
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

188 Drugs Acting on the Motor System
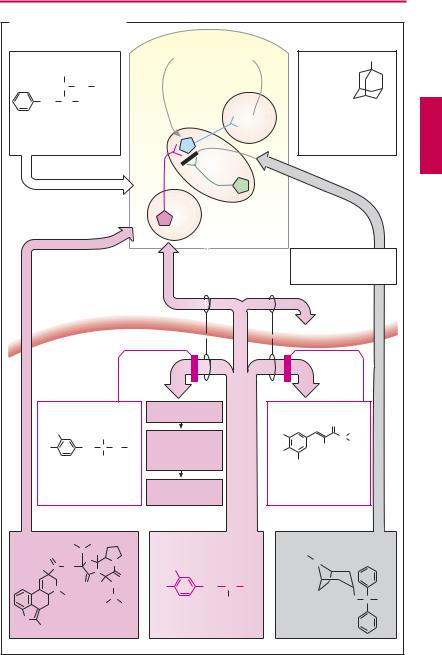
Antiparkinsonian Drugs
The central nervous programming of purposive movements depends on neuronal circuits interconnecting cortical regions, the thalamus, the cerebellum, and the basal ganglia (corpus striatum; subthalamic nucleus). The basal ganglia, in particular, play an important part in the initiation and scaling of movement as well as the programming of target acquisition. A disorder primarily involving basal ganglionic motor function is known as idiopathic Parkinson disease (shaking palsy). The disease typically manifests at an advanced age and is characterized by poverty of movement (akinesia), muscle stiffness (rigidity), tremor at rest, postural instability, gait disturbance, and a progressive impairment in the quality of life. The primary cause of this disease and its syndromal forms is a degeneration of dopamine neurons in the substantia nigra that project to the corpus striatum (specifically, the caudate nucleus and putamen) and exert an inhibitory influence. Cholinergic interneurons in the striatum promote neuronal excitation.
Pharmacotherapeutic measures are aimed at compensating striatal dopamine deficiency or suppressing unopposed cholinergic activity.
L-Dopa. Dopamine itself cannot penetrate the blood–brain barrier; however, its natural precursor, L-dihydroxyphenylalanine (levodopa), is effective in replenishing striatal dopamine levels, because it is transported across the blood–brain barrier via an amino acid carrier and is subsequently decarboxylated by dopa decarboxylase, present in striatal tissue. Decarboxylation also takes place in peripheral organs where dopamine is not needed and is likely to cause undesirable effects (vomiting; hypotension; p.116). Extracerebral production of dopamine can be prevented by inhibitors of dopa decarboxylase (carbidopa, benserazide) that do not penetrate the blood–brain barrier, leaving intracerebral decarboxylation unaffected.
Excessive elevation of brain dopamine levels may lead to undesirable reactions such as involuntary movements (dyskinesias) and mental disturbances.
Dopamine receptor agonists. Striatal dopamine deficiency can be compensated by lysergic acid derivatives such as bromocriptine
(p.116), lisuride, cabergoline, and pergolide and by the non-ergot compounds ropinirole and pramipexole.
Inhibitors of monoamine oxidase-B (MAOB). Monoamine oxidase occurs in the form of two isozymes: MAOA and MAOB. The corpusstriatumisrichinMAOB. Thisisozyme can be inhibited by selegiline. Degradation of biogenic amines in peripheral organs is not affected because MAOA remains functional.
Inhibitor of catecholamine O-methyltrans- ferase (COMT). The CNS-impermeant entacapone inhibits peripheral degradation of L- dopa and thus enhances availability of L- dopa for the brain. Accordingly, it is suitably only for combination therapy with L-dopa.
Anticholinergics. Antagonists at muscarinic cholinoceptors such as benztropine and biperiden (p.110) can be used to suppress the sequelae of the relative predominance of cholinergic activity in the striatum (in particular, tremor). Atropine-like peripheral side effects and impairment of cognitive function limit the tolerable dosage. Complete disappearance of symptoms cannot be achieved.
