
- •VOLUME 4
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •HYDROCEPHALUS, TOOLS FOR DIAGNOSIS AND TREATMENT OF
- •HYPERALIMENTATION.
- •HYPERBARIC MEDICINE
- •HYPERBARIC OXYGENATION
- •HYPERTENSION.
- •HYPERTHERMIA, INTERSTITIAL
- •HYPERTHERMIA, SYSTEMIC
- •HYPERTHERMIA, ULTRASONIC
- •HYPOTHERMIA.
- •IABP.
- •IMAGE INTENSIFIERS AND FLUOROSCOPY
- •IMAGING, CELLULAR.
- •IMAGING DEVICES
- •IMMUNOLOGICALLY SENSITIVE FIELD–EFFECT TRANSISTORS
- •IMMUNOTHERAPY
- •IMPEDANCE PLETHYSMOGRAPHY
- •IMPEDANCE SPECTROSCOPY
- •IMPLANT, COCHLEAR.
- •INCUBATORS, INFANTS
- •INFANT INCUBATORS.
- •INFUSION PUMPS.
- •INTEGRATED CIRCUIT TEMPERATURE SENSOR
- •INTERFERONS.
- •INTERSTITIAL HYPERTHERMIA.
- •INTRAAORTIC BALLOON PUMP
- •INTRACRANIAL PRESSURE MONITORING.
- •INTRAOCULAR LENSES.
- •INTRAOPERATIVE RADIOTHERAPY.
- •INTRAUTERINE DEVICES (IUDS).
- •INTRAUTERINE SURGICAL TECHNIQUES
- •ION-EXCHANGE CHROMATOGRAPHY.
- •IONIZING RADIATION, BIOLOGICAL EFFECTS OF
- •ION-PAIR CHROMATOGRAPHY.
- •ION–SENSITIVE FIELD-EFFECT TRANSISTORS
- •ISFET.
- •JOINTS, BIOMECHANICS OF
- •JOINT REPLACEMENT.
- •LAPARASCOPIC SURGERY.
- •LARYNGEAL PROSTHETIC DEVICES
- •LASER SURGERY.
- •LASERS, IN MEDICINE.
- •LENSES, CONTACT.
- •LENSES, INTRAOCULAR
- •LIFE SUPPORT.
- •LIGAMENT AND TENDON, PROPERTIES OF
- •LINEAR VARIABLE DIFFERENTIAL TRANSFORMERS
- •LITERATURE, MEDICAL PHYSICS.
- •LITHOTRIPSY
- •LIVER TRANSPLANTATION
- •LONG BONE FRACTURE.
- •LUNG MECHANICS.
- •LUNG PHYSIOLOGY.
- •LUNG SOUNDS
- •LVDT.
- •MAGNETIC RESONANCE IMAGING
- •MAGNETOCARDIOGRAPHY.
- •MANOMETRY, ANORECTAL.
- •MANOMETRY, ESOPHAGEAL.
- •MAMMOGRAPHY
- •MATERIALS, BIOCOMPATIBILITY OF.
- •MATERIALS, PHANTOM, IN RADIOLOGY.
- •MATERIALS, POLYMERIC.
- •MATERIALS, POROUS.
- •MEDICAL EDUCATION, COMPUTERS IN
- •MEDICAL ENGINEERING SOCIETIES AND ORGANIZATIONS
- •MEDICAL GAS ANALYZERS
- •MEDICAL PHOTOGRAPHY.
- •MEDICAL PHYSICS LITERATURE
- •MEDICAL RECORDS, COMPUTERS IN
- •MICROARRAYS
- •MICROBIAL DETECTION SYSTEMS
- •MICROBIOREACTORS
- •MICRODIALYSIS SAMPLING
- •MICROFLUIDICS
- •MICROPOWER FOR MEDICAL APPLICATIONS
- •MICROSCOPY AND SPECTROSCOPY, NEAR-FIELD
- •MICROSCOPY, CONFOCAL
- •MICROSCOPY, ELECTRON
- •MICROSCOPY, FLUORESCENCE
- •MICROSCOPY, SCANNING FORCE
- •MICROSCOPY, SCANNING TUNNELING
- •MICROSURGERY
- •MINIMALLY INVASIVE SURGICAL TECHNOLOGY
- •MOBILITY AIDS
- •MODELS, KINETIC.
- •MONITORING IN ANESTHESIA
- •MONITORING, AMBULATORY.
- •MONITORING, FETAL.
- •MONITORING, HEMODYNAMIC
- •MONITORING, INTRACRANIAL PRESSURE
- •MONITORING, NEONATAL.
- •MONITORING, UMBILICAL ARTERY AND VEIN
- •MONOCLONAL ANTIBODIES
- •MOSFET.
- •MUSCLE ELECTRICAL ACTIVITY.
- •MUSCLE TESTING, REHABILITATION AND.
- •MUSCULOSKELETAL DISABILITIES.
12.Giege R, Puglisi JD, Floentz C. In: Cohn WE, Moldave K, eds., Progressin Nucleic Acid Research and Molecular Biology, Vol. 45. Amsterdam: Elsevier; 1993.
13.Hadi Zareie M, Lukins PB. Atomic-resolution STM structure of DNA and localization of the retinoic acid binding site. Biochem Biophy Res Commun 2003;303:153–159.
14.Otero R, et al. Proceedings of the 5th Trends In Nanotechnology (TNT04) CMP Cientifica; 2004. Available at http:// www.phantomsnet.net/files/abstracts/TNT2004/ AbstractKeynoteBesenbacherF.pdf.
15.Available at http://biochem.otago.ac.nz/staff/sowerby/periodicmonolayers.htm. Multiple references listed.
16.Lauhon LJ, Ho W. Single molecule chemistry and vibrational spectroscopy: Pyridine and benzene on Cu(001). J Phys Chem A 2000;104:2463–2467.
17.Image credit in Fig. 11(a): U.S. Department of Energy Human Genome Program, Available at http://www.ornl.- gov/hgmis. This image originally appeared in the 1992 U.S. DOE Primer on Molecular Genetics.
18.Image credit in Fig. 11(b): Mathematical Association of America, Available at http://www.maa.org/devlin/devlin0403. html.
19.Claypool CL, et al. Source of image contrast in STM images of functionalized alkanes on graphite: A systematic functional group approach. J Phys Chem B 1997;101:5978–5995.
20.Faglioni F, Claypool CL, Lewis NS, Goddard WA III. Theoretical description of the STM images of alkanes and substituted alkanes adsorbed on graphite. J Phys Chem B 1997;101:5996–6020.
21.Claypool CL, et al. Effects of molecular geometry on the STM image contrast of methyland bromo-substituted alkanes and alkanols on graphite. J Phys Chem B 1999;103:9690– 9699.
22.Claypool CL, Faglioni F, Goddard WA III, Lewis NS. Tunnel-
ing mechanism implications from an STM study of H3C(CH2)15HC ¼ C ¼ CH(CH2)15CH3 on graphite and C14H29OH on MoS2. J Phys Chem B 1999;103:7077–7080.
23.Villarubia JS. Algorithms for scanned probe microscope image simulation, surface reconstruction and tip estimation. J Res Nat Inst Standards Technol 1997;102: 425–454.
See also BIOSURFACE ENGINEERING; MICROSCOPY, ELECTRON.
MICROSENSORS FOR BIOMEDICAL
APPLICATIONS. See CAPACITIVE MICROSENSORS FOR BIOMEDICAL APPLICATIONS.
