
- •VOLUME 4
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •HYDROCEPHALUS, TOOLS FOR DIAGNOSIS AND TREATMENT OF
- •HYPERALIMENTATION.
- •HYPERBARIC MEDICINE
- •HYPERBARIC OXYGENATION
- •HYPERTENSION.
- •HYPERTHERMIA, INTERSTITIAL
- •HYPERTHERMIA, SYSTEMIC
- •HYPERTHERMIA, ULTRASONIC
- •HYPOTHERMIA.
- •IABP.
- •IMAGE INTENSIFIERS AND FLUOROSCOPY
- •IMAGING, CELLULAR.
- •IMAGING DEVICES
- •IMMUNOLOGICALLY SENSITIVE FIELD–EFFECT TRANSISTORS
- •IMMUNOTHERAPY
- •IMPEDANCE PLETHYSMOGRAPHY
- •IMPEDANCE SPECTROSCOPY
- •IMPLANT, COCHLEAR.
- •INCUBATORS, INFANTS
- •INFANT INCUBATORS.
- •INFUSION PUMPS.
- •INTEGRATED CIRCUIT TEMPERATURE SENSOR
- •INTERFERONS.
- •INTERSTITIAL HYPERTHERMIA.
- •INTRAAORTIC BALLOON PUMP
- •INTRACRANIAL PRESSURE MONITORING.
- •INTRAOCULAR LENSES.
- •INTRAOPERATIVE RADIOTHERAPY.
- •INTRAUTERINE DEVICES (IUDS).
- •INTRAUTERINE SURGICAL TECHNIQUES
- •ION-EXCHANGE CHROMATOGRAPHY.
- •IONIZING RADIATION, BIOLOGICAL EFFECTS OF
- •ION-PAIR CHROMATOGRAPHY.
- •ION–SENSITIVE FIELD-EFFECT TRANSISTORS
- •ISFET.
- •JOINTS, BIOMECHANICS OF
- •JOINT REPLACEMENT.
- •LAPARASCOPIC SURGERY.
- •LARYNGEAL PROSTHETIC DEVICES
- •LASER SURGERY.
- •LASERS, IN MEDICINE.
- •LENSES, CONTACT.
- •LENSES, INTRAOCULAR
- •LIFE SUPPORT.
- •LIGAMENT AND TENDON, PROPERTIES OF
- •LINEAR VARIABLE DIFFERENTIAL TRANSFORMERS
- •LITERATURE, MEDICAL PHYSICS.
- •LITHOTRIPSY
- •LIVER TRANSPLANTATION
- •LONG BONE FRACTURE.
- •LUNG MECHANICS.
- •LUNG PHYSIOLOGY.
- •LUNG SOUNDS
- •LVDT.
- •MAGNETIC RESONANCE IMAGING
- •MAGNETOCARDIOGRAPHY.
- •MANOMETRY, ANORECTAL.
- •MANOMETRY, ESOPHAGEAL.
- •MAMMOGRAPHY
- •MATERIALS, BIOCOMPATIBILITY OF.
- •MATERIALS, PHANTOM, IN RADIOLOGY.
- •MATERIALS, POLYMERIC.
- •MATERIALS, POROUS.
- •MEDICAL EDUCATION, COMPUTERS IN
- •MEDICAL ENGINEERING SOCIETIES AND ORGANIZATIONS
- •MEDICAL GAS ANALYZERS
- •MEDICAL PHOTOGRAPHY.
- •MEDICAL PHYSICS LITERATURE
- •MEDICAL RECORDS, COMPUTERS IN
- •MICROARRAYS
- •MICROBIAL DETECTION SYSTEMS
- •MICROBIOREACTORS
- •MICRODIALYSIS SAMPLING
- •MICROFLUIDICS
- •MICROPOWER FOR MEDICAL APPLICATIONS
- •MICROSCOPY AND SPECTROSCOPY, NEAR-FIELD
- •MICROSCOPY, CONFOCAL
- •MICROSCOPY, ELECTRON
- •MICROSCOPY, FLUORESCENCE
- •MICROSCOPY, SCANNING FORCE
- •MICROSCOPY, SCANNING TUNNELING
- •MICROSURGERY
- •MINIMALLY INVASIVE SURGICAL TECHNOLOGY
- •MOBILITY AIDS
- •MODELS, KINETIC.
- •MONITORING IN ANESTHESIA
- •MONITORING, AMBULATORY.
