
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

266 Hormones
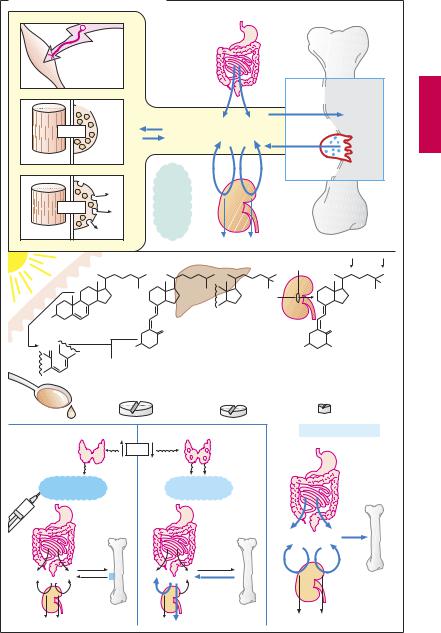
Drugs for Maintaining Calcium Homeostasis
At rest, the intracellular concentration of free calcium ions (Ca2+) is kept at 0.1 µm (see p.132 for mechanisms involved). During excitation, a transient rise of up to 10 µm elicits contraction in muscle cells (electromechanical coupling) and secretion in glandular cells (electrosecretory coupling). The cellular content of Ca2+ is in equilibrium with the extracellular Ca2+ concentration (~ 1000 µm), as is theplasmaprotein-boundfractionofcalcium inblood.Ca2+ maycrystallizewithphosphate to form hydroxyapatite, the mineral of bone. OsteoclastsarephagocytesthatmobilizeCa2+ by resorption of bone. Slight changes in extracellularCa2+ concentration can alterorgan function: thus, excitability of skeletal muscle increasesmarkedlyasCa2+ islowered(e.g.,in hyperventilationtetany).Threehormonesare available to the body for maintaining a constant extracellular Ca2+ concentration.
Vitamin D hormone is derived from vitamin D (cholecalciferol). Vitamin D can also be produced in the body; it is formed in the skin from dehydrocholesterol during irradiation with UV light. When there is lack of solar radiation, dietary intake becomes essential, codliveroilbeingarichsource.Metabolically active vitamin D hormone results from two successive hydroxylations: in the liver at position 25 (†calcifediol) and in the kidney at position 1 (†calcitriol = vitamin D hormone). 1-Hydroxylation depends on the level of calcium homeostasis and is stimulated by parathormone and a fall in plasma levels of Ca2+ andphosphate.VitaminDhormonepromotesenteralabsorptionandrenalreabsorption of Ca2+ and phosphate. As a result of the increased Ca2+ and phosphate concentration in blood, there is an increased tendency for theseionsto be deposited inboneintheform of hydroxyapatite crystals. In vitamin D deficiency, bone mineralization is inadequate (rickets, osteomalacia). Therapeutic use aims at replacement. Mostly,vitaminDisgiven; in liver disease, calcifediol may be indicated, in renal disease, calcitriol. Effective-
ness, as well as rate of onset and cessation of action increase in the order vitamin D < 25- OH-vitamin D < 1,25-di-OH vitamin D. Overdosage may induce hypercalcemia with deposits of calcium salts in tissues (particularly in kidney and blood vessels): calcinosis.
The polypeptide parathormone is released from the parathyroid glands when the plasma Ca2+ level falls. It stimulates osteoclasts to increase bone resorption; in the kidneys it promotes calcium reabsorption, while phosphate excretion is enhanced. As blood phosphate concentration diminishes, the tendency of Ca2+ to precipitate as bone mineral decreases. By stimulating the formation of vitamin D hormone, parathormone has an indirect effect on the enteral uptake of Ca2+ and phosphate. In parathormone deficiency, vitamin D can be used as a substitute that, unlike parathormone, is effective orally. Teriparatide is a recombinant shortened parathormone derivative containing the portion required for binding to the receptor. It can be used in the therapy of postmenopausal osteoporosis and promotes bone formation. While this effect seems paradoxical in comparison with hyperparathyroidism, it obviously arises from the special mode of administration: the once daily s.c. injection generates a quasi-pulsatile stimulation. Additionally, adequate intake of calcium and vitamin D must be ensured.