Amantadine. Early or mild parkinsonian manifestations may be relieved temporarily by amantadine. The underlying mechanisms of action may involve, inter alia, blockade of ligand-gated ion channels of the glutamate/ NMDA subtype, ultimately leading to diminished release of acetylcholine.
Treatment of advanced Parkinson disease requires combined administration of the above drugs for ameliorating the symptoms of this grave condition. Commonly, additional signs of central degeneration develop as the disease progresses.
Antiepileptics 189
A. Antiparkinsonian drugs
Selegiline
CH3
N CH2 C  CH
CH
CH2 CH CH3
Inhibition of dopamine degradation by MAO-B in CNS
Dopaminergic
Cortex
Motor
control  Amantadine NH2 loop
Amantadine NH2 loop
|
GABAergic |
NMDA receptor: |
|
|
Blockade |
|
Pallidum |
of ionophore: |
|
attenuation of |
|
|
|
cholinergic neurons |
|
Cholinergic |
|
S. nigra |
Striatum |
|
Degeneration in |
|
|
Parkinson disease |
|
|
|
|
Inhibition of cholinergic |
|
|
transmission |
|
|
|
|
|
rier |
|
|
|
|
ainbar |
|
|
|
|
br |
|
|
|
|
d- |
|
|
|
|
o |
|
|
|
|
lo |
|
|
|
|
|
B |
|
|
|
|
|
DOPA- decarboxylase
decarboxylase
 COMT
COMT
200 mg
Carbidopa |
|
|
Dopamine |
|
|
||
HO |
|
CH3 |
|
|
|
||
|
|
|
Stimulation of |
mg |
|||
HO |
|
CH2 C |
NH NH2 |
||||
|
peripheral dop- |
||||||
|
|
|
COOH |
2000 |
|||
|
|
|
amine receptors |
||||
|
|
|
|
||||
Inhibition of dopa- |
|
|
|
||||
decarboxylase |
|
Adverse effects |
|
||||
|
|
|
|
|
|
||
|
|
|
Dopamine substitution |
|
|
||
Bromo- |
|
H3C H |
CH3 |
|
L-DOPA |
|
|
criptine O |
|
C |
OH |
|
|
|
|
|
O |
N |
|
|
|
||
|
|
|
HO |
|
|
||
H |
C NH |
|
|
|
|||
N |
|
|
|
||||
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
O |
H |
CH2 |
HO |
CH2 CH NH2 |
|
|
CH3 |
|
C |
|
|
|
|
|
|
|
|
|
COOH |
||
|
|
|
H3C H CH3 |
|
|
||
|
|
|
|
|
|
||
HN |
Dopamine-receptor |
|
|
|
|||
Br |
agonist |
|
|
Dopamine precursor |
|||
Entacapone
|
O |
|
HO |
N |
C2H5 |
|
C2H5 |
|
|
CN |
|
HO
NO2
Inhibition of the peripheral catechol- O-methyltransferase
Benzatropine
H3C
N
O C H
Muscarinic acetylcholine antagonist

190 Drugs Acting on the Motor System
Antiepileptics
Epilepsy is a chronic brain disease of diverse etiology; it is characterized by recurrent paroxysmal episodes of uncontrolled excitation of brain neurons. Involving larger or smaller parts of the brain, the electrical discharge is evident in the electroencephalogram (EEG) as synchronized rhythmic activity and manifests itself in motor, sensory, psychic, and vegetative (visceral) phenomena. As both the affected brain region and the cause of abnormal excitability may differ, epileptic seizures can take on many forms. From a pharmacotherapeutic viewpoint, these may be classified as:
Generalized vs. partial (focal) seizures
Seizures with or without loss of consciousness
Seizures with or without specific modes of
precipitation
The brief duration of a single epileptic fit makes acute drug treatment unfeasible. Instead, antiepileptics are used to prevent seizures and therefore need to be given chronically. Only in the case of status epilepticus (succession of several tonic-clonic seizures), is acute anticonvulsant therapy indicated— usually with benzodiazepines given i.v. or, if needed, rectally.
