
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

286 Antiviral Drugs
Chemotherapy of Viral Infections
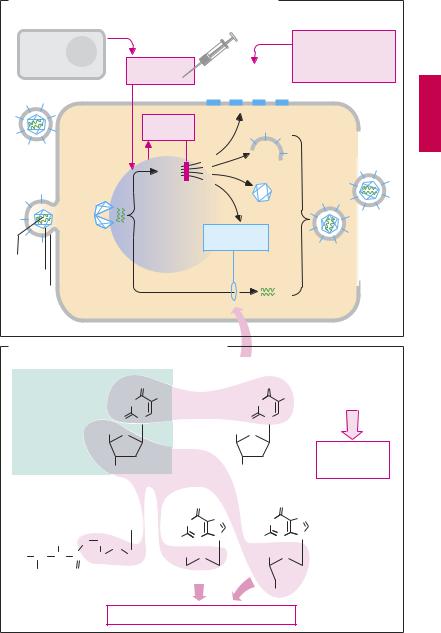
Viruses essentially consist of genetic material (nucleic acids) and a capsular envelope made up of proteins, often with a coat of a phospholipid (PL) bilayer with embedded proteins. They lack a metabolic system and depend on the infected cell for their growth and replication. Targeted therapeutic suppression of viral replication requires selective inhibition of those metabolic processes that specifically serve viral replication in infected cells.
Viral replication as exemplified by herpes simplex viruses (A).
1.The viral particle attaches to the host cell membrane (adsorption) via envelope glycoproteins that make contact with specific structures of the cell membrane.
2.The viral coat fuses with the plasmalemma of host cells and the nucleocapsid (nucleic acid plus capsule) enters the cell interior (penetration).
3.The capsule opens (“uncoating”) near the nuclear pores and viral DNA moves into the cell nucleus. The genetic material of the virus can now direct the cell’s metabolic system.
4a. Nucleic acid synthesis: The genetic material (DNA in this instance) is replicated and RNA is produced for the purpose of protein synthesis.
4b. The proteins are used as “viral enzymes” catalyzing viral multiplication (e.g., DNA polymerase and thymidine kinase), as capsomers, or as coat components, or are incorporated into the host cell membrane.
5.Individual components are assembled into new virus particles (maturation).
6.Release of daughter viruses results in spread of virus inside and outside the or-
ganism.
With herpesviruses, replication entails host cell destruction and development of disease symptoms.
Antiviral mechanisms (A). The organism can disrupt viral replication with the aid of cytotoxic T-lymphocytes that recognize and destroy virus-producing cells (presenting viral proteins on their surface, p.304) or by means of antibodies that bind to and inactivate extracellular virus particles. Vaccinations are designed to activate specific immune defenses.
Interferons (IFN) are glycoproteins that, among other products, are released from virus-infected cells. In neighboring cells, interferon stimulates the production of “antiviral proteins.” These inhibit the synthesis of viral proteins by (preferential) destruction of viral DNA or by suppressing its translation. Interferons are not directed against a specific virus, but have a broad spectrum of antiviral action that is, however, species-specific. Thus, interferon for use in humans must be obtained from cells of human origin, such as leukocytes (IFN-α), fibroblasts (IFN-β), or lymphocytes (IFN-γ). Interferons are used in the treatment of certain viral diseases, as well as malignant neoplasias and autoimmune diseases; e.g., IFN-α for the treatment of chronic hepatitis C and hairy cell leukemia; and IFN-β in severe herpes virus infections and multiple sclerosis.
Virustatic antimetabolites are “false” DNA building blocks (B) or nucleosides. A nucleoside (e.g., thymidine) consists of a nucleobase (e.g., thymine) and the sugar deoxyribose. In antimetabolites, one of the components is defective. In the body, the abnormal nucleosides undergo bioactivation by attachment of three phosphate residues (p.289).
Idoxuridine and congeners are incorporated into DNA with deleterious results. This also applies to the synthesis of human DNA. Therefore, idoxuridine and analogues are suitable only for topical use (e.g., in herpes simplex keratitis).