- •MONITORING, FETAL.
- •MONITORING, HEMODYNAMIC
- •MONITORING, INTRACRANIAL PRESSURE
- •MONITORING, NEONATAL.
- •MONITORING, UMBILICAL ARTERY AND VEIN
- •MONOCLONAL ANTIBODIES
- •MOSFET.
- •MUSCLE ELECTRICAL ACTIVITY.
- •MUSCLE TESTING, REHABILITATION AND.
- •MUSCULOSKELETAL DISABILITIES.
35.Jones S, Wang WZ, Natajaraj C, Khiabani, Stephenson LL, Zamboni WA. HBO inhibits IR induced Neutrophil CD 18 Polarization by a nitric oxide mechanism. Undersea Hyperb Med 2002;35 (Suppl):75.
36.Zamboni WA, Roth AC, Russel RC, Nemiroff PM, Casas L, Smoot EC. Hyperbaric oxygen improves axial skin flap survival when administered during and after total ischemia. J Reconstr Micro 1989;5:343–347.
37.Gimbel M, Hunt TK. Wound healing and hyperbaric oxygen. In: Kindwall EP, Whelan HT, eds. Hyperbaric Medicine Practice, 2nd ed. Flagstaff, AZ: Best; 1999. p 169–204.
38.Bartlett B. Hyperbaric therapy. Radiation Injury 1994:2–3. HBO has been shown to increase angiogenesis and blood flow in previously irradiated tissue or bone. (Marx RE, Ehler WJ, Taypongsak PT, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg 1990; 160:519–524.
39.Carl UM, Feldmeier JJ, Schmitt G, Hartmann KA. Hyperbaric oxygen therapy for late sequelae in women receiving radiation after breast conserving surgery. Int J Radiat Oncol Biol Phys 2001;49:1029–1031.
40.Feldmeier JJ. Hyperbaric oxygen: Does it have a cancer causing or growth enhancing effect. Proceedings of the consensus conference sponsored by the European society for therapeutic radiology and oncology and the European committee for hyperbaric medicine. Portugal, 2001:129–146.
41.Gray KH, Conger AD, Ebert M, Hornsey S, Scott OCA. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26: 638–648.
42.Wada J, Ikeda T, Kamata K, Ebuoka M. Oxygen hyperbaric treatment for carbon monoxide poisoning and severe burn in coal mine gas explosion. Igakunoaymi (Japan) 1965;54–68.
43.Germonpre P, Reper P, Vanderkelen A. Hyperbaric oxygen therapy and piracetam decrease the early extension of deep partial thickness burns. Burns 1996;6:468–473.
44.Cianci P,Sato R, Green B.Adjunctive hyperbaricoxygen reduces length of hospital stay, surgery and the cost of care in severe burns. Undersea Biomed Research Suppl 1991;18:108.
45.Bartlett R. Carbon monoxide poisoning. In: Haddad M, Shannon M, Winchester J, eds. Poisoning and Drug Overdose, 3rd ed. New York: WB Saunders Company; 2002.
46.Bakker DJ, Cramer FS. Hyperbaric Surgery Perioperative Care. Flagstaff, AZ: Best Publishing; 2002.
See also BLOOD GAS MEASUREMENTS; HYPERBARIC OXYGENATION; OXYGEN MONITORING; PULMONARY PHYSIOLOGY; RESPIRATORY MECHANICS AND GAS EXCHANGE; VENTILATORY MONITORING.
HYPERBARIC OXYGENATION
HARRY T. WHELAN
JEFFREY A. NIEZGODA
MATTHEW C. LEWIS
Medical College of Wisconsin
Milwaukee, Wisconsin
ERIC P. KINDWALL
BERNADETTE CABIGAS
St. Luke’s Medical Center
Milwaukee, Wisconsin
INTRODUCTION
Hyperbaric oxygen (HBO) is simply the delivery of molecular oxygen in very high dosage. Even though experience
HYPERBARIC OXYGENATION |
29 |
has shown HBO to be very useful in a number of conditions, the exact mechanism of action at the molecular level is not fully understood. Studies done by Thom et al. (1) demonstrated that elevated oxygen tensions stimulated neuronal nitric oxide synthase (NOS1) and increased steady-state nitric oxide concentration in their microelectrodeimplanted rodents. Buras et al. (2) in their studies with human umbilical vein endothelial cells (HUVEC) and bovine aortic endothelial cells (BAEC) showed that hyperbaric oxygen (HBO) down-regulated intracellular adhesion molecule 1 (ICAM 1) expression via the induction of endothelial nitric oxide synthase (NOS3), which proved beneficial in treating ischemia reperfusion injuries. Other studies talk about interactions between nitric oxide and oxygen species and their role in various disease states. Clearly, interest in HBO is growing.
