
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

214 General Anesthetics
General Anesthesia and General Anesthetic Drugs
General anesthesia is a state of drug-induced reversible inhibition of central nervous function, during which surgical procedures can be carried out in the absence of consciousness, responsiveness to pain, defensive or involuntary movements, and significant autonomic reflex responses (A).
The required level of anesthesia depends on the intensity of the pain-producing stimuli, i.e., the degree of nociceptive stimulation. The skillful anesthetist, therefore, dynamically adapts the plane of anesthesia to the demands of the surgical situation. Originally, anesthesia was achieved with a single anesthetic agent (e.g., diethyl ether, first successfully demonstrated in 1846 by W. T. G. Morton, Boston). To suppress defensive reflexes, such a “monoanesthesia” necessitates a dosage in excess of that needed to cause unconsciousness, thereby increasing the risk of paralyzing vital functions, such as cardiovascular homeostasis (B). Modern anesthesia employs a combination of different drugs to achieve the goals of surgical anesthesia (balanced anesthesia). This approach reduces the hazards of anesthesia. In (C) are listed examples of drugs that are used concurrently or sequentially as anesthesia adjuncts. Neuromuscular blocking agents are covered elsewhere in more detail. Recall that “curarization” of the patient necessitates artificial ventilation. However, the use of neuromuscular blockers is making an essential contribution to risk reduction in modern anesthesia. In the following, some special methods of anesthesia are considered before presentation of the anesthetic agents.
Neuroleptanalgesia can be considered a special form of combination anesthesia: the short-acting opioid analgesic fentanyl is combined with a strongly sedating and af- fect-blunting neuroleptic. Because of major drawbacks, including insuf cient elimination of consciousness and extrapyramidal
motor disturbances, this procedure has become obsolete.
In regional anesthesia (spinal anesthesia) with a local anesthetic (p.202), nociceptive conduction is interrupted. Since consciousness is preserved, this procedure does not fall under the definition of anesthesia.
According to their mode of application, general anesthetics in the narrow sense are divided into inhalational (gaseous, volatile) and injectable agents.
Inhalational anesthetics are administered in and, for the most part, eliminated via respired air. They serve especially to maintain anesthesia (p.216)
Injectable anesthetics (p.218) are frequentlyemployedfor induction. Intravenous injection andrapidonsetofaction areclearly more agreeable to the patient than is breathing a stupefying gas. The effect of most injectable anesthetics is limited to a few minutes. This allows brief procedures to be carried out or preparation of the patient for inhalational anesthesia (intubation). Administration of the volatile anesthetic must then be titrated in such a manner as to counterbalance the waning effect of the injectable agent. Increasing use is now being made of injectable, instead of inhalational, anesthetics during prolonged combined anesthesia (e.g., propofol; total intravenous anaes- thesia—TIVA).

General Anesthesia and General Anesthetic Drugs |
215 |
A. Goals of surgical anesthesia
Muscle relaxation |
Loss of consciousness |
Automatic stabilization |
Motor |
Pain and |
Autonomic |
reflexes |
suffering |
reflexes |
|
Nociception |
|
|
|
|
Analgesia |
|
|
Pain stimulus |
|
|
B. Traditional monoanesthesia vs. modern balanced anesthesia |
|||
Monoanesthesia |
|
|
|
e.g., diethyl ether |
|
For |
|
Reduced pain sensitivity, |
|
unconsciousness: |
|
|
e.g., halothane |
||
analgesia |
|
or propofol |
|
|
For |
If needed, |
|
Loss of consciousness |
muscle |
||
autonomic |
|||
relaxation |
|||
|
stabilization: |
||
|
e.g., pan- |
atropine, |
|
Muscle relaxation |
curonium |
esmolol |
|
|
|
For |
|
|
|
analgesia |
|
Paralysis of |
|
e.g., N2O |
|
vital centers |
|
or fentanyl |
|
C. Regimen for balanced anesthesia |
|
||
Premedication |
Induction |
Maintenance |
Recovery |
|
|
N |
|
|
Muscle |
2 |
|
Fentanyl |
O |
|
|
|
Midazolam Isoflurane |
|
|
|
|
|
Neostigmine |
|
|
Pancuronium |
Fentanyl |
|
|
|
neuromuscular |
|
relaxation, |
|
|
analgesia |
|
|
|
Diazepam |
|
|
blockAnalgesia |
|
|
|
|
|
|
|
reversal |
|
unconsciousness |
Muscle relaxation |
|
|
|
intubation |
|
|
|
|
of |
anxiolysis |
|
|
Analgesia |
|
|
|
|
|
|
|
Unconsciousness |