The initiation of an epileptic attack involves “pacemaker” cells; these differ from other nerve cells by their unstable resting membrane potential; i.e., a depolarizing membrane current persists after the action potential terminates.
Therapeutic interventions aim to stabilize neuronal resting potential and, hence, to lower excitability. In specific forms of epilepsy, initially a single drug is tried to achieve control of seizures, valproate usually being the drug of first choice in generalized seizures, and carbamazepine being preferred for partial (focal), especially partial complex, seizures. Dosage is increased until seizures are no longer present or adverse effects become unacceptable. Only when monotherapy with different agents proves inadequate
can change-over to a second-line drug or combined use (“add on”) be recommended (B), provided that the possible risk of pharmacokinetic interactions is taken into account (see below). The precise mode of action of antiepileptic drugs remains unknown. Some agents appear to lower neuronal excitability by several mechanisms of action. In principle, responsivity can be decreased by inhibiting excitatory or activating inhibitory neurons. The transmitters utilized by most excitatory and inhibitory neurons are glutamate and γ-aminobutyric acid (GABA), respectively (p.193A).
Glutamate receptors comprise three subtypes, of which the NMDA subtype has the greatest therapeutic importance. (N-methyl- D-aspartate is a synthetic selective agonist.) This receptor is a ligand-gated ion channel that, upon stimulation with glutamate, permits entry of both Na+ and Ca2+ into the cell. Valproic acid inhibits both Na+ and Ca2+ channels. The antiepileptics lamotrigine, phenytoin, and phenobarbital inhibit, among other things, the release of glutamate. Felbamate is a glutamate antagonist.
Benzodiazepines and phenobarbital augment the activation of the GABAA receptor by physiologically released amounts of GABA (B) (see pp.193, 222). Chloride influx is increased, counteracting depolarization. Progabide is a direct GABA-mimetic but not an approved drug. Tiagabine blocks removal of GABA from the synaptic cleft by decreasing its reuptake. Vigabatrin inhibits GABA catabolism. Gabapentin augments the availability of glutamate as a precursor in GABA synthesis (B).

Antiepileptics 191
A. Epileptic attack, EEG, and antiepileptics
Drugs used in the treatment of status epilepticus:
Benzodiazepines, e.g., diazepam
Waking state |
|
|
|
|
EEG |
|
|
|
Epileptic attack |
|||
mV |
|
|
|
|
|
|
|
|
mV |
|
|
|
150 |
|
|
|
|
|
|
|
|
150 |
|
|
|
100 |
|
|
|
|
|
|
|
|
100 |
|
|
|
50 |
|
|
|
|
|
|
|
|
50 |
|
|
|
0 |
|
|
|
|
|
|
|
|
0 |
|
1 s |
|
1 s |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Drugs used in the prophylaxis of epileptic seizures |
|
|
|
|
||||||
|
|
|
H |
O |
|
|
H |
O |
|
|
CH3 |
|
|
|
|
|
|
|
N |
|
|
|
|
||
|
|
|
C |
|
|
C |
|
|
|
C2H5 |
||
5 |
|
|
N |
C2H5 |
O |
C |
O |
C |
|
|||
|
O |
C |
|
|
C |
|
||||||
N |
|
|
|
|
N |
|
|
N |
O |
|||
C |
|
|
N |
C |
|
|
|
|
|
|||
|
|
|
|
H |
|
|
H |
|
|
|||
O |
NH2 |
|
H |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Carbamazepine |
|
Phenobarbital |
|
Phenytoin |
|
Ethosuximide |
||||||
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
H3C |
CH3 |
|
|
CH2 |
NH2 |
|
HC |
|
|
H2C NH2 |
||
|
|
|
HC |
NH2 |
|
|||||||
H2C |
CH2 |
|
|
|
|
|
|
CH2 |
|
|||
|
|
C |
|
|
CH2 |
|
|
|||||
H2C H |