Boerema (3) introduced hospital use of the hyperbaric chamber in the late 1950s in Holland, simply to maintain a semblance of normoxia in patients undergoing cardiac surgery. Heart–lung machines had not yet been invented, and the use of the chamber made certain kinds of cardiac surgery possible for the first time. Boerema felt that if enough oxygen could be driven physically into solution in the tissues, which he termed ‘‘drenching’’, the circulation to the brain could be interrupted longer than 3–4 min. It also rendered surgery on many pediatric patients less risky. For example, if the normal arterial pO2 in a patient with Tetralogy of Fallot was 38 mmHg, placing him in the chamber might raise it to 94 mmHg. Operating on the patient under hyperbaric conditions posed much less risk of ventricular fibrillation when the heart or great vessels were manipulated.
This idea caught on quickly, and soon large surgical hyperbaric chambers were built in Glasgow, New York, Los Angeles, Chicago, Minneapolis, and at Boston Children’s Hospital. By the early 1960s, however, heart–lung machines became more common, and the need to do surgery in the hyperbaric chamber diminished substantially. Many large surgical chambers were left to gather dust or were dismantled, as hospital floor space is always at a premium. During this time the surgeons, who had been doing most of the research, left the field. Of the nondiving conditions, only carbon monoxide poisoning and gas gangrene seemed to be likely candidates for hyperbaric oxygen treatment based on credible research.
In 1969, however, a double-blind controlled study on the use of hyperbaric oxygen in senility was published in The New England Journal of Medicine. Results seemed promising, and this initiated the propagation of hyperbaric quackery. The original investigators made no sweeping claims for the research, but simply felt that the area merited further investigation. Eventually, further research showed that the results of the study reported in the New England Journal article were a statistical anomaly and could not be reproduced. However, hyperbaric enthusiasts seized upon the earlier report, and senility began to be treated in hyperbaric chambers, along with a host of other diseases. Most of these were not in medical centers. Fly-by-night ‘‘clinics’’ suddenly appeared claiming to cure anything and everything. Patients were treated for skin wrinkles, loss of sexual vigor, and a host of other
30 HYPERBARIC OXYGENATION
maladies. As there were few investigators doing good research in the area at that time, the field fell into disrepute.
Fortunately, a few legitimate investigators persisted in their work, looking at the effects of hyperbaric oxygen in greater detail. Soon it became clear that under hyperbaric conditions oxygen had some unusual effects. The Undersea and Hyperbaric Medical Society created a committee to investigate the field. After careful study, the committee laid down guidelines for what should be reimbursed by third-party payers and what conditions should be considered investigational. Their report appeared in 1977 and was adopted as a source document for Blue Cross/Blue Shield (4). About the same time, Jefferson C. Davis of the United States Air Force School of Aerospace Medicine edited the first textbook in hyperbaric medicine (5). It was only then that a firm scientific basis was reestablished for the field, leading to increased acceptance by the medical community. The number of chambers operating in hospitals has risen dramatically from only 37 in 1977 to > 500 today. The Undersea and Hyperbaric Medical Society (www.UHMS.org) and the American College of Hyperbaric Medicine (www.ACHM.org) have taken responsibility for setting standards in this field and for encouraging additional research. At this time, 13 clinical disorders have been approved for hyperbaric treatment. They include air or gas embolism, carbon monoxide poisoning, clostridial myonecrosis, crush injury or compartment syndrome, decompression sickness, problem wounds, severe blood loss anemia, necrotizing soft tissue infections, osteomyelitis, radiation tissue damage, skin grafts or flaps, thermal burns and brain abscess.