MICROSURGERY
KEITH J. REBELLO
The Johns Hopkins University
Applied Physics Lab
Laurel, Maryland
INTRODUCTION
Microsurgery is a specialized surgical technique whereby a microscope is used to operate on tiny structures within the body. Fine precision microinstruments are used to manipulate tissue with or without robotic or computer control.
MICROSURGERY 523
This technique has allowed for significant advances in surgery especially for operations involving the inner ear, eye, brain, nerves, and small blood vessels.
HISTORY OF THE SURGICAL MICROSCOPE
The compound microscope is generally agreed upon to have been invented in the 1590s by the two Dutch opticians Hans and Zacharias Janssen. Their device consisted of a sliding tube with two aligned lenses. In 1624 Galileo Galilei, the famous astronomer and mathematician, demonstrated an inverted telescope to his colleagues of the Lincean Academy. One of them, Giovanni Faber, named it a microscope from the Greek words micro, meaning small, and scope, meaning to aim or shoot. Microscope lenses were improved in the seventeenth century by Antonie van Leeuwenhoek, a Dutch linen draper who originally was interested in counting the number of threads per square inch in his reams of cloth. How he constructed his spherical lenses still remains a mystery to this day. In the eighteen century, fine and course adjustments as well as tube inclination were added by Robert Hooke, who first discovered the cell. The microscope was further improved by Joseph Jackson Lister a British wine merchant, school visitor, and histologist, the father of Lord Joseph Lister whom is credited as stating the era of modern surgery. His innovations included the developed achromatic objective lens corrected for chromatic and spherical aberrations and stands designed to reduce vibrations. His jointly published work with Dr. Joseph Hodgkins in 1827, redefined the understanding at the time of arteries, muscles, nerves, and the brain.
In 1848 Carl Zeiss, a German machinist opened a microscope workshop. Ernst Abbe´, a physicist working with Zeiss, derived new mathematical formulas and theories that allowed the optical properties to be mathematically predicted for the first time. Prior lenses had always been made by craftsmen who learned their trade by trial and error. Abbe´’s advancements enabled Zeiss to become the first mass producer of high quality microscopes.
USE IN SURGERY
Edwin Theodor Saemisch, a German ophthamologist, used loupes in surgery in 1876, but although the microscope was being used in the laboratory medical research environment it was not used the operating room. Zeiss manufactured a binocular microscope specifically designed for dissecting which was used for ophthalmological examinations of the cornea and anterior chamber of the eye. It was not until 1921 that Carl Olof Nylen, a Swedish, otologist and tennis olympian, used his homebuilt monocular microscope for the first time in ear surgery on a case of chronic otis media, a type of ear infection. His monocular microscope was quickly replaced in 1922 by a binocular microscope developed by adding a light source to a Zeiss dissecting microscope by his chief surgeon, Gunnar Holmgren. He used it to treat diseases otosclerosis, the abnormal growth of temporal bone in the middle ear.

524 MICROSURGERY
Despite these early successes the surgical microscope was seldom used due to its limited field of vision, very short focal distance, poor light quality, and instability. It was not until the 1950s that the surgical microscope started to become more widely adopted. In 1953, Zeis released the Zeiss OpMi 1(Zeiss Operating Microscope Number One), which was specially designed for otological procedures. Its superior coaxial lighting, stability, and ease of operation enabled the advent of tympanoplasty operations to repair ruptured ear drums as well as widespread use in temporal bone surgery.
The success the microscope was having in otology soon spread to other disciplines as well. In the early 1950s, Jose´ Ignacio Barraquer adapted a slip lamp to the Zeiss otological surgical microscope for ocular microsurgery. By the 1960s, J. I. Barraquer, Joaqu´ın Barraquer, and Hans Littman of Zeiss, had further modified the surgical microscope and refined microsurgical techniques to make ocular maneuvers in glaucoma microsurgery easier to perform. During this same time frame, Richard Troutman also had Zeiss make a special microscope for his ophthalmic procedures. He made many advances and is credited as adding electric and hydraulic control to surgical microscopes, but possibly his greatest innovation was the first variable magnification, or zoom, surgical microscope.
Around this time neurosurgeons also began using the surgical microscope in the operating room. In 1957, Theodore Kurze removed a neuriloma tumor from the seventh cranial nerve, and then later anastomized it to the hypoglossal nerve. He also developed techniques to use the surgical microscope for aneurysm surgeries. Recognizing that sterilization was a major problem, he developed the use of ethylene oxide gas to sterilize his surgical microscopes for use in the operating room. Dr. R. M. Peardon Donaghy established the first microsurgical training lab at the University of Vermont, where many surgeons were trained. He collaborated with the vascular surgeon Julius Jacobson to remove occlusions from cerebral arteries. Jacobson and his colleague Ernesto Suarez, were responsible for developing small vessel anastomoses techniques. Their procedures required another surgeon’s assistance. To meet this need Jacobson invented the diploscope that allowed two surgeons to view the same operative field. Later he and his colleagues worked with Hans Littman of Zeiss to develop a commercial surgical microscope with a beamsplitter enabling two surgeons to operate at the same time. A modern day version of the Zeiss dual head microscope is shown in Fig. 1.
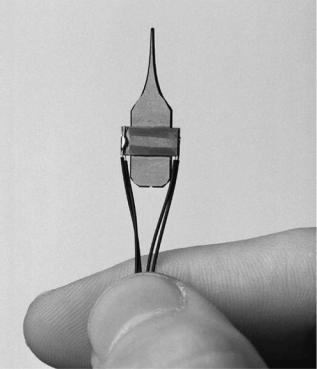
Inspired by the work of the neuroscientists plastic surgeon also started using the surgical microscope. Harold Buncke was one of the first plastic surgeons to use the microscope for digit/limb replantation and free-flap autoplantation. Buncke also developed many of the tools microsurgeons use by borrowing technology from the jewelry, watchmaking, and microassembly industries (Fig. 2.)
The 1960s saw the techniques applied to neurosurgery. Breakthroughs in microneurosurgery included the repair of peripheral nerve injuries, intracranial aneurysm surgeries, embolectomies of middle cerebral arteries, middle cerebral artery bypasses. One on the visionaries of this time was M. G. Yasargil a Turkish neurosurgeon from
Figure 1. Zeiss OpMi Vario S8 surgical microscope. (Courtesy of Carl Zeiss.)
Switzerland. Trained in Donaghy’s training lab he further refined and improved the surgical techniques.
The next advancements came with the development of minimally invasive surgical techniques. Traditional surgical techniques used relatively large incisions to allow the surgeon full access to the surgical area. This type of operation, called open surgery, enables the surgeon’s hands and instruments to come into direct contact with organs and tissue, allowing them to be manipulated freely. These operations are classified as first generation surgical techniques and most surgeons are trained in this manner. While the large incision gives the surgeon a wide range of motion to do very fine controlled procedures, it also causes substantial trauma to the patient. In fact, the majority of trauma is caused by the incisions the surgeon uses to get access to the surgical site instead of the actual
Figure 2. Modern day microsurgical tools. (Courtesy of WPI Inc.)
surgical procedure itself. For example, in a conventional open-heart cardiac operation, the rib cage must be cracked and split exposing the heart muscle. This trauma not only increases pain to the patient, but adds to recovery times increasing hospital stays, in turn increasing costs.