Chemotherapy of Viral Infections |
287 |
A. Virus multiplication and modes of action of antiviral agents |
|
|||
Virus- |
|
|
|
Specific immune |
infected |
|
|
|
defense |
cell |
Glycoprotein |
|
e.g., cytotoxic |
|
|
|
T-lymphocytes |
||
|
Interferon |
Proteins with |
||
|
|
|||
|
|
|
|
|
1. Adsorption |
|
|
antigenic properties |
|
|
|
|
|
|
|
|
Antiviral |
|
|
|
|
proteins |
synthesis |
|
|
|
|
6. Release |
|
|
|
RNA |
Protein |
|
|
|
|
||
2. Penetration |
|
|
|
|
|
4a. Nucleic acid |
|
|
|
3. Uncoating |
synthesis |
Viral DNA |
|
|
polymerase |
|
|||
|
|
|||
DNA |
4b. |
|
||
|
|
|
|
|
Capsule |
|
|
DNA |
|
Envelope |
|
|
|
|
|
|
|
5. Maturation |
|
|
|
|
|
|
B. Chemical structure of virustatic antimetabolites |
|
|
|
|
||||||||
Correct: |
|
|
|
|
|
|
Antimetabolites = incorrect DNA building blocks |
|||||
|
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
R: -I Idoxuridine |
||
Thymidine |
|
|
|
|
|
CH3 |
incorrect base |
|
R |
|
||
|
|
|
HN |
HN |
|
-CF3 Trifluridine |
||||||
|
|
|
|
|
|
|
||||||
Thymine |
|
|
|
O |
N |
|
|
|
O |
N |
|
|
|
|
HOCH2 O |
|
|
|
HOCH2 O |
|
|
|
|||
|
|
|
|
|
|
|
|
|
||||
Desoxyribose |
|
|
|
|
|
|
|
|
|
|
Insertion into |
|
|
|
|
|
|
incorrect sugar |
|
|
|
DNA instead |
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
OH |
|
|
|
|
OH |
|
|
of thymidine |
|
|
|
|
|
|
|
|
Acyclovir |
|
|
|
Ganciclovir |
|
|
|
|
|
|
|
|
O |
|
|
O |
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
Guanine |
|
HN |
N |
HN |
N |
|
|||
|
|
|
|
|
|
|
||||||
NH2 |
|
O |
CH2 |
|
|
H2N N |
N |
H2N |
N |
N |
Guanine |
|
|
O |
|
|
|||||||||
|
|
|
|
|
HOCH2 O |
|
HOCH2 O |
|
|
|||
H3C CH CH |
C |
H2C |
|
CH2 |
|
|
|
|||||
CH3 |
O |
|
|
|
|
|
|
|
|
|
|
|
Valaciclovir, |
|
|
|
|
|
|
|
|
|
OH |
|
|
prodrug |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inhibition of viral DNA polymerase |
|
|
|||||

288 Antiviral Drugs
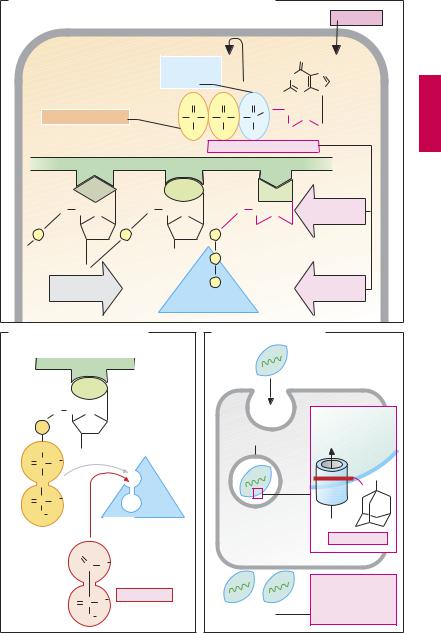
Among virustatic antimetabolites, aciclovir (A) has both specificity of the highest degree and optimal tolerability because it undergoes bioactivation only in infected cells, where it preferentially inhibits viral DNA synthesis. (1) A virally coded thymidine kinase (specific to herpes simplex and varicel- la-zoster viruses) performs the initial phosphorylation step; the remaining two phosphate residues are attached by cellular kinases. (2) The polar phosphate residues render aciclovir triphosphate membraneimpermeable and cause it to accumulate in infected cells. (3) Aciclovir triphosphate is a preferred substrate of viral DNA polymerase; it inhibits enzyme activity and, following its incorporation into viral DNA, induces strand breakage because it lacks the 3′-OH group of deoxyribose that is required for the attachment of additional nucleotides. The high therapeutic value of aciclovir is evident in severe infections with herpes simplex viruses (e.g., encephalitis, generalized infection) and varicella-zoster viruses (e.g., severe herpes zoster). In these cases, it can be given by i.v. infusion. Aciclovir may also be given orally despite its incomplete (15–30%) enteral absorption. In addition, it has topical uses. Because host DNA synthesis remains unaffected, adverse effects do not include bone marrow depression.
