
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

144 Antithrombotics
Prophylaxis and Therapy of Thromboses
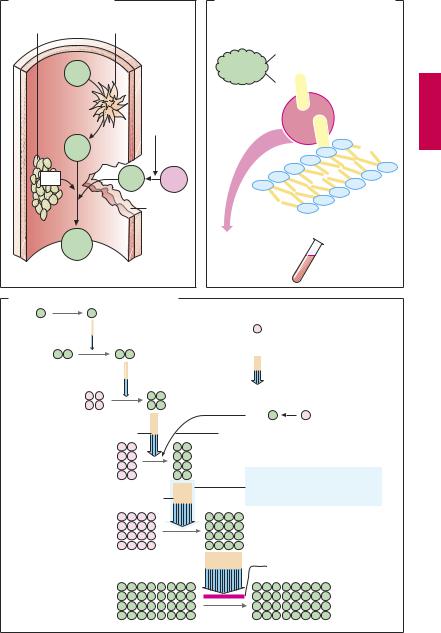
Upon vascular injury, the coagulation system is activated: thrombocytes and fibrin molecules coalesce into a “plug” that seals the defect and halts bleeding (hemostasis). Unnecessary formation of an intravascular clot—a thrombosis—can be life-threatening. If the clot forms on an atheromatous plaque in a coronary artery, myocardial infarction is imminent; a thrombus in a deep leg vein can be dislodged and carried into a lung artery and can cause pulmonary embolism.
Drugs that decrease the coagulability of blood, such as coumarins and heparin (A) are employed for the prophylaxis of thromboses. In addition, attempts are directed, by means of acetylsalicylic acid, at inhibiting the aggregation of blood platelets, which are prominently involved in intra-ar- terial thrombogenesis (p.152). For the therapy of thrombosis, drugs are used that dissolve the fibrin meshwork—fibrinolytics (p.150).
An overview of the coagulation cascade and sites of action for coumarins and heparin is shown in (A). There are two ways to initiate the cascade (B): (1) conversion of factor XII into its active form (XIIa, intrinsic system) at intravascular sites denuded of endothelium; (2) conversion of factor VII into VIIa (extrinsic system) under the influence of a tissue-derived lipoprotein (tissue thromboplastin). Both mechanisms converge via factor X into a common final pathway.
The clotting factors are protein molecules. “Activation” mostly means proteolysis (cleavage of protein fragments) and, with the exception of fibrin, conversion into proteinhydrolyzing enzymes (proteases). Some activated factors require the presence of phospholipids (PL) and Ca2+ for their proteolytic activity. Conceivably, Ca2+ ions cause the adhesion of factor to a phospholipid surface, as depicted in (B). Phospholipids are contained in platelet factor 3 (PF3), which is released from aggregated platelets, and in tissue
thromboplastin (A). The sequential activation of several enzymes allows the aforementioned reactions to “snowball” (symbolized in C by increasing number of particles), culminating in massive production of fibrin.
Ca2+-chelators (B) prevent the enzymatic activity of Ca2+-dependent factors; they contain COO– groups that bind Ca2+ ions (C): citrate and EDTA (ethylenediaminetetraacetic acid) form soluble complexes with Ca2+; oxalate precipitates Ca2+ as insoluble calcium oxalate. Chelation of Ca2+ cannot be used in vivo for therapeutic purposes because Ca2+ concentrations would have to be lowered to a level incompatible with life (hypocalcemic tetany). These compounds (sodium salts) are, therefore, used only for rendering blood incoagulable outside the body. This effect can be reversed at any time by addition of Ca2+ ions.
In vivo, the progression of the coagulation cascade can be inhibited as follows (C):
1.Coumarin derivatives decrease the blood concentrations of inactive factors II, VII, IX and X, by inhibiting their synthesis in the liver.
2.The complex consisting of heparin and antithrombin III neutralizes the protease activity of activated factors; unlike unfractionated heparin, low-molecular-weight heparin fragments or the “minimal molecular subunit of heparin,” fondaparinux, inhibit only activated factor Xa when complexed with antithrombin III.