In 1985, Muhe performed the first laparoscopic cholecystectomy, or gallbladder removal surgery with a fiberoptic scope. The technique he performed is commonly called minimally invasive surgery but also goes by other names, such as micro, keyhole, microscopic, telescopic, less invasive, and minimal access surgery. This microsurgical technique is based on learnings from gynecological pelviscopies and arthroscopic orthopedic operations along with the previous advances made in otology, opthamology, neurosurgery, and reconstructive microsurgeries. It has subsequently been applied to many other surgical areas, such as general surgery, urology, thoracic surgery, plastic surgery, and cardiac surgery. These procedures are classified as second generation surgeries as trauma to the patient is drastically reduced by the reducing or eliminating incisions. The shorter hospital stays and faster recovery times for the patient reduce the cost of a minimally invasive procedure 35% compared to its open surgery counterpart.
In a minimally invasive cardiac operation a few small holes, access points, or ports are punctured into the patient and trocars are inserted. A trocar consists of a guiding cannula or tube with a valve–seal system to allow the body to be inflated with carbon dioxide. This is done so that the surgeon has enough room to manipulate his instruments at the surgical site. An endoscope is inserted into one of the trocar ports to allow the surgeon a view the surgical site. Various other surgical instruments, such as clippers, scissors, graspers, shears, cauterizers, dissectors, and irrigators were miniaturized and mounted on long poles so that they can be inserted and removed from the other trocar ports to allow the surgeon to perform the necessary tasks at hand.
While minimally invasive surgery has many advantages to the patient, such as reduced postoperative pain, shorter hospital stays, quicker recoveries, less scarring, and better cosmetic results, there are a number of new problems for the surgeon. The surgeon’s view is now restricted and does not allow him to see the entire surgical area with his eyes. While the operation is being performed he must look at a video image on a monitor rather than at his hands. This is not very intuitive and disrupts the natural hand–eye coordination we all have been accustomed to since childhood. The video image on the monitor is also only two dimensional (2D) and results in a loss of our binocular vision eliminating the surgeon’s depth perception. While performing the procedure the surgeon does not have direct control of his own field of view. A surgical assistant holds and maneuvers the endoscopic camera. The surgeon has to develop his own language to command the assistant to position the scope appropriately, which often leads to orientation errors and unstable camera handling, especially during prolonged procedures. Since the images from the camera are magnified, small motions, such as the tremor in a surgical assistant’s hand or even their heartbeat can cause the surgical team to experience motion induced nausea. To combat the endoscopic problems, some
MICROSURGERY 525
surgeons choose to manipulate the endoscope themselves. This restricts them to using only one hand for delicate surgical procedures and makes procedures even more complicated.
The surgeon also loses the freedom of movement he has in open surgery. The trocar ports are fixed to the patient’s body walls by pressure and friction forces. This constrains the instrument’s motion in two directions and limits the motion of the tip of the instrument to four degrees of freedom (in–out, left–right, up–down, and rotation). The trocars also act as pivot points and cause the surgical instruments to move in the opposite direction to the surgeon’s hands. When the surgeon is moving left, the image on the monitor is moving to the right. The amount of this opposite movement also depends on the depth of the introduction of the instrument. Again because of the pivot point the deeper an instrument is inserted into the body the more the surgeon’s movement is amplified. Even a small movement made by the surgeon on the outside of the patient can translate to a very large movement on the inside of the patient. The seals and valves in the trocars also impede movements which hinders the smoothness of motions into and out of the patient and greatly reduces the already limited tactile feedback the surgeon experiences. These movement behaviors and lack of tactile feedback are counter to what the surgeon is used to in open surgery and require long training to develop the technical skills to perform these operations.
Performing a minimally invasive procedure has been likened to writing your name holding the end of an 18 in. (45.72 cm) pencil (1). The surgeon loses three-dimensional (3D) vision, dexterity, and the sense of touch. The instruments are awkward, counterintuitive, and restricted in movement. The lack of tactile feedback prevents the surgeon from knowing how hard he or she is pulling, cutting, twisting, suturing, and so on. These factors cause a number of adjustments to be made by the surgeon, which requires significant retraining on how to do the procedures in a minimally invasive manner. These difficulties encountered by the surgeon cause degradation in surgical performance compared to open surgery which limits surgeons to performing only simpler surgical procedures.
In an attempt to address some of these shortcomings and allow the surgeon more control during operations a third generation of surgical procedures, robotic surgery was developed. Although these types of procedures are commonly referred to as robotic surgery, the operations themselves are not completely automated and are still carried out by a surgeon. For this reason, robotic surgery is also referred to as computer aided or computer assisted surgery.
The robotic technology was originally developed for telerobotic applications in the late 1980s for the Defense Advanced Research Project Administration (DARPA) by researchers at SRI International. The surgeon of the future would allow surgeons from remote command centers to operate on injured soldiers in the battlefield. In 1995, this technology was spun off into a new company named Intuitive Surgical to commercialize the technology for use in the hospital environment. Near the same time Dr. Yulan Wang was developing robotic technology for NASA to allow

526 MICROSURGERY
Figure 3. Intuitive Surgical da Vinci robotic surgery system. (Copyright #2005 Intuitive Surgical, Inc.)
surgeons on earth to deal with medical emergencies on the international space station. He formed Computer Motion in 1989. Both of these companies merged in 2003, and Intuitive Surgical is now the leader in Robotic Surgery. In 2002, Dr. Fredric Mol, one of the original founders of Intuitive Surgical, founded Hansen Medical which brings computerized robotic control of catheters to electrophysiology and interventional cardiac procedures.
Current robotic surgery systems have a number of benefits over conventional minimally invasive surgery. Figure 3 shows an Intuitive Surgical da Vinci robotic system. In this arrangement the surgeon sits comfortably at a computer console instead of having to stand throughout the entire procedure, which can last up to 5 h long. A three-armed robot takes his place over the patient. One arm holds an endoscope while the other two hold a variety of surgical instruments. The surgical team can also look at a video monitor to see what the surgeon is seeing. The surgeon looks into a stereo display in much the same way as looking though a surgical microscope and manipulates joystick actuators located below the display. This simulates the natural hand–eye alignment he is used to in open surgery, (Fig. 4). Since computers are used to control the robot and are already in the operating room, they can be used to give the surgeon superhuman like abilities. Accuracy is improved by employing tremor cancellation algorithms to filter the surgeon’s hand movements. This type of system can eliminate or reduce the inherent jitter in a surgeon’s hands for operations where very fine precise control is needed. Motion scaling also improves accuracy by translating large, natural movements into extremely precise, micromovements. A wide variety of surgical instruments or end effectors are available including graspers, cutters, cauterizers, staplers, and so on. Both companies provide end effectors that have special wrist like joints at their tips enabling full seven degree of freedom movements inside the patient, (Fig. 5), but still lack tactile feedback.
These robotic advances allow surgeons to perform more complex procedures, such as reconstructive cardiac operations like coronary bypass and mitral valve repair that cannot be performed using other minimally invasive techniques.
Figure 4. Intuitive Surgical stereo display and joysticks. (Copyright #2005 Intuitive Surgical, Inc.)
Figure 5. Multidegrees-of-freedom end effector. (Copyright
#2005 Intuitive Surgical, Inc.)

