagonist at the interleukin-1 receptor; it is used in rheumatoid arthritis (p.332).
III. Disruption of cell metabolism with inhibition of proliferation. At dosages below those needed to treat malignancies, some cytostatics are also employed for immunosuppression; e.g., azathioprine, methotrexate, and cyclophosphamide. The antiproliferative effect is not specific for lymphocytes and involves both T and B cells.
Mycophenolate mofetil has a more specific effect on lymphocytes than on other cells. It inhibits inosine monophosphate dehydrogenase, which catalyzes purine synthesis in lymphocytes. It is used in acute tissue rejection responses.

Inhibition of Immune Responses |
305 |
A. Immune reaction and immunosuppressives |
|
|
|
||
Antigen |
Macrophage |
|
Virus-infected cell, |
Glucocorticoids |
|
|
|
|
transplanted cell. |
|
|
|
|
|
tumor cell |
|
|
|
|
|
|
|
Inhibition of |
|
Phagocytosis |
Synthesis of |
|
cytokine |
|
|
|
synthesis, |
|||
|
Degradation |
"foreign" proteins |
|||
|
e. g., |
||||
|
Presentation |
Presentation |
|
||
|
|
|
|||
|
|
|
|
|
IL-1 IL-2 |
|
MHC II |
|
MHC I |
|
|
|
|
IL-1 |
|
|
|
|
|
|
T-cell |
|
Muromonab- |
|
|
|
|
CD3 |
|
|
|
|
receptor |
|
|
|
|
|
|
|
|
|
CD4 |
CD3 |
CD8 |
CD3 |
monoclonal |
MHC II |
antibody |
||||
|
|||||
Uptake |
T-Helper- |
|
|
|
|
Degradation |
|
|
|
||
Presentation |
cell |
|
|
|
Calcineurin |
|
|
|
|
|
inhibitors |
B-Lymphocyte |
Interleukins |
IL-2 |
T-Lymphocyte |
Inhibition of |
|
|
|
|
|
|
|
|
|
|
|
|
cytokine |
|
|
|
|
|
synthesis |
|
|
|
|
|
IL-2 |
|
|
|
|
|
Daclizumab |
Proliferation |
|
|
|
|
Basiliximab |
and |
|
|
|
|
IL-2 receptor |
|
|
|
|
blockade |
|
differentiation |
|
|
|
|
|
into plasma cells |
|
|
|
|
|
|
|
|
|
|
Sirolimus |
|
|
|
|
|
Suppression |
|
|
|
|
|
of IL-2 effect |
|
|
|
Cytotoxic |
|
|
|
|
|
T-lymphocytes |
|
|
|
|
|
|
|
Cytotoxic |
|
|
|
|
|
antiproliferative |
|
Lymphokines |
|
|
substances |
|
|
|
|
|
||
|
Chemotaxis |
|
|
|
Azathioprine |
|
|
|
|
|
|
|
|
|
|
|
Methotrexate |
|
|
|
|
|
Cyclo- |
|
Immune reaction: |
Elimination of |
|
phosphamide |
|
Antibody-mediated |
delayed |
|
|
Mycophenolate |
|
immune reaction |
hypersensitivity |
“foreign” cells |
|
mofetil |
|

306 Immune Modulators
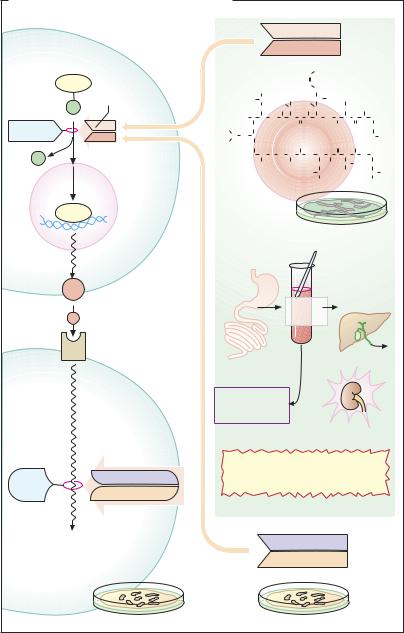
IV. Anti-T-cell immune serum is obtained from animals immunized with human T- lymphocytes. The antibodies bind to and damage T cells and can thus be used to attenuate tissue rejection.
Ciclosporin is of fungal origin; it is a cyclic peptide composed of 11, in part atypical, amino acids. Therefore, orally administered ciclosporin is not degraded by gastrointestinal proteases. In T-helper cells, it inhibits the production of interleukin-2 by interfering at the level of transcriptional regulation. Normally, “nuclear factor of activated T cells,” (NFAT) promotes the expression of interleu- kin-2.Thisrequiresdephosphorylationofthe precursor,phosphorylatedNFAT,bythephosphatase calcineurin, enabling NFAT to enter the cell nucleus from the cytosol. Ciclosporin binds to the protein cyclophilin in the cell interior; the complex inhibits calcineurin, hence the production of interleukin-2.