3.Hirudin and its derivatives (bivalirudin, lepirudin) block the active center of thrombin.
Prophylaxis and Therapy of Thromboses |
145 |
A. Activation of clotting |
|
|
B. Inhibition of clotting by removal of Ca2+ |
|
Platelets |
Endothelial defect |
Clotting factor |
|
|
|
|
|
|
|
|
|
|
COO– |
|
XII |
|
|
COO– |
|
|
|
|
|
|
|
|
Tissue |
+ |
+ |
|
|
kinase |
Ca |
|
|
|
thrombo- |
|
|
XIIa |
|
|
|
– |
|
|
– |
– |
|
|
|
|
|
|
|
|
|
– |
|
PF3 |
VIIa |
VII |
– |
|
|
|
|
– |
|
|
|
Vessel |
|
Phospholipids, |
|
|
rupture |
|
|
|
|
|
e.g., PF3 |
|
|
|
|
|
|
Fibrin |
|
|
Ca2+ chelation |
|
|
|
|
Citrate |
only in vitro |
|
|
|
EDTA |
|
|
|
|
|
|
|
|
|
Oxalate |
|
C. Inhibition of clotting cascade in vivo |
|
|
||
XII |
XIIa |
|
|
|
|
|
Synthesis susceptible to |
|
|
|
inhibition by coumarins |
XI |
XIa |
|
Reaction susceptible to |
|
|
|
|
|
|
|
inhibition by heparin– |
|
|
|
antithrombin complex |
|
IX |
IXa |
|
|
|
VIIa |
VII |
|
VIII + Ca2+ + Pl |
Ca2+ + Pl (phospholipids) |
|
|
X |
Xa |
|
|
|
Susceptible to inhibition also |
|
|
|
by low-molecular-weight |
|
|
V + Ca2+ + Pl |
heparin and fondaparinux |
|
|
Prothrombin II |
IIa Thrombin |
|
|
|
|
Hirudin |
|
Fibrinogen I |
|
Ia Fibrin |

146 Antithrombotics
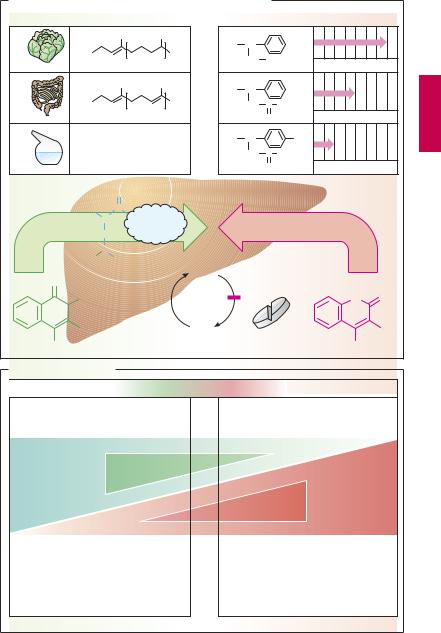
VitaminKAntagonistsand VitaminK
Vitamin K promotes the hepatic γ-carboxy- lation of glutamate residues on the precursors of factors II, VII, IX, and X. Carboxyl groups are required for Ca2+-mediated binding to phospholipid surfaces (p.144). There are several vitamin K derivatives of different origins: K1 (phytomenadione) from chlorophyllous plants; K2 from gut bacteria; and K3 (menadione) synthesized chemically. All are hydrophobic and require bile acids for absorption.
Oral anticoagulants. Structurally related to vitamin K, 4-hydroxycoumarins act as ”false” vitamin K and prevent regeneration of reduced (active) vitamin K from vitamin K epoxide, hence the synthesis of vitamin K- dependent clotting factors
Coumarins are well absorbed after oral administration. Their duration of action varies considerably. Synthesis of clotting factors depends on the intrahepatocytic concentration ratio between coumarins and vitamin K. The dose required for an adequate anticoagulant effect must be determined individually for each patient (monitoring of the International Normalized Ratio, INR).
