
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

88 Drugs Acting on the Sympathetic Nervous System
Adrenoceptor Subtypes and Catecholamine Actions
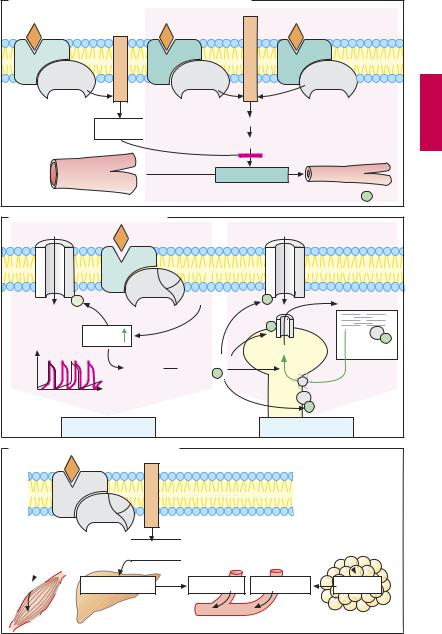
The biological effects of epinephrine and norepinephrine are mediated by nine different adrenoceptors (α1A,B,D, α2A,B,C, β1, β2, β3). To date, only the classification into α1, α2, β1 and β2 receptors has therapeutic relevance.
Smooth Muscle Effects
The opposing effects on smooth muscle (A) of α- and β-adrenoceptor activation are due to differences in signal transduction. α1-Re- ceptor stimulation leads to intracellular release of Ca2+ via activation of the inositol trisphosphate (IP3) pathway. In concert with the protein calmodulin, Ca2+ can activate myosin kinase, leading to a rise in tonus via phosphorylation of the contractile protein myosin († vasoconstriction). α2-Adrenocep- tors can also elicit a contraction of smooth muscle cells by activating phospholipase C (PLC) via the βγ-subunits of G1 proteins.
cAMP inhibits activation of myosin kinase. Via stimulatory G-proteins (Gs), β2-receptors mediate an increase in cAMP production († vasodilation).
Vasoconstriction induced by local application of α-sympathomimetics can be employed in infiltration anesthesia (p.204) or for nasal decongestion (naphazoline, tetrahydrozoline, xylometazoline; p.94, 336, 338). Systemically administered epinephrine is important in the treatment of anaphylactic shock and cardiac arrest.
Bronchodilation. β2-Adrenoceptor-medi- ated bronchodilation plays an essential part in the treatment of bronchial asthma and chronic obstructive lung disease (p.340). For this purpose, β2-agonists are usually given by inhalation; preferred agents being those with low oral bioavailability and low risk of systemic unwanted effects (e.g., fenoterol, salbutamol, terbutaline).
Tocolysis. The uterine relaxant effect of β2- adrenoceptor agonists, such as fenoterol, can be used to prevent premature labor. β2-Vaso- dilation in the mother with an imminent drop in systemic blood pressure results in reflex tachycardia, which is also due in part to the β1-stimulant action of these drugs.
Cardiostimulation
By stimulating β-receptors, and hence cAMP production, catecholamines augment all heart functions including systolic force, velocity of myocyte shortening, sinoatrial rate, conduction velocity, and excitability. In pacemaker fibers, cAMP-gated channels (“pacemaker channels”) are activated, whereby diastolic depolarization is hastened and the firing threshold for the action potential is reached sooner (B). cAMP activates protein kinase A, which phosphorylates different Ca2+ transport proteins. In this way, contraction of heart muscle cells is accelerated, as more Ca2+ enters the cell from the extracellular space via L-type Ca2+ channels and release of Ca2+ from the sarcoplasmic reticulum (via ryanodine receptors, RyR) is augmented. Faster relaxation of heart muscle cells is effected by phosphorylation of troponin and phospholamban.
In acute heart failure or cardiac arrest, β- mimetics are used as a short-term emergency measure; in chronic failure they are not indicated.
Metabolic Effects
Via cAMP, β2-receptors mediate increased conversion of glycogen to glucose (glycoge- nolysis) in both liver and skeletal muscle. From the liver, glucose is released into the blood. In adipose tissue, triglycerides are hydrolyzed to fatty acids (lipolysis mediated by β2- and β3-receptors), which then enter the blood.
Adrenoceptor Subtypes and Catecholamine Actions |
89 |
A. Effects of catecholamines on vascular smooth muscle
Relaxation |
Contraction |
|
|
cyclase-Ad |
|
|
C |
|
|
β 2 |
|
α 1 |
|
Phospholipase |
α 2 |
|
|
|
|
|
|
|
|||
Gs |
α |
|
Gq |
α |
|
βγ |
Gi |
|
|
|
|
|
cAMP

In
hib
itio
n
IP3
Ca2+/Calmodulin
Myosin-Kinase
|
Myosin |
|
|
|
Myosin- P |
B. Cardiac effects of catecholamines |
|
|
|
|
|
|
|
|
|
Ca channel |
|
|
β |
Ad-cyclase |
|
|
|
|
Gs |
|
|
|
|
Pacemaker |
+ |
|
|
P |
Ca2+ |
channels |
|
|
|
|
|
|
cAMP |
|
RyR |
P |
|
|
|
|
Ca2+ |
P |
|
|
|
|
|
Troponin |
|
|
Protein |
Phosphory- |
|
|
|
|
kinase A |
lation |
P |
|
|
|
|
|
|
|
Ca-ATPase |
|
|
|
|
P |
Phospholamban |
|
Positive chronotropic |
|
|
Positive inotropic |
|
C. Metabolic effects of catecholamines
Gs |
cyclase-Ad |
β |
|
 cAMP
cAMP