Remember that hyperbaric oxygen was introduced initially into hospitals in order to simply maintain normoxia or near-normoxia in patients undergoing surgery. It was only later, and quite serendipitously that researchers discovered that oxygen under increased atmospheric pressure gained some of the attributes of a pharmacologic agent. Oxygen begins to act like a drug when given at pressures of 2 atm or greater. For example, oxygen under pressure can terminate lipid peroxidation in vivo (6), it can enhance the bacteriocidal capabilities of the normal leukocyte (7,8), and it can stimulate the growth of new capillaries in chronically ischemic tissue, such as in the diabetic foot, or in tissue that has undergone heavy radiation. It can reduce intracranial pressure on the order of 50% within seconds of its initiation, and this effect is additive to that of hypocapnia (9–11). HBOT can increase the flexibility of red cells, augmenting the effects of pentoxifylline (12). It can decrease edema formation by a factor of 50% in postischemic muscle and prevent sec- ond-degree burn from advancing to full-thickness injury (13–15). Hyperbaric oxygen has also been shown to hasten functional recovery of traumatized peripheral nerves by almost 30% following repair. Many of these discoveries have been made only in the last decade.
In a number of these areas, we are beginning to understand the basic mechanisms of action, but overall very little is understood at the molecular level. It is anticipated that studies involving nitric oxide synthase will provide insight regarding the elusive molecular mechanistic
explanation. Also, many contributions to our understanding have come from advances made in the biochemistry of normal wound healing. We understand that normal oxygen pressures are 80–90-mmHg arterially, that oxygen enters our tissues from the capillaries, and that at this interface carbon dioxide (CO2) is removed. Under hyperbaric conditions, all of this changes. At a chamber pressure of 2.4 atm (ATA), the arterial oxygen pressure (pO2) reaches 1500 mmHg, immediately saturating the red blood cells (RBCs). Upon reaching the tissues, these RBCs never unload their oxygen. At this high partial pressure of gas, oxygen diffuses into the tissues directly from the plasma. Returning to the heart, the RBCs are bathed in plasma with a pO2 of 150–200 mmHg. Tissue oxygen requirements are completely derived from the plasma. In theory, one might think that this condition could prove fatal, as red cells no longer can carry CO2 away from the tissues. However, we are fortunate that CO2 is 50 times more soluble in plasma than are oxygen and nitrogen, and the body has a very capable buffering system which overcomes the loss of the Haldane effect, which is the increase in CO2 carrying capacity of deoxygenated red cells (16).
Another factor to be considered is the actual part of the circulatory system that overcomes the loss of the Haldane effect. Traditionally, we think of this exchange occurring in the capillaries. Under very high pressures, however, computer modeling has shown that nitrogen exchange under pressure (as in deep sea divers) is probably complete by the time the blood reaches the arteriolar level. Whether this is true when hyperbaric oxygen is breathed has not yet been determined. The rate of metabolism under hyperbaric conditions appears to be unchanged, and the amount of CO2 produced appears to be about the same as when breathing air. It would be interesting to know just at what level oxygen exchange is accomplished in the tissues, as this might have practical implications when treating people with severe capillary disease.
Oxygen can be toxic under pressure. Pulmonary toxicity and lung damage can be seen at oxygen pressures > 0.6 atm during chronic exposure. Central nervous system (CNS) toxicity can manifest as generalized seizure activity when oxygen is breathed at pressures of 3 atm or greater. The CNS toxicity was first observed by Paul Bert in 1878, and is termed the ‘‘Paul Bert Effect’’ (17). Despite years of research into this phenomenon, the exact underlying or molecular cause of the seizure has not yet been discovered. There is a generalized vasoconstriction that occurs when oxygen is breathed at high pressure, reducing blood flow to muscle, heart, and brain by a factor of20%, as a defense against toxic quantities of oxygen. The exact mechanism responsible for this phenomenon is not fully understood.
Central nervous system oxygen toxicity was evaluated by the Royal Navy. The purpose of this research was to determine the time until convulsion so that combat swimmers would know their endurance limits under various conditions. Volunteer research subjects swam in a test tank using closed-circuit oxygen rigs until convulsion occurred and thus established safe oxygen tolerance boundaries.
Also related to the effect of oxygen, the ‘‘off ’’ phenomenon (18) was first described by Donald in 1942. He observed that seizures sometimes occurred when the chamber pressure was reduced or when a diver surfaced and oxygen breathing under pressure was suddenly terminated. Lambertsen (19) provided a description of this type of seizure activity:
The convulsion is usually but not always preceded by the occurrence of localized muscular twitching, especially about the eyes, mouth and forehead. Small muscles of the hands may also be involved, and incoordination of diaphragm activity in respiration may occur. After they begin, these phenomena increase in severity over a period which may vary from a few minutes to nearly an hour, with essentially clear consciousness being retained. Eventually an abrupt spread of excitation occurs and the rigid tonic phase of the convulsion begins. Respiration ceases at this point and does not begin again until the intermittent muscular contractions return. The tonic phase lasts for about 30 seconds and is accompanied by an abrupt loss of consciousness. It is followed by vigorous clonic contractions of the muscle groups of the head and neck, trunk and limbs. As the incoordinated motor activity stops, respiration can proceed normally.