Indications. Hydroxycoumarins are used for the prophylaxis of thromboembolism as, for instance, in atrial fibrillation or after heart valve replacement.
The most important adverse effect is bleeding. With coumarins, this can be counteracted by giving vitamin K1. However, coagulability of blood returns to normal only after hours or days, when the liver has resumed synthesis and restored suf cient blood levels of carboxylated clotting factors. In urgent cases, deficient factors must be replenished directly (e.g., by transfusion of whole bloodor ofprothrombin concentrate).
Other notable adverse effects include: at the start of therapy, hemorrhagic skin necroses and alopecia; with exposure in utero, disturbances of fetal cartilage and bone for-
mation and CNS injury (due to bleeding); enhanced risk of retroplacental bleeding.
Possibilities for Interference (B)
Adjusting the dosage of a hydroxycoumarin calls for a delicate balance between the opposing risks of bleeding (effect too strong) and of thrombosis (effect too weak). After the dosage has been titrated successfully, loss of control may occur if certain interfering factorsare ignored. If the patientchanges dietary habits and consumes more vegetables, vitamin K may predominate over the vitamin K antagonist. If vitamin K-producing gut flora is damaged in the course of antibiotic therapy, the antagonist may prevail. Drugs that increase hepatic biotransformation via enzyme induction (p.38) may accelerate elimination of a hydroxycoumarin and thus lower its blood level. Inhibitors of hepatic biotransformation (e.g., the H2 blocker cimetidine) augment the action of hydroxycoumarins. Apart from pharmacokinetic alterations, pharmacodynamic interactions must be taken into account. Thus, acetylsalicylic acid is contraindicated because (a) it retards hemostasis by inhibiting platelet aggregation and (b) itmaycause damage tothe gastric mucosawith erosion of blood vessels.
Vitamin K Antagonists and Vitamin K |
147 |
A. Vitamin K-antagonists of the coumarin type and vitamin K |
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
Duration of action/days |
||
|
|
|
CH3 |
|
CH3 |
R = |
CH |
|
|
|
|
|
|
R = |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
CH3 |
|
|
|
||
Vit. K1 |
Phytomenadione |
3 |
|
Phenprocoumon |
||||||||
|
|
|
|
|
||||||||
|
|
|
CH3 |
|
CH3 |
R = |
CH |
|
|
|
|
|
|
R = |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
CH3 |
|
CH2 |
C |
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Vit. K2 |
|
|
|
|
1 – 12 |
|
|
O |
|
Warfarin |
|
|
|
|
|
|
|
|
|
|
|
||||
|
R = H |
|
|
|
|
R = |
CH |
|
|
NO2 |
|
|
|
|
|
|
|
|
CH2 C CH3 |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Vit. K3 |
Menadione |
|
|
|
|
O |
|
Acenocoumarol |
||||
|
|
|
O |
Carboxylation of glutamine residues |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2N |
CH |
C |
|
|
|
|
|
|
|
|
|
|
|
II, VII, IX, X |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
HOOC |
CH |
|
|
|
|
|
|
|
|
|
|
|
|
COOH |
|
Vit. K- |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
Epoxid |
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
O |
O |
|
R |
|
|
|
|
Vit. K |
4-Hydroxy- |
|
|
R |
||
OH |
|
|
|
|
|
|
|
OH |
|
|||
Vit. K derivates |
|
|
coumarin derivatives |
|
||||||||
B. Possible interactions
Risk of thrombosis |
Optimal adjustment |
Risk of bleeding |
|
|
|
Increased intake of vitamin K-rich food
Increase
Vitamin K effect
Decrease
Inhibition of enteral coumarin absorption by adsorbents, e.g., antacids, medicinal charcoal
Acceleration of hepatic coumarin metabolism: enzyme induction, e.g., by carbamazepine, rifampicin
Damage to vitamin K-producing intestinal bacteria by antibiotics
Decrease
Hydroxycoumarin effect
Increase
Inhibition of hepatic coumarin metabolism, e.g., by cimetidine, metronidazole