216 General Anesthetics
Inhalational Anesthetics
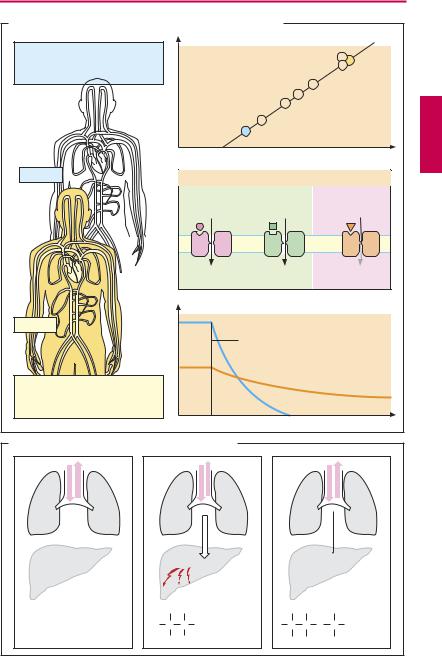
The mechanism of action of inhalational anesthetics is not known in detail. In the first instance, the diversity of chemical structures (inert gas xenon; hydrocarbons; halogenated hydrocarbons) possessing anesthetic activity appeared to argue against the involvement of specific sites of action. The correlation between anesthetic potency and lipophilicity of anesthetic drugs (A) pointed to a nonspecific uptake into the hydrophobic interior of the plasmalemma, with a resultant impairment of neuronal function. Meanwhile, several lines of evidence support an interaction with membrane proteins; among these ligand-gated ion channel proteins assume special importance. Experimental studies favor the idea that anesthetics enhance the effectiveness of inhibitory GABA and glycine receptors, while attenuating responsiveness to stimulation of excitatory glutamate receptors.
Anesthetic potency can be expressed in terms of the minimal alveolar concentration (MAC) at which 50% of patients remain immobile following a defined painful stimulus (skin incision). Whereas the poorly lipophilic nitrous oxide must be inhaled in high concentrations, much smaller concentrations are required in the case of the more lipophilic halothane.
The rates of onset and cessation of action vary widely among different inhalational anesthetics and also depend on the degree of lipophilicity. In the case of nitrous oxide, elimination from the body is rapid when the patient is ventilated with normal air. Owing to the high partial pressure in blood, the driving force for transfer of the drug into expired air is large and, since tissue uptake is minor, the body can be quickly cleared of nitrous oxide. In contrast, with halothane, partial pressure in blood is low and tissue uptake is high, resulting in a much slower elimination.
Given alone, nitrous oxide (N2O, “laughing gas”) is incapable of producing anesthesia of
suf cient depth for surgery, even when taking up 80% of the inspired air volume (O2 20% vol. is necessary!). It has good analgesic ef cacy that can be exploited when it is used in conjunction with other anesthetics. As a gas, N2O can be administered directly; it is not metabolized appreciably and is cleared entirely by exhalation (B).
Halothane (boiling point [BP] 50οC), enflurane (BP 56οC), isoflurane (BP 48οC) and the newer substances, desflurane and sevoflurane, have to be vaporized by special devices. Part of the administered halothane (up to 20%) is converted into hepatotoxic metabolites (B). Liver damage may result from halothane anesthesia. With a single exposure, the risk involved is unpredictable; however, the risk increases with the frequency of exposure and the shortness of the interval between successive exposures (estimated incidence 1 in 35 000 procedures).
Degradation products of enflurane or isoflurane (fraction biotransformed < 2%) probablydonotplayany role in anestheticaction.
Halothane exerts a hypotensive effect (vasodilation and negative inotropic effect). Enflurane and isoflurane cause less circulatory depression. Halothane sensitizes the myocardium to catecholamines. This effect is much less pronounced with enflurane and isoflurane. Unlike halothane, enflurane and isoflurane have a muscle-relaxant effect that is additive with that of nondepolarizing neuromuscular blockers.
Desflurane is a close structural relative of isoflurane, but has low lipophilicity and a low rate of biotransformation (0.02%). This permits rapid induction and recoveryas well as good control of anesthetic depth. The newest member of this group, sevoflurane, is similarly fast-acting and convenient to control but has a higher rate of biotransformation (up to 5%) and lower incidence of laryngospasm and cough.
|
Inhalational Anesthetics |
217 |
|
A. Lipophilicity, potency and elimination of N2O and halothane |
|
|
|
Anesthetic potency |
|
|
|
Low potency |
Halothane |
|
|
high partial pressure needed |
|
||
|
Chloroform |
||
relatively little binding to tissue |
|
||
|
Isoflurane |
|
|
|
|
|
|
|
Enflurane |
|
|
|
Diethyl ether |
|
|
|
Cyclopropane |
|
|
Nitrous oxide |
|
|
|
N2O |
Xenon |
|
|
|
Lipophilicity |
|
|
N2O |
Potential effects |
|
|
Enhancement |
Inhibition |
|
|
Cl- |
Cl- |
Na+, Ca2+ |
|
GABAA |
Glycine |
NMDA |
|
receptor |
receptor |
receptor |
|
Partial pressure in tissue |
|
|
|
Halothane |
|
|
|
|
Termination of intake |
|
|
High potency |
|
|
|
low partial pressure sufficient |
|
|
|
relatively high binding in tissue |
|
|
|
|
Time |
|
|
B. Elimination routes of different volatile anesthetics |
|
|
|
|
|
|
|||||
|
|
|
Metabolite |
|
|
Metabolite |
|||||
|
|
|
|
15 – 20% |
|
|
|
0.2% |
|
||
N2O |
Nitrous oxide |
F |
Br |
|
|
F |
H |
|
F |
|
|
C |
C |
H |
F |
C |
C |
O |
C |
H |
|||
|
F |
||||||||||
H5C2OC2H5 |
Ether |
F |
Cl |
Halothane |
|
F |
Cl |
|
F |
Isoflurane |
|