MEMS
Around the same time that minimally invasive surgery was being developed, there was a turning point in microelectromechanical systems (MEMS). This a technology was developed from the integrated circuit industry to create miniature sensors and actuators. Originally, these semiconductor processes and materials were used to build electrical and mechanical systems, but have now expanded to include biological, optical, fluidic, magnetic, and other systems as well. The term MEMS originated in the United States and typically contain a moving or deformable object. In Europe, this technology goes by the name microsystems technology or microstructures technology (MST) and also encompasses the method of making these devices, which is referred to as micromachining. In Japan and Asia MEMS are called micromachines when mechanisms and motion are involved. The MEMS devices first were used in medical applications in the early 1970s with the advent of the silicon micromachined disposable blood pressure sensor (2), but it was not until the mid-1980s when more complicated mechanical structures, such as gears and motors, were able to be fabricated.
FABRICATION TECHNOLOGIES
The fabrication of MEMS devices is based on the merger of semiconductor microfabrication processes and micromachining techniques to create the desired microstructural components. There are four major processes that are used to fabricate MEMS devices: bulk micromachining, surface micromachining, LIGA, and precision machining. Combinations of these technologies are what allow MEMS to be highly miniaturized and integratable with microelectronics. These processes are very sensitive to impurities and environmental conditions such as temperature, humidity, and air quality. Typically, these fabrication steps are performed inside a cleanroom (Fig. 6). Bulk micromachining, surface micromachining, and LIGA have the added advantage of being able to be batch fabricated. This allows many devices to be made in parallel at the same time greatly reducing device cost.
Bulk micromachining utilizes wetor dry-etch processes to form 3D structures out of the substrate. These subtractive processes produce isotropic or anisotropic etch profiles in material substrates, which are typically but not limited to silicon wafers. Bulk micromachining can create large MEMS structures on the micrometers (mm) to millimeters (mm) scale (tens of mm-to-mm thick). Commercial applications of bulk micromachining have been available since the 1970s. These applications include pressure sensors, inertial sensors such as accelerometers and gyros, and microfluidic channels and needles for drug delivery.
In surface micromachining, MEMS are formed on the surface of the substrate using alternating layers of structural and sacrificial materials. These film materials are repeatedly deposited, patterned, and etched to form structures that can then be released by removing sacrificial layers. The release process allows for the fabrication of complex movable structures that are already assembled,
MICROSURGERY 527
Figure 6. Cleanroom fabrication facility. (Courtesy of Intel Corp.)
such as motors, switches, resonators, cantilevers, and so on. Surface micromachined structures are typically limited to thicknesses of 2–6 mm and because they use much of the same technology as is used in the integrated circuit industry are readily integrated with electronics. Because so much technology is shared with the IC industry silicon wafers are typical substrates with thousands of devices being able to be fabricated at once (Fig. 7).
Lithographie, Galvanik, Abformung (LIGA) is a German acronym that means lithography, electroforming, and molding. The technology was originally developed in the late-1970s to fabricate separation nozzles for uranium enrichment. This technology uses X rays to fabricate devices with very high aspect ratios. A synchrotron radiation source is used to define small critical dimensions in a poly(methyl methyacrylate) (PMMA), mold that can then be electroplated to form high aspect ratio metallic structures. Many parts can be batch fabricated in this manner, but assembly is usually still a serial process.
Precision machining technology, such as micro-EDM (microelectro discharge machining), laser micromachining, and micro stereo lithography, is also used to form complex structures out of metal, plastic, and ceramics that the previous fabrication technologies may be incapable of. Precision machining is typically a serial process, but is

528 MICROSURGERY
Figure 7. Silicon wafer in the fabrication process. (Courtesy of Intel Corp.)
often better able to deal with the varied shapes and substrates of microsurgical instruments.
Micro EDM is a form of spark machining used to shape conductive materials, such as silicon and metals. An EDM erodes material by creating a controlled electric discharge between an electrode and the substrate. It is a noncontact process and there is no direct mechanical cutting force applied to the substrate. Dielectric fluid is used to remove the erosion particles, as well as to keep the substrate material from oxidizing. Micro-EDMs can be used to make holes, channels gears, shafts, molds, dies, stents, as well as more complex 3D parts such as accelerometers, motors, and propellers (3).
Lasers can be used to both deposit and remove material. Laser ablation vaporizes material through the thermal noncontact interaction of a laser beam with the substrate. It allows for the micromachining of silicon and metals, as well as materials that are difficult to machine using other techniques such as diamond, glass, soft polymers, and ceramics. Laser direct writing and sintering is a maskless process where a laser beam is used to directly transfer metal materials onto a substrate. This can be used to form metal traces on nonplaner surfaces, which reduces the need for wires on surgical tools (4).
Micro stereo lithography processes generate 3D structures made out of ultraviolet (UV) cured polymers. It is an additive process where complex 3D structures are made from stacks of thin 2D polymer slices that have been hardened from a liquid bath. Conventional systems were limited in that they were a serial process where only one part could be made at a time. MicroTEC has developed a batch fabricated wafer level process called rapid material product development (RMPD), which is capable of constructing structures out of 100 different materials including plastics, sol–gels, and ceramics (5).
APPLICATIONS
The inclusion of MEMS technology in microsurgery, will allow for smaller more miniaturized surgical tools that not
Figure 8. Strain gauges fabricated on surgical sharps. (Courtesy of Verimetra, Inc.)
only overcome many of the limitations of microsurgical procedures, but allow for new more advanced operations to be performed. MEMS are just now being incorporated into microsurgical tools and coming on the market. Most are still at the research level, but the industry is moving in this direction as the need for smaller smarter tools increases.
HAPTIC FEEDBACK
One of the key areas for improvement in microsurgery is tactile feedback. The lack of tactile sensing limits the effectiveness these procedures. Recent work in robotic feedback for minimally invasive surgery has concentrated on force feedback techniques using motors and position encoders to provide tactile clues to the surgeon. In these approaches, the sense element is far removed from the sense area. Verimetra, Inc. has developed strain gauge force sensor fabrication technology which uses the surgical tools themselves as a substrate (6). Prior efforts have focused on fabrication of sensors on silicon, polyimide, or some other substrate followed by subsequent attachment onto a surgical tool with epoxy, tape, or some other glue layer. Attaching a sensor in this manner limits performance, introduces sources of error, limits the sensor’s size, and further constrains where the sensor can be placed. By eliminating glue and adhesion layers improved sensitivity and reduces errors due to creep. Figure 8 shows strain gauges fabricated on surgical sharps. Figure 9 is a cut away SEM image of a strain gauge and temperature sensor embedded inside of a robotic microforcep. While this microfabrication technology is an improvement in sensor technology, wires are still used to connect the sensor to the outside world. Reliability and the added complexity of adding wires to surgical end effectors with high degrees
Figure 9. Strain gauges and temperature sensor embedded in robotic microgripper. (Courtesy of Verimetra, Inc.)
of freedom limit the effectiveness of the technology. Shortrange wireless technology compatible with the operating room environment need to be developed to overcome these limitations.
Recently tactile feedback has been shown to be able to be added to noncontact lasers. Based on optical distance measurements, the systems synthesize haptic feedback through a robotic arm held by the surgeon when the focal point of the laser is coincident with a real surface. This gives the operator the impression of touching something solid. By increasing the power of the laser such a system could also be used for cutting or ablation.