Within the wound healing community, current doctrine holds that a tissue pO2 of 30–40 mmHg is necessary for adequate wound healing (20,21). Below 30 mmHg, fibroblasts are unable to replicate or produce collagen. Additionally, when the pO2 drops < 30 mmHg, leukocytes are unable to utilize oxidative mechanisms to kill bacteria. We have noted that the tissue pO2 is critical, but that the actual quantity of oxygen consumed in wound healing is relatively small. The amount of oxygen used to heal a wound is only 10% of that required for brain metabolism.
Production of new collagen is also a requirement for capillary ingrowth or proliferation (22). As capillaries advance, stimulated by angiogenic growth factor, they must be supported by an extracellular collagen matrix to facilitate ingrowth into tissue. In the absence of new collagen, capillary ingrowth cannot occur. This effect is crucial in treating radionecrosis (23–25), where the tissue is primarily hypovascular, and secondarily hypoxic and hypocellular. It has been discovered that when collagen production can be facilitated, new capillaries will invade the previously irradiated area, and healing will then occur. The tissue pO2 rises to 80% of normal and plateaus; however, this is sufficient for healing and will even support bone grafting. Historically, the only means of managing radionecrosis was to excise the radiated area and bring in fresh tissue with its own blood supply. New collagen formation and capillary ingrowth also account for the rise in tissue pO2, which can be achieved in patients with diabetic foot lesions.
It is now well understood that the stimulus for growth factor production by the macrophage is hypoxia and/or the presence of lactic acid (26,27). Wounds managed in hyperbaric units are typically ischemic and hypoxic. Periods of relative hypoxia, required for the stimulation
HYPERBARIC OXYGENATION |
31 |
of growth factor production, exist between hyperbaric treatments.
Surprisingly, oxygen levels remain high in tissues for longer than one would expect following hyperbaric treatment. In a study by George Hart (28) at Long Beach Memorial Hospital, a mass spectrometer probe was inserted in the unanesthetized thigh tissues of normal volunteers. Muscle and subcutaneous tissue pO2 values in study subjects remained significantly elevated for 2–3 h following hyperbaric oxygen treatment. Arterial pO2 was also measured and found to rise immediately and significantly under hyperbaric conditions but returned to normal levels within a couple of minutes upon egress from the chamber (Fig. 1). Thus, multiple daily HBO treatments can maintain useful oxygen levels for up to 12 h/day.
Mention has been made of enhanced leukocyte killing of bacteria under hyperbaric conditions. Jon Mader of the University of Texas-Galveston (29) carried out a rather simple, but elegant, experiment to demonstrate this. The fascinating part of this study is that in the evolution of the human body, a leukocyte has never been exposed to a partial pressure of 150 mmHg while in tissues. This level is impossible to attain breathing air. Nevertheless, when one artificially raises the pO2 far beyond the leukocyte’s normal functional parameters, it becomes even more lethal to bacteria. This is an anomaly, as one rarely can improve on Mother Nature. Of some interest in this regard is that if one bites one’s tongue, one is never concerned about possible infection, even though it is a human bite. Similarly, hemorrhoidectomies rarely, if ever, become infected. The reason is that the pO2 of the tissues in and around the oral cavity are very high, and the pO2 in hemorrhoidal veins is nearly arterial. Tom Hunt has shown it is impossible to infect tissue that is injected with raw staphylococci if the pO2 in the same tissue is > 50 mmHg. Both he and David Knighton have described oxygen as an antibiotic (30,31).
The reduction of intracranial pressure is facilitated by vasoconstriction. Experimentally, Rockswold has shown that mortality can be halved in victims of closed head injury with Glasgow Coma Scales in the range of 4–6. One of the major mechanisms here is a reduction of intracranial pressure while continuing to oxygenate hypoxic brain (32–36). Sukoff et al. (37) administered 100% O2, 1.5 ATA 60 min every 24 h (maximum of 7 h) to severely brain injured patients. This resulted in a 50% reduction in mortality.