The polypeptide calcitonin is secreted by thyroid C-cells during imminent hypercalcemia. It lowers elevated plasma Ca2+ levels by inhibiting osteoclast activity. Its uses include hypercalcemia and osteoporosis. Remarkably, calcitonin injection may produce a sustained analgesic effect that alleviates pain associated with bone diseases (Paget disease, osteoporosis, neoplastic metastases) or Sudek syndrome.
Hypercalcemia can be treated by (1) administering 0.9% NaCl solution plus furosemide (if necessary) † renal excretion ⁄; (2) the osteoclast inhibitors calcitonin and clodronate (a biphosphonate) † bone Ca mobilizationø; (3) glucocorticoids.
Drugs for Maintaining Calcium Homeostasis |
267 |
A. Calcium homeostasis of the body |
|
|
|
|
|
|||||
|
Electrical |
|
|
|
|
|
|
|
|
|
|
excitability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bone trabeculae |
|
|
|
1 x 10-7M |
|
|
|
|
|
|
Hydroxyapatite crystals |
|
|
~ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ca2+ |
|
cell function |
|
1 x 10-3M |
Ca2+ + PO43- |
Ca10(PO4)6(OH)2 |
||||
|
|
|
|
|||||||
Muscle cell |
Gland cell |
|
|
|
|
|
|
|
||
|
|
|
on |
~1 |
Ca |
|
|
|
Osteoclast |
|
|
|
~10-5M |
Effect |
|
|
|
||||
|
|
|
x |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
10- |
|
|
|
|
|||
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
Albumin |
M |
|
|
|
|
Ca2+ |
|
|
|
Globulin |
|
|
|
|
||
Contraction |
Secretion |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
Skin |
|
|
|
|
|
Parathyroid hormone, Ca2+ , PO3- |
||
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
25 |
|
|
|
25 |
OH |
|
OH |
|
|
|
|
|
|
|
|
|
||
1 |
|
|
|
|
|
|
|
|
|
|
HO |
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7-Dehydrocholesterol |
|
|
CH2 |
|
|
|
CH2 |
|||
H2C |
|
|
HO |
1 |
|
|
|
|
HO |
OH |
|
|
|
|
|
|
|
||||
|
|
Cholecalciferol |
|
|
25-Hydroxychole- |
|
1,25-Dihydroxychole- |
|||
|
|
(vitamin D3) |
|
|
calciferol |
|
calciferol (calcitriol) |
|||
|
|
50 – 5000 g/day |
(calcifediol) |
|
0.5 – 2 g/day |
|||||
|
|
|
|
|
|
|
50 – 2000 g/day |
|
|
|
Cod liver oil |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vit. D-Hormone |
Parafollicular |
|
|
Ca2+ |
|
|
|
|
Parathyroid |
|
|
cells of |
|
|
|
|
|
|
glands |
|
|
|
thyroid |
|
|
|
|
|
|
|
|
|
|
Calcitonin |
|
|
|
Parathyroid |
|
|
||||
|
|
|
hormone |
|
|
|
||||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Ca2+ + PO3- |
|
|
|
|
|
|
|
|
|
|
|
4 |

268 Antibacterial Drugs
Drugs for Treating Bacterial Infections
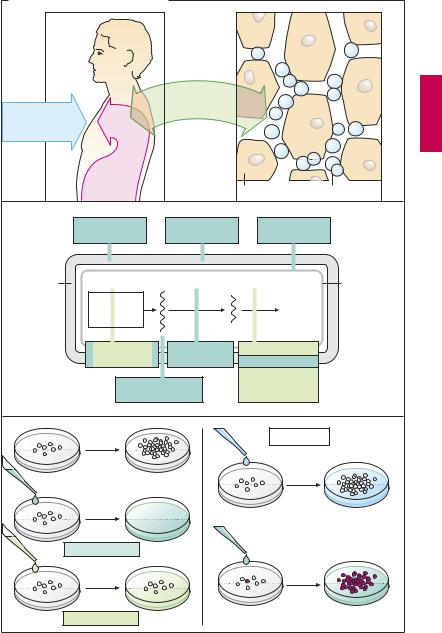
When bacteria overcome the cutaneous or mucosal barriers and penetrate into body tissues, a bacterial infection is present. Frequently the body succeeds in removing the invaders, without outward signs of disease, by mounting an immune response. However, certain pathogens have evolved a sophisticated counterstrategy. Although they are taken up into host cells via the regular phagocytotic pathway, they are able to forestall the subsequent fusion of the phagosome with a lysosome and in this manner can escape degradation. Since the wall of the sheltering vacuole is permeable to nutrients (amino acids, sugars), the germs are able to grow and multiply until the cell dies and the released pathogens can infect new host cells. This strategy is utilized, e.g., by Chlamydia and Salmonella species, Mycobacterium tuberculosis, Legionella pneumophila, Toxoplasma gondii, and Leishmania species. It is easy to see that targeted pharmacotherapy is especially dif cult in such cases because the drug cannot reach the pathogen until it has surmounted first the cell membrane and then the vacuolar membrane. If bacteria multiply faster than the body’s defenses can destroy them, infectious disease develops, with inflammatory signs, e.g., purulent wound infection or urinary tract infection. Appropriate treatment employs substances that injure bacteria and thereby prevent their further multiplication, without harming cells of the host organism (1).