TISSUE SENSING
Taking haptic feedback one step further is the ability to distinguish between different types of tissue in the body. Tissue sensing is of vital importance to a surgeon. Before making an incision into tissue, the surgeon must identify what type of tissue is being incised, such as fatty, muscular, vascular, or nerve tissue. This becomes more complicated because the composition and thickness of different human tissues varies from patient to patient. Failure to properly classify tissue can have severe consequences. For example, if a surgeon fails to properly classify a nerve and cuts it, then the patient can suffer effects ranging from a loss of feeling to loss of motor control. If a neurosurgeon cuts into a blood vessel while extracting a tumor severe brain damage may occur. The identification and classification of different types of tissue during surgery, and more importantly during the actual cutting operation, will lead to the creation of smart surgical tools. If a surgical tool senses that it is too close to or about to cut the wrong type of tissue it can simply turn itself off.
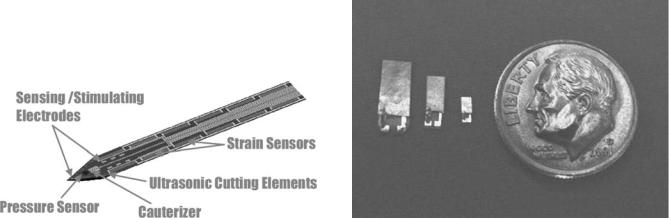
Verimetra, Inc. has developed a device called the data knife, Fig. 10. It is a scalpel, which is outfitted with different strain sensors along the edges of the blade to sense the amount of force being applied. The resistance of the tissue is one of the signals used for classifying tissue. Pressure sensors are used to measure the characteristics of material surrounding the blade. The pressure of the surrounding fluid can be used to help classify the type or location of tissue. Electrodes are used to measure the impedance of different types of tissue, as well as being used to identify nerves by picking up their electrical signals. The tool provides the real-time feedback surgeons
MICROSURGERY 529
have been asking for during surgery, and can also be used to record data for later use for tracking purposes.
Sensing the density of tissue can also be used to assist the surgeon in identifying tissue. In open cardiac bypass operations, the surgeons insert their hands inside the body to palpate arteries. For cardiac bypass surgery, surgeons select the bypass location by feeling where the fat and fatty plaque is located in your arteries with their fingers. The lack of tactile feedback in minimally invasive surgery, prevents them from using this technique. The MEMS devices have been developed for the palpation of tissue using strain gauges (7), diaphragms (8), micropositioners (9,10), and load cells (11) and have shown the ability to measure blood pressure, pulse, different kinds of arterial plaque, and distinguish between colon, bowel, stomach, lung, spleen, and liver tissue.
Piezoelectric transducers can also be used to measure density. Macroscale transducers are frequently used in imaging applications to differentiate between tumors, blood vessels, and different types of tissue. These transducers both emit and receive sound waves. By vibrating at a high frequency sound waves are emitted in the direction of the object of interest. The density of the impinged object can then be measured based on the signal that is reflected back by that object. Sound waves are reflected off the interfaces between different types of tissue and returned to the transducer. Present ultrasound systems tend to be large and are not well suited for incorporation into microsurgical devices. The MEMS technology is well suited for this application and many ultrasonic MEMS sensors have been developed for imaging (12–16).
Microelectromechanical systems ultrasound devices for density measurements are shown in Fig. 11. They have been shown to be able to detect the location of bone in tissue and are being applied to atrial fibrillation surgeries. Atrial fibrillation is what causes irregular heartbeats and leads to one out of every six strokes. Drugs can be used to treat this condition, but have dangerous side effects including causing a switch from atrial fibrillation to the more dangerous ventricle fibrillation. Pacemakers and other electrical control devices can be used, but they do not always work for all patients. The most effective treatment is the surgical
Figure 10. Data Knife smart scalpel. (Courtesy of Verimetra, |
Figure 11. Ultrasound transducers next to a dime. (Courtesy of |
Inc.) |
Verimetra, Inc.) |
530 MICROSURGERY
MAZE procedure, but it is an incredibly invasive treatment. The patient is put on a heart lung machine and then their heart is stopped. Next, the surgeon takes a scalpel and actually cuts all the way through the heart making lesions, which physically separate the heart muscle. These lesions break the electrical connections in the heart. The heart is then sewn back together. Recently, there have been a variety of different methods used to address the problem. Instead of physically cutting all the way through the heart with a scalpel, surgeons are using radio frequency, microwave, cryo, and laser energy to create transmural lesions. Transmurality means that the lesions go all the way through the tissue, breaking the heart’s electrical connections. One of the problems surgeons encounter is to know how deep the ablation is or if it is transmural. If the lesions are not completely transmural or just partially transmural then the undesirable electrical signals may still be transmitted to the heart muscle. The MEMS ultrasound technology or micromachined electrodes can be used to measure the transmurality.
Temperature can be used to detect if a surgical device is close to a blood vessel, or if the surgical tool is in a diseased or infected area. Temperature can also be used to monitor the usage of a surgical device, by monitoring the time at which the device is at body temperature. Usage is just one of many areas where Auto-ID technologies will benefit microsurgery (17). They can be used to make sure that only the correct surgical tool is used for a procedure and if that tool has been properly sterilized. Keeping track of how many times, how long, and what was done with a surgical tool will improve the reliability and effectiveness of surgical procedures and will greatly impact the entire medical industry.
TRACKING SYSTEMS
Traditionally, a surgeon uses an endoscope in minimally invasive surgery to determine where the surgical instruments are located in the patient. The view the surgeon has of the surgical area is not ideal and the position of surgical instruments outside of the camera view is not known. Ideally, the surgeon would like to know the position and orientation of each of his instruments. Computer-aided surgery has enabled the surgeon to overlay magnetic resonance imaging (MRI) or computed artial tomography (CAT) scan images of the patient with position and orientation data taken during surgery to create 3D models that the surgeon can use to better visualize the surgical procedure. Computers can be used to simulate the procedure beforehand allowing the surgeon to practice difficult operations ahead of time.
Current technology in this area is predominately optical in nature. Markers are placed on the ends of the surgical instruments that are located outside of the body, as well as on specific locations on the patient’s body. A computer registers the location of the surgical tools with the reference markers on the patient so that images of the patient’s body can be aligned with the surgical tools. This is done through the use of visible and infrared (IR) cameras. The tips of the surgical tools that are located inside of the body
are then extrapolated. The markers must not interfere with the surgery in any way, and therefore should be as small and lightweight as possible. While these systems are wireless and do not have cords that can get tangled on the surgeon or on the surgical tools, there must be an unobstructed path from the markers to the camera systems. The surgeon must be careful not to block the markers themself or with other surgical instruments. Precision is compromised because the location of the surgical tips is extrapolated and does not take into account bending of the surgical tools. Markers on the outside of the body do not take into account compression of the tissue.
MEMS based ultrasound tracking systems have been developed to address these issues (18). Constellations of ultrasound sensors can also be placed on the surgical tools themselves to determine position thereby eliminating errors from extrapolation. Reference markers can now be placed inside of the body, closer to the surgical area so that they are less affected by compression and movement of the patient.
Position and orientation can also be estimated using accelerometers and gyroscopes. The signal outputs can be integrated to determine or predict the distance traveled by a surgical tool. Conventional MEMS accelerometers have accuracies in the milligram range, which are not sufficient for measuring accurately the relatively small displacements made during surgery (19). More accurate inertial sensors need to be developed before they can be integrated into surgical tools.
Magnetic field sensors can also be used to determine position and orientation of surgical tools (20,21). A three axis magnetoeffect sensor has been developed for determining the location of catheters. Currently this is done by continually taking X rays of the patient. However X rays only provide a 2D snapshot, a 3D image would be more helpful for navigation.