Glycogenolysis |
Glycogenolysis |
Glucose |
Fatty acids |
Lipolysis |
Glucose |
|
|
|
|

90 Drugs Acting on the Sympathetic Nervous System
Structure–Activity Relationships of Sympathomimetics
Owing to its equally high af nity for all α- and β-receptors, epinephrine does not permit selective activation of a particular receptor subtype. Like most catecholamines, it is also unsuitable for oral administration (catechole is a trivial name for o-hydroxyphe- nol). Norepinephrine differs from epinephrine by its high af nity for α-receptors and low af nity for β2-receptors. The converse holds true for the synthetic substance, isoproterenol (isoprenaline) (A).
Norepinephrine |
† α, β1 |
|
Epinephrine |
† |
α, β1 β2 |
Isoproterenol |
† |
β1, β2 |
Knowledge of structure–activity relationships has permitted the synthesis of sympathomimetics that display a high degree of selectivity at adrenoceptor subtypes.
Direct-acting sympathomimetics (i.e. adrenoceptor agonists) typically share a phenlethylamine structure. The side chain β- hydroxyl group confers af nity for α- and β- receptors. Substitution on the amino group reduces af nity for α-receptors, but increases it for β-receptors (exception: α-ago- nist phenylephrine), with optimal af nity being seen after the introduction of only one isopropyl group. Increasing the bulk of amino substituents favors af nity for β2-re- ceptors (e.g., fenoterol, salbutamol). Both hydroxyl groups on the aromatic nucleus contribute to af nity; high activity at α-re- ceptorsisassociated withhydroxylgroupsat the 3 and 4 positions. Af nity for β-receptors is preserved in congeners bearing hydroxyl groups at positions 3 and 5 (orciprenaline, terbutaline, fenoterol).
The hydroxyl groups of catecholamines are responsible for the very low lipophilicity of these substances. Polarity is increased at physiological pH owing to protonation of the amino group. Deletion of one or all hydroxyl groups improves the membrane penetrability at the intestinal mucosa–blood barrier and the blood–brain barrier. Accordingly,
these noncatecholamine congeners can be given orally and can exert CNS actions; however, this structural change entails a loss in af nity.
Absence of one or both aromatic hydroxyl groups is associated with an increase in indirect sympathomimetic activity, denoting the ability of a substance to release norepinephrine from its neuronal stores without exerting an agonist action at the adrenoceptor (p.92).
A change in position of aromatic hydroxyl groups (e.g., in orciprenaline, fenoterol, or terbutaline) or their substitution (e.g., salbutamol) protects against inactivation by COMT (p.87). Introduction of a small alkyl residue at the carbon atom adjacent to the amino group (ephedrine, methamphetamine) confersresistance to degradation by MAO(p.87); replacement on the amino groups of the methyl residue with larger substituents (e.g., ethyl in etilefrine) impedes deamination by MAO. Accordingly, the congeners are less subject to presystemic inactivation.
Since structural requirements for high affinity on the one hand and oral applicability on the other do not match, choosing a sympathomimetic is a matter of compromise. If the high af nity of epinephrine is to be exploited, absorbability from the intestine must be foregone (epinephrine, isoprenaline). If good bioavailability with oral administration is desired, losses in receptor af nity must be accepted (etilefrine).

Structure–Activity Relationships of Sympathomimetics |
91 |
A. Interaction between epinephrine and the β 2-adrenoceptor |
|
|
||||||
|
|
|
|
|
β 2 Adrenoceptor |
6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phe 290 |
|
|
|
|
|
|
|
|
Asn 293 |
Ser207 |
|
|
|
|
|
Phe |
|
|
|
|
|
Asp |
|
Ser |
Asn |
|
|
5 |
|
|
|
|
|
|
|
||
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
|
|
|
|
|
|
|
Asp113 |
|
Ser204 |
|
|
|
|
|
|
|
Ser203 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
HO |
|
|
|
|
3 |
|
|
|
|
|
|
|
+ |
|
Epinephrine |
|
|
HO |
|
CH |
CH2 |
NH2 |
CH3 |
|
|
|
|
|
OH |
|
|
Epinephrine |
|
|
B. Structure–activity relationship of epinephrine |
|
|
||
|
|
Catecholamine |
|
|
|
|
O-methyltransferase |
|
|
|
|
(COMT) |
|
|
Lack of penetrability |
HO |
|
|
|
|
|
+ |
Metabolic |
|
through membrane |
|
|
||
barriers |
HO |
CH CH2 |
NH2 CH3 |
reaction sites |
|
||||
|
|
OH |
|
|
(poor enteral absorbability |
|
Monoamine oxidase |
|
|
and CNS penetrability) |
|
|
(MAO) |
|
C . Direct sympathomimetics
Receptor subtype selectivity of direct sympathomimetics
α 1 |
|
|
|
α 2 |
|
|
β 1 |
|
β 2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Epinephrine |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Norepinephrine |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dobutamine |
|
|
|
||
|
|
|
|
|
|
|
|||
Phenylephrine |
|
|
|
|
|
|
|
|
Fenoterol |
|
|
|
|
|
|
|
|
||
|
|
|
|
Clonidine |
|
|
Salbutamol |
||
|
|
|
|
|
|
Terbutaline |
|||
|
|
|
Brimonidine |
|
|
Salmeterol |
|||
|
|
|
Naphazoline |
|
|
Formoterol |
|||
|
|
|
Oxymetazoline |
|
|
|
|||
|
|
|
Xylometazoline |
|
|
|
|||