CH2 |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
CH2 |
|
|||||
|
|
CH2 |
|
|
CH2 |
|
|
|||||
C |
|
|
|
|
|
|
COOH |
|||||
COOH |
|
|
COOH |
|
COOH |
|
||||||
|
|
|
|
|
|
|
||||||
Valproic acid |
|
Gabapentin |
Vigabatrin |
|
GABA |
|||||||
B. Indications for antiepileptics |
|
|
|
|
Focal |
|
I. |
II. |
III. Choice |
seizures |
Simple |
Carbam- |
Valproic acid, |
Primidone, |
|
||||
|
seizures |
azepine |
Phenytoin |
Phenobarbital |
|
Complex or |
+ Lamotrigine or Vigabatrin or Gabapentin |
||
|
secondarily |
|
|
|
|
generalized |
|
|
|
Generalized |
Tonic-clonic |
Valproic acid |
Carbamazepine, |
Lamotrigine, |
attacks |
attack (grand mal) |
|
Phenytoin |
Primidone, |
|
Tonic attack |
|
|
Phenobarbital |
|
|
|
|
|
|
Clonic attack |
+ Lamotrigine or Vigabatrin or Gabapentin |
||
|
Myoclonic attack |
|
|
|
|
Absence |
|
Ethosuximide |
|
|
|
|
|
|
|
seizure |
|
+ Lamotrigine or Clonazepam |
|
|
|
|
||

192 Drugs Acting on the Motor System
The tricyclic carbamazepine, its analogue oxycarbazepine, and phenytoin enhance inactivation of voltage-gated sodium and calcium channels and limit the spread of electrical excitation by inhibiting sustained high-frequency firing of neurons.
Ethosuximide blocksaneuronalT-typeCa2+ channel (A); and represents a special class becauseitiseffectiveonlyinabsenceseizures.
All antiepileptics are likely, albeit in different degrees, to produce adverse effects. Sedation, dif culty concentrating, and slowing ofpsychomotordrive encumber practically all antiepileptic therapy. Moreover, cutaneous, hematological, and hepatic changes may necessitate a change in medication. Phenobarbital, primidone, and phenytoin may lead to osteomalacia (vitamin D prophylaxis) or megaloblastic anemia (folate prophylaxis). During treatment with phenytoin, gingival hyperplasia may develop in ~ 20% of patients. Valproic acid (VPA) is less sedating than other anticonvulsants. Tremor, gastrointestinal upset, and weight gain are frequently observed; reversible hair loss is a rarer occurrence. Its hepatotoxicity should be kept in mind.
Adverse reactions to carbamazepine include nystagmus, ataxia, and diplopia, particularly if the dosage is raised too fast. Gastrointestinal problems and skin rashes are frequent. It exerts an antidiuretic effect (sensitization of collecting ducts to vasopressin).
Valproate, carbamazepine, and other anticonvulsants pose teratogenic risks. Despite this, treatment should continue during pregnancy, as the potential threat to the fetus by a seizure is greater. However, it is mandatory to apply the lowest dose affording safe and effective prophylaxis. Concurrent high-dose administration of folate may prevent neural tube defects.
Carbamazepine, phenytoin, phenobarbital, and other anticonvulsants induce hepatic enzymes responsible for drug biotransformation; valproate is a potent inhibitor. Combinations between anticonvulsants or with other drugs may result in clinically impor-
tant interactions (plasma level monitoring!).
Carbamazepine is also used to treat trigeminal neuralgia and neuropathic pain.
For the often intractable childhood epilepsies, various other agents are used including ACTH and the glucocorticoid dexamethasone. Multiple (mixed) seizures associated with the slow spike-wave (Lennox– Gastaut) syndrome may respond to valproate, lamotrigine, and felbamate, the last being restricted to drug resistant seizures owing to its potentially fatal liver and bone marrow toxicity.