In valaciclovir, the hydroxyl group is esterified with the amino acid L-valine (p.287B). This allows utilization of an enteral dipeptide transporter, leading to an enteral absorption rate almost double that of aciclovir. Subsequent cleavage of the valine residue yields aciclovir.
Famciclovir is an antiherpetic prodrug (active species penciclovir) with good oral bioavailability.
Ganciclovir (structure on p.287B) is used in the treatment of severe infections with cytomegaly viruses (also belonging to the herpes group); these do not form thymidine kinase, phosphorylation being initiated by a different viral enzyme. Ganciclovir is less well tolerated and, not infrequently, produ-
ces leukopenia and thrombopenia. It is infused or administered orally as a valine ester (valganciclovir).
Foscarnet represents a diphosphate analogue. Incorporation of nucleotide into a DNA strand entails cleavage of a diphosphate residue. Foscarnet inhibits DNA polymerase by interacting with its binding site for the diphosphate group. Indications: systemic therapy in severe cytomegaly infections in AIDS patients; local therapy of herpes simplex infections.
Drugs against influenza viruses (C). Amantadine specifically affects the replication of influenza A (RNA) viruses, the causative agents of true influenza. These viruses are endocytosed into the cell. Release of viral RNA requires protons from the acidic content of endosomes to penetrate into the virus. Amantadine blocks a channel protein in the viral coat that permits influx of protons. Thus, “uncoating” is prevented. The drug is used for prophylaxis and, hence, must be taken before the outbreak of symptoms. It is also an antiparkinsonian drug (p.188).
Neuraminidase inhibitors prevent the release of influenza A and B viruses. Normally, the viral neuraminidase splits off N-acetyl- neuraminic (sialic) acid residues on the cellular surface coat, thereby enabling newly formed viral particles to be detached from the host cell. Zanamivir is given by inhalation; oseltamivir is suitable for oral administration because it is an ester prodrug. Possible uses include treatment and prophylaxis of influenza virus infections.
Chemotherapy of Viral Infections |
289 |
A. Activation of acyclovir and inhibition of viral DNA synthesis |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Acyclovir |
Infected cell: |
|
|
Viral |
|
|
|
|
|
|
O |
|
|
herpes simplex |
|
|
thymidine |
|
|
|
HN |
|
N |
|||
or varicella-zoster |
|
|
kinase |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
N |
||||
|
|
|
|
|
O |
|
O |
|
H2N |
N |
||
|
|
|
|
|
|
|
O |
|
|
|
||
Cellular kinases |
|
|
HO |
P |
O |
P |
O |
O |
CH2 |
|
||
|
|
P |
H2C |
O |
CH2 |
|||||||
|
|
|
|
|
O– |
|
O– |
|
O– |
|
||
|
|
|
|
|
|
|
Active antimetabolite |
|
||||
|
|
|
Viral DNA template |
|
|
|
|
|
||||
|
Base |
|
|
Base |
|
|
|
Base |
|
|
||
|
5' |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
DNA-chain |
|
O |
CH2 O |
|
O |
CH2 O |
|
|
|
O |
CH2 O |
|
||
|
|
|
|
|
termination |
|||||||
P |
3' |
P |
|
3' |
|
|
P |
|
|
|
|
|
|
|
|
|
HO |
|
|
P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
DNA |
|
|
|
Viral |
|
P |
|
|
|
|
Inhibition |
|
synthesis |
|
|
|
|
|
|
|
|
||||
|
|
DNA polymerase |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|||||
B. Inhibitor of DNA-polymerase: |
||||
Foscarnet |
|
|
||
|
|
|
Base |
|
|
|
O |
CH2 O |
|
|
P |
|
3´ |
|
|
O |
|
HO |
|
O |
P |
O |
|
|
|
|
|||
|
O |
|
|
|
O |
P |
O |
|
|
|
O |
|
|
Viral |
|
|
|
|
|
|
|
|
|
DNA polymerase |
|
|
|
O |
|
|
|
|
C |
O |
|
|
|
O P |
Foscarnet |
|
|
|
O |
|
|
|
|
O |
|
C. Prophylaxis for viral flu |
|
|
Influenza A virus |
|
Viral channel |
|
protein |
Endosome |
H+ |
|
|
|
NH2 |
Inhibition of |
Amantadine |
uncoating |
|
|
Neuraminidase |
Inhibition of |
inhibitors |
|
|
release |
|