A paper published by Mathieu (38) has shown that the flexibility index of red cells can be changed from 23.2 to 11.3 within 15 hyperbaric treatments. This increase in flexibility can prove quite useful in people with narrowed capillaries. However, whether this phenomenon plateaus at 15 treatments, its duration and underlying mechanism are still unknown.
Nylander et al. (39) demonstrated that following complete occlusion of the blood flow to rat leg for 3 h, postischemic edema could be reduced by 50% if the animals are promptly treated with hyperbaric oxygen. He also demonstrated that the mechanism for this was preservation of adenosine triphosphate (ATP) in the cells, which provides the energy for the cells to maintain their osmolarity. Cianci
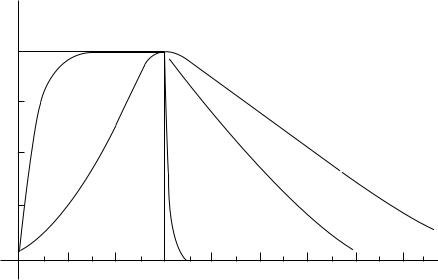
32 HYPERBARIC OXYGENATION
Pressure (in atmospheres)
Figure 1. Arterial, muscle and subcuta-
Oxygenation
2
1
|
Sub |
1 Plug |
Muscle |
|
H
B
O
1 |
2 |
cutaneous
3 |
4 |
neous pO2 after HBO treatment.
(40,41). Yamaguchi, and others have underscored the importance of ATP in preventing edema in burn. Treatment twice daily has shown to be more efficacious than treatment once a day.
Zamboni (42) and Gingrass, working at the University of Southern Illinois, have shown the effects of hyperbaric oxygen on speeding functional return in peripheral nerve repair and grafting. At 6 weeks, there is a 28% improvement of function in the affected leg of these rats.
Niezgoda (43) performed a randomized and doubleblinded study in human volunteers investigating the effect of hyperbaric oxygen in a controlled burn wound model. He demonstrated statistically significant decreases in edema formation and wound exudate in the hyperbaric oxygen treated group.
Finally, the mechanism for the effects of hyperbaric oxygen in carbon monoxide poisoning (44,45) is now better understood. Traditionally, it was felt that the mere presence of carboxyhemoglobin blocked transport of oxygen to the tissues. However, studies by Goldbaum et al. (46) at the Armed Forces Institute of Pathology in 1975 lead us to different conclusions. Impairment of cytochrome A3 oxidase and lipid peroxidation occurring following a reperfusion injury are now suggested as the primary pathways in the pathophysiology causing fatality. Stephen Thom (47– 50) at the University of Pennsylvania pioneered research in this area. It appears that as carbon monoxide levels fall, the products of lipid peroxidation rise, indicating that brain damage is occurring only during the resuscitative phase, thus becoming reperfusion injury. Thom demonstrated that a period of hypotension (even though it may only be a matter of seconds) is enough to initiate lipid peroxidation. Oxygen at 1 atm has no effect on halting the process. However, oxygen at 3 atm terminates lipid peroxidation. Patients who have been treated acutely with hyperbaric oxygen rarely exhibit signs of delayed deterioration, reported in 30–40% of severe cases treated only with normobaric oxygen. The probable mechanism for this
Time (in hours)
is the ability of hyperbaric oxygen at three ATA to terminate lipid peroxidation.
Finally, in many ways it seems paradoxical that oxygen at high pressure, which intuitively would seem to provide more substrate for free-radical formation, still benefits tissues from crush injury and postischemic states. But ironically, it is precisely this hyperbaric environment that promotes certain so-called reactive oxygen species with inherent protective qualities (51). More studies are certainly needed to investigate the underlying pharmacologic benefits afforded by hyperbaric oxygen. We have only just begun to explore and utilize a treatment modality whose time has come.
BIBLIOGRAPHY
1.Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol 2003;284:H1230–1239.
2.Buras JA, Stahl GL, Svoboda KKH, Reenstra WR. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol 2000;278:C292–C302.
3.Boerema I, Kroll JA, Meijne NG, Lokin E, Kroon B, Huiskes JW. High atmospheric pressure as an aid to cardiac surgery. Arch Chir Neerl 1956;8:193–211.
4.Kindwall EP. Report of the committee on hyperbaric oxygenation. Bethesda: Undersea Medical Society; Bethesda 1977.