EYE SURGERY
The leading cause of vision loss in adults > 60 are cataracts. The word cataract comes from the Greek meaning waterfall and was originally thought to be caused by opaque material flowing, like a waterfall, into the eye. The condition is actually caused by the clouding of the eye’s intraocular lense. In the eye, light passes through the lens that focuses it onto the retina. The retina converts the light into electrical signals that are then interpreted by the brain to give us our vision. The lens is a hard crystalline material made mostly of water and protein. The protein is aligned in such a way to allow light to pass through and focus on the retina. When proteins in the lens clump together, the lens begins to cloud and block light from being focused on the retina. This causes vision to become dull and blurry, which is commonly referred to as a cataract.
Much like other surgery in other parts of the body, cataract surgery has followed down the minimally invasive path for the same benefits. Cataract surgery is one of the earliest known surgical procedures. The earliest evidence is the written Sanskrit writings of the Hindu surgeon
Susrata dating from the fifth century BC. He practiced a type of cataract surgery known as couching or reclination, in which a sharp instrument was inserted into eye and pushed the clouded lens out of the way. This displacement of the lens enabled the patient to see better. Although vision was still blurred without corrective lenses, many famous people underwent this procedure including the artists Michelangelo, Rembrandt, and Renoir. Couching was still performed until the mid-twentieth century in Africa and Asia.
In 1748, Jacques Daviel of Paris introduced extracapsular surgery where the lens was removed from the eye. Later, very thick pairs of glasses were used to focus the light onto the retina and restore sight, but the glasses were cumbersome and caused excessive magnification and distortion.
By 1949, Dr. Harold Ridley of England, used PMMA as the first intraocular lens. He discovered that PMMA was biocompatible with the eye while treating WWII fighter pilots whose eyes were damaged by shattering plastic from their windshields. In the 1960s and 1970s, extracapsular surgery became the preferred treatment. A large incision (10–12 mm) was made in the eye to remove and replace the lense. This procedure minimized problems with image size, side vision, and depth perception, but the large incisions required longer hospitalization, recovery time, and stitches.
Today, cataracts are removed with a procedure called phacoemulsication with 1.5 million operations performed yearly. A hollow ultrasonically driven titanium needle is inserted into the anterior chamber of the eye. Ultrasonic energy is then used to liquefy the hard lens and it is then aspirated out of the eye. A foldable lens made of acrylic or silicone is inserted through a (1–3 mm hole) as a replacement. Since the incision size has been reduced compared to conventional extracapsular surgery, hospitalization, general anesthesia, sutures and bandages have all been eliminated. The reduction in incision size has also reduced the risk of infection and postoperative refractions.
During the procedure the surgeon cannot see directly under the needle as the lens is broken up and aspirated. A thin clear membrane or capsule surrounds the lense. The posterior capsule tissue underneath the lens is very delicate and easily cut compared the crystalline lens. To prevent the soft underlying tissue from damage requires a skilled surgeon whom has performed many procedures. If the posterior capsule is ruptured it can lead to glaucoma, infection, or blindness. As the size of the incision has decreased, heat damage to surrounding tissue from the ultrasonic tip has increased that can alter the characteristics of the tissue and change its appearance. In addition positive intraocular pressure must be maintained by balancing the flow of infusion fluid at positive pressure and the aspirated cataract lens fragments. If pressure is not maintained the anterior chamber can collapse. Pressure is currently maintained by sensors located many feet from the surgical area. This distance creates delays in the feedback loop, which can cause dangerous pressure fluctuations leading to damage to the iris and cornea.
Recently, micromachined silicon ultrasonic surgical tools for phacoemulsifiaction have been developed by Lal’s
MICROSURGERY 531
Figure 12. Ultrasonic phacoemulsification tool. (Courtesy of Jeff Miller/University of Wisconsin-Madison.)
research group (22,23) (Fig. 12). Piezoelectric material is attached to a micromachined silicon needle. The needle has a fluid channel for aspiration as well as a horn for amplifying the ultrasonic displacement. These silicon devices are able to generate higher stroke velocities and lower heat generation than their conventional titanium counterparts. High levels of integration has been achieved by integrating pressure and flow sensors directly on the needle for maintaining intraocular pressure, reducing delays and making the phacoemulsification procedure safer.
To prevent damage to the posterior capsule a piezoelectric sensor has been integrated into a phacoemulsification hand piece and undergone clinical trials (24). The device can determine tissue hardness by measuring the impressed loading on the needle tip or by monitoring the resonant frequency at which the ultrasonic system oscillates. Both of these approaches have proven successful in determining when a hard to soft tissue transition has occurred during a phacoemulsification procedure. This technology enables a surgeon to get real-time feedback on the type of tissue he is cutting, and can be applied to other types of surgical procedures such as tumor extraction as well.
Insertion of a replacement lense requires precise movements by the surgeon. Serious postoperative vision problems may occur if the lens is inserted incorrectly and needs to be removed. Precision piezoelectric micromotors have been developed for intraocular delivery of replacement lenses after cataract removal (25). These inchworm actuators use a glider and clamp arrangement to generate large forces over small displacements. An electrostatic clamp made of an oxide dielectric layer sandwiched

532 MICROSURGERY
between two silicon wafers layer locks the micromotor in place while a PZT actuator generates the force. The inertia of a mass is used to move the clamp. Forces of 3.0 N and step sizes of 100 nm to 10 mm have been reported.
In eye surgery there are many times when precision cutting is required. Highly sharpened steel, ceramic, or diamond scalpel blades are used, but are expensive to produce costing up to $1000 a piece. Disposable silicon micromachined scalpels are an attractive alternative. They can be batch fabricated to reduce cost and sharpened to an atomic edge along their crystal planes. They are already being used in Russia at the Fyodorov Eye Care Center in nonpenetrating deep sclerectomy operations for the treatment of glaucoma (26). BD (Bekton, Dikenson, and Company) is producing Atomic Edge silicon blades for cataract surgery (27). Soon they will be used for eye operations and eventually migrate to procedures on other parts of the body. Smaller incisions made by sharper blades result in less bleeding and tearing. An added advantage of silicon blades is that sensors and electronics can be directly fabricated on them during fabrication. Integrating cauterizing electrodes on the blade itself will prevents the patient from bleeding as well as let the surgeon more clearly see the surgical area.
CATHETERS/GUIDEWIRES/STENTS
Cardiac catheterizations can be referred to as a noninvasive surgical procedure. Specialized tubes or catheters are threaded up through blood vessels in the groin, arm, or neck to an area of the body which needs treatment. The problem is then treated from the inside of the blood vessel. The advantage of these approaches is that the procedures require very small incisions, hospital stays are usually one night or less and the discomfort and recovery times afterwards are minimal. For patients with more complicated ailments, catheter treatments can be used in combination with minimally invasive or open surgery to give the best possible results at the lowest possible risk. Catheters, guidewires, and stents currently represent the most widespread use of MEMS technology in surgery.
Diagnostic catheterizations, which are used to measure pressure in different parts of the body, take blood samples, and to perform detailed angiograms of the heart, can be performed by injecting X-ray dye through the catheters. The MEMS pressure sensors are now commonly found on catheter devices and are the most mature MEMS technology in this area. Even smaller designs are being sold for placement on guidewires, such as those made by Silex Microsystems, shown next to a 30 gauge needle (Fig. 13). Each sensor is but 100 mm thick, 150 mm wide, and 1300 mm long (28). MEMS ultrasound sensors are also starting to be used for both forward looking (16) and side looking intravascular imaging (12,13).