Benzodiazepines are the drugs of choice for status epilepticus (see above); however, development of tolerance renders them less suitable for long-term therapy. Clonazepam is used for myoclonic and atonic seizures. Clobazam, a 1,5-benzodiazepine exhibiting an increased anticonvulsant/sedative activity ratio, has a similar range of clinical uses. Personality changes and paradoxical excitement are potential side effects.
Clomethiazole can also be effective for controlling status epilepticus but is used mainly to treat agitated states, especially alcoholic delirium tremens and associated seizures.
Topiramate, derived from D-fructose, has complex, long-lasting anticonvulsant actions that cooperate to limit the spread of seizure activity; it is effective in partial and generalized seizures and as add-on in Lennox–Gas- taut syndrome.
It should be noted that certain drugs (e.g., neuroleptics, isoniazid, and high-dose β-lac- tam antibiotics) lower seizure threshold and are therefore contraindicated in epileptic patients.
Outlook: Among the newer antiepileptics, gabapentin, oxycarbazepine, lamotrigine, and topiramate are now endorsed as primary monotherapeutics for both partial and generalized seizures. Their pharmacokinetic characteristics are generally more desirable than those of the older drugs.

|
Antiepileptics |
193 |
|
A. Neuronal sites of action of antiepileptics |
|
|
|
Na+ Ca2+ |
Excitatory neuron |
|
|
NMDA- |
|
|
|
receptor |
|
Inhibition of |
|
|
|
|
|
|
Glutamate |
glutamate |
|
NMDA-receptor- |
release: |
|
|
|
|
||
antagonist |
|
phenytoin, |
|
felbamate, |
|
lamotrigine |
|
|
phenobarbital |
|
|
valproic acid |
|
|
|
Ca2+-channel |
|
|
|
T-Type- |
|
|
|
calcium |
Voltage |
|
|
channel blocker |
|
|
|
ethosuximide, |
dependent |
Inhibition of |
|
(valproic acid) |
Na+-channel |
|
|
action |
|
||
|
|
|
|
GABAA- |
|
potentials |
|
|
carbamazepine |
|
|
receptor |
|
|
|
|
valproic acid |
|
|
GABA |
|
|
|
CI- |
|
phenytoin |
|
Gabamimetics: |
|
|
|
|
|
|
|
|
benzodiazepine |
|
|
|
barbiturates |
|
|
Inhibitory |
vigabatrin |
|
|
tiagabine |
|
|
|
neuron |
|
|
|
gabapentin |
|
|
|
B. Sites of action of antiepileptics in GABAergic synapse |
|
|||||||
Benzodiazepines |
GABAA- |
|
|
Tiagabine |
||||
|
|
|
|
|
||||
Allosteric |
|
β |
receptor |
|
|
|
||
α |
α |
|
|
|
|
Inhibition |
||
enhance- |
|
|
|
|
||||
|
|
|
|
|
||||
ment of |
|
γ |
β |
α |
β |
α |
Chloride |
of GABA |
GABA |
|
|
|
|
γ |
β |
channel |
reuptake |
action |
|
|
|
|
|
|
|
|
Barbiturates |
|
|
|
|
|
|
|
|
Progabide |
|
|
|
GABA- |
|
|||
GABA- |
|
|
GABA |
|
|
transaminase |
|
|
mimetic |
|
|
|
|
|
Vigabatrin |
||
|
|
|
|
|
|
|
|
|
|
|
|
Glutamic acid |
|
|
Succinic |
Inhibitor |
|
|
|
|
decarboxylase |
|
|
of GABA- |
||
|
|
|
|
|
|
semialdehyde |
transaminase |
|
|
|
|
|
|
|
Succinic acid |
|
|
|
|
Glutamic |
|
|
|
|
|
|
|
|
acid |
|
|
|
|
|
|
|
|
|
|
|
Gabapentin |
|
||
Ending of |
|
|
|
|
Improved utilization |
|
||
inhibitory |
|
|
|
|
of GABA precursor: |
|
||
neuron |
|
|
|
|
glutamate |
|
||