5.Davis JC, Hunt TK. Hyperbaric oxygen therapy. Undersea Medical Society; Bethesda 1977.
6.Thom SR. Molecular mechanism for the antagonism of lipid peroxidation by hyperbaric oxygen. Undersea Biom Res (Suppl) 1990;17:53–54.
7.Andersen V, Hellung-Larsen P, Sorensen SF. Optimal oxygen tension for human lymphocytes in culture. J Cell Physiol 1968;72:149–152.
8.Gadd MA, McClellan DS, Neuman TS, Hansbrough JF. Effect of hyperbaric oxygen on murine neutrophil and T-lymphocyte functions. Crit Care Med 1990;18:974–979.
9.Hayakawa T, Kanai N, Kuroda R, et al. Response of cerebrospinal fluid to hyperbaric oxygenation. J Neurol Neurosurg Psych 1971;34:580–356.
10.Miller JD, Fitch W, Ledingham IM, et al. Reduction of increased intracranial pressure. Neurosurgery 1970;33: 287–296.
11.Sukoff MH, Ragatz RE. Hyperbaric oxygenation for the treatment of acute cerebral edema. Neurosurgery 1982;10: 29–38.
12.Nemiroff PM. Synergistic effects of pentoxifylline and hyperbaric oxygen on skin flaps. Arch Otolaryngol Head Neck Surg 1988;114:977–981.
13.Cianci P, Lueders H, Shapiro R, Sexton J, Green B. Current status of adjunctive hyperbaric oxygen in the treatment of thermal wounds. In: Proceedings of the second Swiss symposium on hyperbaric medicine; Baker DJ, JS, editors. Foundation for Hyperbaric Medicine; Basel: 1988. p 163–172.
14.Grossman AR. Hyperbaric oxygen in the treatment of burns. Ann Plast Surg 1978;1:163–171.
15.Hart GB, O’Reilly RR, Broussard ND, Cave RH, Goodman DB, Yanda RL. Treatment of burns with hyperbaric oxygen. Surg Gynacol Obstet 1974;139:693–696.
16.Coburn RF, Forster RE, Kane PB. Considerations of the physiological variables that determine the blood carboxyhemoglobin concentrations in man. J Clin Invest 1965;44: 1899– 1910.
17.Bert P. Barometric Pressure. 1879. p 579. (translated by Hitchcock MS, Hitchcock FA) Bethesda: Reprinted by the Undersea and Hyperbaric Medicine Society; 1978.
18.Donald KW. Oxygen poisoning in man. Br Med J 1947; 712–717.
19.Lambertsen CJ. In: Fenn WO, Rahn H, editors. Handbook of Physiology, Respiration. Washington, D.C.: American Physiological Society; Section 3, Volume II. p 1027–1046.
20.Knighton DR, Hunt TK, Scheuenstuhl H, Halliday B, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science 1983;221:1283–1285.
21.Knighton DR, Oredsson S, Banda MJ, Hunt TK. Regulation of
repair: hypoxic control of macrophage mediated angiogenesis. In: Hunt TK, Heppenstall RB, Pines E, Rovee D, editors. Soft
and hard tissue repair. New York: Praeser; 1948. |
p 41–49. |
22.Knighton DR, Hunt TK, Thakral KK, Goodson WH. Role of platelets and fibrin in the healing sequence, an in vivo study of angiogenesis and collagen synthesis. Ann Surg 1982;196: 379–388.
23.Davis JC. Soft tissue radiation necrosis: The role of hyperbaric oxygen. HBO Rev 1987;2(3):153–167.
24.Davis JC, et al. Hyperbaric oxygen: A new adjunct in the management of radiation necrosis. Arch Otol 1979;105:58–61.
25.Hart GB, Mainous EG. The treatment of radiation necrosis with hyperbaric oxygen (OHP). 1976;37:2580–2585.
26.Jensen JA, Hunt TK, Scheuenstuhl H, Banda MJ. Effect of lactate, pyruvate, and physician on secretion of angiogenesis and mitogenesis factors by macrophages. Lab Invest 1986;54: 574–578.
27.Knighton DR, Schumerth S, Fiegel VD. Microenvironmental regulation of macrophage growth factor production. In preparation.
28.Hart GB, Wells CH, Strauss MB. Human skeletal muscle and subcutaneous tissue carbon dioxide, nitrogen and oxygen gas tension measurement under ambient and hyperbaric conditions. J App Res Clin Exper Therap Spring 2003;3(2).