To provide the doctor with more information to make better decisions, additional MEMS sensors are needed to gather additional data for the diagnosis, monitoring of procedures, as well as for checking results of completed operations. Many other types of MEMS sensors are being researched to measure blood flows, pressures, temperatures, oxygen content, and chemical concentrations for placement on diagnostic catheters (29,30).
Figure 13. MEMS pressure sensors next to a 30-gauge needle (Courtesy of Silex Microsystems, Jarfalla, Sweden.)
Heart disease continues to be the leading cause of death in the United States. Interventional catheterization is an increasingly more common way to treat blood vessels which have become occluded (blocked) or stenotic (narrowed) by calcified artherosclerotic plaque deposits. Blood vessels that have become occluded or stenotic may interrupt blood flow, which supplies oxygen and cause heart attacks or strokes. Occluded or stenotic blood vessels may be treated with a number of medical procedures including angioplasty and atherectomy. Angioplasty techniques, such as percutaneous transluminal coronary angioplasty (PTCA), also known as balloon angioplasty are relatively noninvasive methods of treating restrictions in blood vessels. A balloon catheter is advanced over a guidewire until the balloon is positioned in the restriction of a diseased blood vessel. The balloon is then inflated compressing the atherosclerotic plaque. Frequently, the wall of the vessel is weakened after inflation and a metal stent in is expanded and deployed against the plaque. The stent helps keep the vessel open. During an atherectomy procedure, the stenotic lesion is mechanically cut or abraded away from the blood vessel wall using an atherectomy catheter.
Microelectrochemical systems pressure sensors can be used to measure the pressure in the balloons during inflation, to make sure damage due to over inflation is minimized. The MEMS temperature sensors can be integrated on catheters and guidewires to determine the location of inflamed plaque. The inflammation causes artery walls in the damaged area to have an increased temperature up to 38C higher than healthy tissue. Approximately 20–50% of all patients undergoing these therapeutic procedures to clear blocked coronary arteries will suffer restenosis (reblockage) within 6 months of the initial procedure. Drug coated stents have significantly lowered these rates and have been approved for use in Europe for a few years. They are expected to be approved in the United States later this year. The MEMS laser micromachining technology is used in the fabrication of conventional stents and drug coated stents (31). Stainless steel micromachining technology has also been developed at the University of Virginia for piercing structure drug delivery/gene therapy stents for the treatment of restenosis (32). There is potentially a large opportunity for MEMS in embedding sensors into stents to
create a smart stents, which would be able to alert doctors when restenosis occurs or other problems occur (33). The MEMS rotary cutting devices have been fabricated for atherectomy procedures (34), but are not widely used because cut up plaque particles can flow downstream and cause strokes.
FETAL SURGERY
Fetal surgical techniques were first pioneered at the University of California San Francisco (UCSF) in the 1980s to operate on babies while they were still in the womb. The success rate of treating certain birth defects is higher the earlier they are addressed in fetal development. Initially open surgical techniques were used with an incision through the mother’s abdomen to allow direct access for the surgeon’s hands. After the surgery was complete, the womb was sutured and the mother delivered the baby weeks or months later. In 1981, the first fetal urinary tract obstruction procedure was performed at UCSF. Lately, minimally invasive surgical and robotic surgical techniques have been used that have reduced the risk of complications and premature labor. Other fetal procedures have also been developed to treat cojoined twins, hernias, spina bifida, tumors, and heart defects. One area of interest is in fetal heart.
The development of the cardiovascular system in a developing fetus is typically completed by the twelfth week of gestation. At this stage primary heart defects are small and if repaired will prevent defects from becoming more severe as the heart changes to adapt to the normalization blood flows and pressures in the later periods of gestation.
Fetal heart surgery can be used to treat hypoplastic heart syndrome (HHS), which was often considered fatal. This syndrome causes the heart’s left side to fail to grow properly and is caused by an obstruction which restricts blood flow. The heart requires the mechanical stress of blood flow to grow properly, and thus fails to develop normally. Today, three open surgeries are required to allow the remaining single (right) ventricle to support both the pulmonary and systemic circulations. The long-term survival for these patients into adulthood is significantly < 50%. Preserving both ventricles would result in the best chances of long-term survival.
An interventional catheter can be used to treat HHS in a minimally invasive manner. The balloon catheter must first penetrate in through the mother’s abdominal wall, the placenta and then the fetus’s chest into its heart. It must then locate the fetus’s tiny heart and expand to open the blockage. Afterward, the surgeon needs to know whether the operation has succeeded enough for the baby to develop normally. Verimetra, Inc., Carnegie Mellon University, and Children’s Hospital of Pittsburgh have embedded a MEMS flow sensor and a series of micromachined bar codes on the tip of a balloon catheter. The bar codes enable the catheter to be visualized with ultrasound allowing surgeons to know its exact position. The flow sensor is a thermistor. As blood flow increases the temperature measured by the thermistor decreases and in this manner blood flow changes as the catheter progresses through the heart’s vessels can be monitored. This allows for the measurement of blood flow at or near a constriction
MICROSURGERY 533
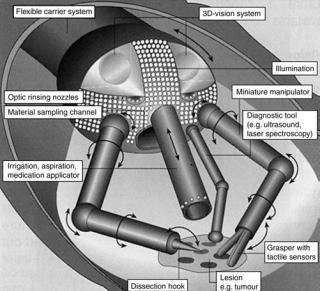
Figure 14. Highly integrated microsurgical probe. (Courtesy of Prof. Dr. M. Schurr, novineon Healthcare Technology Partners GmbH and Prof. G. Buess, Universita¨tsklinikums Tbingen.)
and then again after the procedure to open the constriction has been performed.
FUTURE SYSTEMS
In 1959, Richard Feynman gave his famous talk There’s Plenty of Room at the Bottom (35). In it, he talked about being able to put a surgeon in a blood vessel which would be able to look around ones heart. Initially, new microsurgical tools will focus on measuring or detecting a specific parameter be it pressure, blood flow, velocity, temperature, and so on. The MEMS sensor systems will continue to be refined and improved leading to the integration of multiple sensor systems on surgical tool followed by tools with multiple functions. Multifunction surgical tools will reduce the number of tool insertions and removals reducing patient risks. Eventually, this will lead to highly integrated probes, which will do everything a surgeon needs, such as the concept shown in Fig. 14 (36). These tools will fit through a standard 5-mm port and have built in 3D cameras for visualization, biopsy samplers with microfluidic processing capability to do tissue analysis, ultrasound transducers, and tactile sensors for feedback to the surgeon.
BIBLIOGRAPHY
1.Diamiano R. Next up: Surgery by remote control. NY Times, Apr. 4, 2000; pp. D1.
2.Blazer E, Koh W, Yon E. A miniature digital pressure transducer. Proceedings of the 24th Annual Conference on Engineering Medicine and Biology. 1971. pp 211.
3.Peirs J, et al. A microturbine made by micro-electro-discharge machining. Proc 16th Eur Conf Solid-State Transducers 2002; 790–793.
4.Pique A, et al. Laser direct-write of embedded electronic components and circuits. Presented at Photon Processing
534 MICROSURGERY
in Microelectronics and Photonics IV, Jan 24–27 2005, San Jose, (CA); 2005.