The breakthroughs in modern transplantation medicine are largely attributable to the introduction of ciclosporin. It is now also employed in certain autoimmune diseases, atopic dermatitis, and other disorders.
The predominant adverse effect of ciclosporin is nephrotoxicity. Its dosage must be titrated so that blood levels are neither too high (risk of renal injury) nor too low (rejection reaction). To complicate the problem, ciclosporin is a substance dif cult to manage therapeutically. Oral bioavailability is incomplete. Back-transport of the drug into the gut lumen occurs via the P-glycoprotein ef ux pump, in addition to metabolization by cytochrome oxidases of the 3A subfamily. Hepatic CYP3A4 enzymes contribute to presystemic elimination and are responsible for elimination of systemically available ciclosporin. Diverse drug interactions may occur by interference with CYP3A and P-glycopro- tein. For optimal dosage adjustment, monitoring of plasma levels is mandatory.
Drug-mediated suppression of transplant rejection entails long-term treatment. Protracted immunosuppression carries an in-
creased risk of malignomas. Risk factors for cardiovascular diseases may be adversely af- fected—a critical and important concern in long-term prognosis.
Tacrolimus is a macrolide antibiotic from
Streptomyces tsukubaensis. In principle, it acts like ciclosporin. At the molecular level, however, its “receptor” is not cyclophilin but a so-called FK-binding protein. Tacrolimus is likewise used to prevent allograft rejection. Its epithelial penetrability is superior to that of ciclosporin, allowing topical application in atopic dermatitis.
Sirolimus (rapamycin) is another macrolide, produced by Streptomyces hydroscopicus. Its immunosuppressant action, evidently, does not appear to involve inhibition of calcineurin. It forms a complex with the FK protein, imparting a special conformation on it; and the complex then inhibits the mTOR (mammalian target of rapamycin) phosphatase. The latter operates in the signaling path leading from the interleukin-2 receptor to activation of mitosis in lymphocytes. Thus, sirolimus inhibits lymphocyte proliferation. It is approved for the prevention of transplant rejection.
Inhibition of Immune Responses |
307 |
A. Calcineurin inhibitors and sirolimus (rapamycin)
Activated T-helper lymphocyte
Cyclophilin
Ciclosporin
NFAT Immunophilin/drug
complex
P
Calcineurin
P
NFAT
DNA
Synthesis
|
|
CH3 |
|
|
|
H3C |
CH |
|
H3C |
|
|
|
|
|
|
|
CH2 |
H3C |
|
|
H3C N CH |
CO |
N |
|
H3C |
CO |
|
|
|
|
|
|
|
|
CH |
CH2 CH |
|
|
|
H3C |
N |
|
|
|
|
OC |
D |
N |
CO |
|
CH |
|||
|
|
|
H |
|
CH3
CH3
|
|
|
HC |
|
|
|
|
|
|
|
|
|
|
|
|
|
HC |
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
HO |
CH |
CH3 |
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH |
|
H3C |
CH |
|
|
CH2 |
|
|
CH3 |
|
|
||
CH |
C |
N |
CH |
CO |
N CH |
C |
N CH2 |
|
|
||||
|
O |
|
|
|
H |
O |
CO |
|
|
||||
|
|
|
|
|
|
|
|
|
|
N |
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
O |
H |
|
|
|
|
|||
CH |
N |
CO |
CH |
N |
C CH N CO CH |
|
|
||||||
CH3 |
|
|
|
CH2 |
CH3 |
|
CH |
CH3 |
CH2 |
|
|
||
|
|
|
H3C |
CH |
|
|
CH3 |
|
|
CH |
CH3 |
|
|
|
|
|
|
CH3 |
|
|
|
|
|
CH3 |
|
|
|
Ciclosporin
Measurement!
IL-2 |
and other |
|
|
|
lymphokines |
Plasma |
|
|
CYP3A |
CYP3A |
|
|
con- |
||
|
P-glyco- |
centration |
|
|
protein |
|
|
|
IL-2 |
|
|
|
receptor |
|
|
FK-binding protein
mTOR
Sirolimus
Lymphocyte proliferation
Inhibition of transplant
rejection
Nephrotoxicity
Long-term adverse effects Neoplasia, hypertension, hyperlipidemia, hyperglycemia
FK-binding protein
Tacrolimus