Specific damage to bacteria is particularly feasible when a substance interferes with a metabolic process that occurs in bacterial but not in host cells. Clearly this applies to inhibitors of cell wall synthesis, since human or animal cells lack a cell wall. The points of attack of antibacterial agents are schematically illustrated in a grossly simplified bacterial cell, as depicted in (2).
In the following sections, plasmalemmadamaging polymyxins and tyrothricin are
not considered further. Because of their poor tolerability, they are suitable only for topical use.
The effect of antibacterial drugs can be observed in vitro (3). Bacteria multiply in a growth medium under controlled conditions. If the medium contains an antibacterial drug, two results can be discerned: (a) bacteria are killed—bactericidal effect; or
(b) bacteria survive, but do not multiply— bacteriostatic effect. Although variations may occur under therapeutic conditions, the different drugs can be classified according to their primary mode of action (color tone in 2 and 3).
When bacterial growth remains unaffected by an antibacterial drug, bacterial resistance is present. This may occur because of certain metabolic characteristics that confer a natural insensitivity to the drug on a particular strain of bacteria (natural resistance). Depending on whether a drug affects only few or numerous types of bacteria, the terms narrow-spectrum (e.g., penicillin G) or broad-spectrum (e.g., tetracyclines) antibiotic are applied. Naturally susceptible bacterial strains can be transformed under the influence of antibacterial drugs into resistant ones (acquired resistance), when a random genetic alteration (mutation) gives rise to a resistant bacterium. Under the influence of the drug, the susceptible bacteria die off, whereas the mutant multiplies unimpeded. The more frequently a given drug is applied, the more probable the emergence of resistant strains (e.g., hospital strains with multiple resistance)!
Resistance can alsobe acquired when DNA responsible for nonsusceptibility (so-called resistance plasmid) is passed on from other resistant bacteria by conjugation or transduction.
Drugs for Treating Bacterial Infections |
269 |
A. Principles of antibacterial therapy |
|
|
|
|
|
|
Anti- |
|
|
|
|
bacterial |
|
|
Bacterial |
|
drugs |
|
|
|
|
|
|
|
invasion: |
|
|
|
|
infection |
|
|
|
|
|
|
Selective |
|
|
|
|
antibacterial |
|
|
|
|
toxicity |
|
|
|
Immune |
|
|
|
1. |
defenses |
|
Body cells |
Bacteria |
|
Penicillins |
Bacitracin |
Polymyxins |
|
|
Cephalosporins |
Vancomycin |
Tyrothricin |
|
Cell wall |
DNA |
RNA |
|
Cell |
|
Tetrahydro- |
|
|
membrane |
|
|
|
|
|
|
folate |
|
Protein |
|
|
synthesis |
|
|
|
Bacterium |
Sulfonamides |
Rifampicin |
Tetracyclines |
|
Trimethoprim |
Aminoglycosides |
|
||
|
|
|||
|
|
|
Chloramphenicol |
|
|
“Gyrase-inhibitors” |
Erythromycin |
|
|
|
Clindamycin |
|
||
|
Nitroimidazoles |
|
||
2. |
|
|
||
|
|
|
|
|
|
1 day |
|
Resistance |
|
|
|
|
|
|
Antibiotic |
|
|
|
|
|
|
Insensitive strain |
|
|
|
Bactericidal |
|
|
|
3. |
Bacteriostatic |
Sensitive strain with |
Selection |
|
resistant mutant |
|
|||