5.Go¨tzen R. Growing up, additive processes in MEMS fabrication and packaging. Presented at Machines and Processes for Micro-scale and Meso-scale Fabrication, Metrology and Assembly, Gainesville, FL; 2003.
6.Rebello KJ, Lebouitz K, Migliuolo M. MEMS tactile sensors for surgical instruments. MRS Symp. Proc.: Biomicroelectromech. Systems (BioMEMS). 2003;773:55–60.
7.Menciassi A, et al. Force feedback-based microinstrument for measuring tissue properties and pulse in microsurgery. Presented at 2001 IEEE International Conference on Robotics and Automation (ICRA), May 21–26 2001, Seoul; 2001.
8.Rebello KJ, et al. MEMS based technology for endoscopic assessment of coronary arterial hardness. Presented at the 5th Annual NewEra Cardiac Care Conference, Dana Point (CA); 2002.
9.Bicchi A, et al. Sensorized minimally invasive surgery tool for detecting tissutal elastic properties. Presented at Proceedings of the 1996 13th IEEE International Conference on Robotics and Automation. Pt. 1 (of 4), Apr. 22–28 1996, Minneapolis, (MN); 1996.
10.Rosen J, Hannaford B, MacFarlane MP, Sinanan MN. Force controlled and teleoperated endoscopic grasper for minimally invasive surgery—experimental performance evaluation. IEEE Trans Biomed Eng 1999;46:1212–1221.
11.Scilingo EP, Bicchi A, De Rossi D, Iacconi P. Haptic display able to replicate the rheological behaviour of surgical tissues. Presented at Proceedings of the 1998 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Pt. 4 (of 6), Oct. 29-Nov. 1 1998, Hong Kong, China; 1998.
12.Fleischman A. et al. Miniature high frequency focused ultrasonic transducers for minimally invasive imaging procedures. Sensors Actuators, A: Phys 2003;103:76–82.
13.Zara JM, Bobbio SM, Goodwin-Johansson S, Smith SW. Intracardiac ultrasound scanner using a micromachine (MEMS) actuator. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control 2000;47:984–993.
14.Daft C, et al. Microfabricated ultrasonic transducers monolithically integrated with high voltage electronics. Presented at 2004 IEEE Ultrasonics Symposium, Aug. 23–27 2004, Montreal, Queeue, Canada; 2004.
15.Chang JK, et al. Development of endovascular microtools. J Micromech Microeng 2002;12:824–831.
16.Degertekin FL, Guldiken RO, Karaman M. Micromachined capacitive transducer arrays for intravascular ultrasound. Presented at MOEMS Display and Imaging Systems III, Jan. 24–25 2005, San Jose, (CA); 2005.
17.Brock D. Smart medicine: The application of Auto-ID technology to healthcare. Auto-ID Center, MIT, Cambridge, (MA); 2002.
18.Tatar F, Mollinger JR, Bastemeijer J, Bossche A. Time of flight technique used for measuring position and orientation of laparoscopic surgery tools. Presented at Sensors, 2004. Proceedings of IEEE; 2004.
19.Fang C-M, Lee S-C. A research of robotic surgery technique by the use of MEMS accelerometer. Presented at Engineering in Medicine and Biology, 2002. 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society. EMBS/BMES Conference; 2002. Proceedings of the Second Joint, 2002.
20.Tanase D, et al. 3D position and orientation measurements with a magnetic sensor for use in vascular interventions. Presented at Biomedical Engineering, 2003. IEEE EMBS Asian-Pacific Conference on; 2003.
21.Totsu K, Haga Y, Esashi M. Three-axis magneto-impedance effect sensor system for detecting position and orientation of catheter tip. Sensors Actuators, A: Phys 2004;111:304–309.
22.Chen X, Lal A. Integrated pressure and flow sensor in siliconbased ultrasonic surgical actuator. Presented at Ultrasonics Symposium, 2001 IEEE; 2001.
23.Son I-S, Lal A. Feedback control of high-intensity silicon ultrasonic surgical actuator. Presented at Solid-State Sensors, Actuators and Microsystems, 2005. Digest of Technical Papers. TRANSDUCERS ’05. The 13th International Conference on; 2005.
24.Polla DL, et al. Microdevices in medicine. Annu Rev Biomed Eng 2000;2:551–76.
25.Polla D, et al. Precision micromotor for surgery. Presented at Microtechnologies in Medicine and Biology, 1st Annual International, Conference On. 2000; 2000.
26.Kozlova TV, Shaposhnikova NF, Scobeleva VB, Sokolovskaya TV. Non-penetrating deep sclerectomy: Evolution of the method and prospects for development (review). Ophthalmosurgery 2000;3:39–54.
27.Angunawela R, Von Mohrenfels CW, Marshall J. A new age of cataract surgery. Cataract & Refractive Surgery Today I 2005; 36–38.
28.Kalvesten JE, Smith L, Tenerz L, Stemme G. First surface micromachined pressure sensor for cardiovascular pressure measurements. Presented at Proceedings of the 1998 IEEE 11th Annual International Workshop on Micro Electro Mechanical Systems, Jan. 25–29 1998, Heidelberg, Ger; 1998.
29.Tanase D, Goosen JFL, Trimp PJ, French PJ. Multi-para- meter sensor system with intravascular navigation for catheter/guide wire application. Presented at Transducers’01 Eurosensors XV, Jun. 10–14 2001; Munich, 2002.
30.Haga Y, Esashi M. Biomedical microsystems for minimally invasive diagnosis and treatment. Proc IEEE Biomed App Mems Microfluidics 2004;92:98–114.
31.Kathuria YP. An overview on laser microfabrication of biocompatible metallic stent for medical therapy. Presented at Laser-Assisted Microand Nanotechnologies 2003, Jun. 29– Jul. 3 2003, St. Petersburg, Russian Federation; 2004.
32.Reed ML. Micromechanical systems for intravascular drug and gene delivery. Presented at BioMEMS 2002 Conference, Boston; 2002.
33.Goldschmidt-Clermont P, Kandzari D, Khouri S, Ferrari M. Nanotechnology needs for cardiovascular sciences. Biomed Microdevices 2001;3:83–88.
34.Ruzzu A, et al. A cutter with rotational-speed dependent diameter for interventional catheter systems. Presented at Micro Electro Mechanical Systems, 1998. MEMS 98. Proceedings., The Eleventh Annual International Workshop on, 1998.
35.Feynman RP. There’s plenty of room at the bottom. J Microelectromech Systs 1992;1:60–66.
36.Schurr MO, Heyn S-P, Ment W, Buess G. Endosystems— Future perspectives for endoluminal therapy. Minimally Invasive Ther Allied Technol 1998;7:37–42.
Further Reading
Taylor RH, Lavallee S, Burdea GC, Mosges R. Computer Integrated Surgery: Technology and Clinical Application. Cambridge, (MA): MIT Press; 1996.
Zenati M. Robotic heart surgery. Cardiol Rev 2001;9(5):1–8. Davies B. A review of robotics in surgery. Proc Inst Mech Eng
2000;214:129–140.
Madou M. Fundamentals of Microfabrication: The Science of Miniturization. 2nd ed. Boca Raton, (FL): CRC Press; 2002.
Kovacs GTA. Micromachined Transducers Sourcebook. Boston, MA: McGraw-Hill; 2002.
See also ENDOSCOPES; FIBER OPTICS IN MEDICINE; INTRAUTERINE SURGICAL TECHNIQUES; NANOPARTICLES; RADIOSURGERY, STEREOTACTIC.