29.Wang J, Corson K, Mader J. Hyperbaric oxygen as adjunctive therapy in vibrio vulnificus septicemia and cellulites. Undersea Hyperbaric Med Spring 2004, 31(1):179–181.
30.Hunt TK, Linsey M, Grislis G, Sonne M, Jawetz E. The effect of differing ambient oxygen tensions on wound infections. Ann Surg 1975;181:35–39.
HYPERBARIC OXYGENATION |
33 |
31.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. A comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg 1986;121:191–195.
32.Miller JD, et al.The effect of hyperbaric oxygen on experimentally increased intracranial pressure. J Neurosurg 1970;32: 51–54.
33.Miller JD, Ledingham IM. Reduction of increased intracranial pressure: Comparison between hyperbaric oxygen and hyperventilation. Arch Neurol 1971;24:210–216.
34.Mogami H, et al.Clinical application of hyperbaric oxygenation in the treatment of acute cerebral damage. J Neurosurg 1969;31:636–643.
35.Sukoff MH, et al.The protective effect of hyperbaric oxygenation in experimental cerebral edema. J Neurosurg 1968;29: 236–241.
36.Sukoff MH, Ragatz RE. Hyperbaric oxygen for the treatment of acute cerebral edema. Neurosurgery 1982;10(1):29–38.
37.Sukoff MH. Effects of hyperbaric oxygenation [comment]. J Neurosurg 2001;94(3):403–411.
38.Mathieu D, Coget J, Vinkier L, Saulnier F, Durocher A, Wattel F. Red blood cell deformability and hyperbaric oxygen therapy. (Abstract) HBO Rev 1985;6:280.
39.Nylander G, Lewis D, Nordstrom H, Larsson J. Reduction of postischemic edema with hyperbaric oxygen. Plast Reconstr Surg 1985;76:596–601.
40.Cianci P, Lueders HW, Lee H, Shapiro RL, Sexton J, Williams C, Green B. Adjunctive hyperbaric oxygen reduces the need for surgery in 40-80% burns. J Hyper Med 1988;3: 97–101.
41.Cianci P, Lueders HW, Lee H, Shapiro RL, Sexton J, Williams C, Green B. Hyperbaric oxygen and burn fluid requirements: Observations in 16 patients with 40-80% TBSA burns. Undersea Biomed Res (Suppl) 1988;15:14.
42.Zamboni WA, Roth AC, Russell RC, Nemiroff PM, Casa L, Smoot C. The effect of acute hyperbaric oxygen therapy on axial pattern skin flap survival when administered during and after total ischemia. J Reconst Microsurg 1989;5: 343–537.
43.Niezgoda JA, Cianci P. The effect of hyperbaric oxygen on a burn wound model in human volunteers. J Plast Reconstruct Surg 1997;99:1620–1625.
44.Brown SD, Piantadosi CA. Reversal of carbon monoxide-cyto- chrome C oxidase binding by hyperbaric oxygen in vivo. Adv Exp Biol Med 1989;248:747–754.
45.End E, Long CW. Oxygen under pressure in carbon monoxide poisoning. J Ind Hyg Toxicol 1942;24:302–306.
46.Goldblum LR, Ramirez RG, Absalon KB. Joint Committee on Aviation Pathology XII. What is the mechanism of carbon monoxide toxicity? Aviat Space Environ Med 1975;46(10): 1289–1291.
47.Thom SR. Antagonism of carbon monoxide-mediated brain lipid peroxidation by hyperbaric oxygen. Toxicol Appl Pharmacol 1990;105:340–344.
48.Thom SR, Elbuken ME. Oxygen-dependent antagonism of lipid peroxidation. Free Rad Biol Med 1991;10:413–426.
49.Thom SR. Carbon-monoxide mediated brain lipid peroxidation in the rat. J Appl Physiol 1990;68:997–1003.
50.Thom SR. Dehydrogenase conversion to oxidase and lipid peroxidation in brain after carbon monoxide poisoning. J Appl Physiol 1992;73:1584–1589.
51.Thom SR, Bhopale V, Fisher D, Manevich Y, Huang PL, Buerk DG. Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: An oxidative stress response. J Neurobiol 2002;51:85–100.
See also HYBERBARIC MEDICINE; OXYGEN MONITORING.
