
- •VOLUME 4
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •HYDROCEPHALUS, TOOLS FOR DIAGNOSIS AND TREATMENT OF
- •HYPERALIMENTATION.
- •HYPERBARIC MEDICINE
- •HYPERBARIC OXYGENATION
- •HYPERTENSION.
- •HYPERTHERMIA, INTERSTITIAL
- •HYPERTHERMIA, SYSTEMIC
- •HYPERTHERMIA, ULTRASONIC
- •HYPOTHERMIA.
- •IABP.
- •IMAGE INTENSIFIERS AND FLUOROSCOPY
- •IMAGING, CELLULAR.
- •IMAGING DEVICES
- •IMMUNOLOGICALLY SENSITIVE FIELD–EFFECT TRANSISTORS
- •IMMUNOTHERAPY
- •IMPEDANCE PLETHYSMOGRAPHY
- •IMPEDANCE SPECTROSCOPY
- •IMPLANT, COCHLEAR.
- •INCUBATORS, INFANTS
- •INFANT INCUBATORS.
- •INFUSION PUMPS.
- •INTEGRATED CIRCUIT TEMPERATURE SENSOR
- •INTERFERONS.
- •INTERSTITIAL HYPERTHERMIA.
- •INTRAAORTIC BALLOON PUMP
- •INTRACRANIAL PRESSURE MONITORING.
- •INTRAOCULAR LENSES.
- •INTRAOPERATIVE RADIOTHERAPY.
- •INTRAUTERINE DEVICES (IUDS).
- •INTRAUTERINE SURGICAL TECHNIQUES
- •ION-EXCHANGE CHROMATOGRAPHY.
- •IONIZING RADIATION, BIOLOGICAL EFFECTS OF
- •ION-PAIR CHROMATOGRAPHY.
- •ION–SENSITIVE FIELD-EFFECT TRANSISTORS
- •ISFET.
- •JOINTS, BIOMECHANICS OF
- •JOINT REPLACEMENT.
- •LAPARASCOPIC SURGERY.
- •LARYNGEAL PROSTHETIC DEVICES
- •LASER SURGERY.
- •LASERS, IN MEDICINE.
- •LENSES, CONTACT.
- •LENSES, INTRAOCULAR
- •LIFE SUPPORT.
- •LIGAMENT AND TENDON, PROPERTIES OF
- •LINEAR VARIABLE DIFFERENTIAL TRANSFORMERS
- •LITERATURE, MEDICAL PHYSICS.
- •LITHOTRIPSY
- •LIVER TRANSPLANTATION
- •LONG BONE FRACTURE.
- •LUNG MECHANICS.
- •LUNG PHYSIOLOGY.
- •LUNG SOUNDS
- •LVDT.
- •MAGNETIC RESONANCE IMAGING
- •MAGNETOCARDIOGRAPHY.
- •MANOMETRY, ANORECTAL.
- •MANOMETRY, ESOPHAGEAL.
- •MAMMOGRAPHY
- •MATERIALS, BIOCOMPATIBILITY OF.
- •MATERIALS, PHANTOM, IN RADIOLOGY.
- •MATERIALS, POLYMERIC.
- •MATERIALS, POROUS.
- •MEDICAL EDUCATION, COMPUTERS IN
- •MEDICAL ENGINEERING SOCIETIES AND ORGANIZATIONS
- •MEDICAL GAS ANALYZERS
- •MEDICAL PHOTOGRAPHY.
- •MEDICAL PHYSICS LITERATURE
- •MEDICAL RECORDS, COMPUTERS IN
- •MICROARRAYS
- •MICROBIAL DETECTION SYSTEMS
- •MICROBIOREACTORS
- •MICRODIALYSIS SAMPLING
- •MICROFLUIDICS
- •MICROPOWER FOR MEDICAL APPLICATIONS
- •MICROSCOPY AND SPECTROSCOPY, NEAR-FIELD
- •MICROSCOPY, CONFOCAL
- •MICROSCOPY, ELECTRON
- •MICROSCOPY, FLUORESCENCE
- •MICROSCOPY, SCANNING FORCE
- •MICROSCOPY, SCANNING TUNNELING
- •MICROSURGERY
- •MINIMALLY INVASIVE SURGICAL TECHNOLOGY
- •MOBILITY AIDS
- •MODELS, KINETIC.
- •MONITORING IN ANESTHESIA
- •MONITORING, AMBULATORY.
- •MONITORING, FETAL.
- •MONITORING, HEMODYNAMIC
- •MONITORING, INTRACRANIAL PRESSURE
- •MONITORING, NEONATAL.
- •MONITORING, UMBILICAL ARTERY AND VEIN
- •MONOCLONAL ANTIBODIES
- •MOSFET.
- •MUSCLE ELECTRICAL ACTIVITY.
- •MUSCLE TESTING, REHABILITATION AND.
- •MUSCULOSKELETAL DISABILITIES.
38.Tsai-Goodman B, et al. Development of a system to record cardiac output continuously in the newborn. Pediatr Res. 1999;46(5):621–625.
39.Hermansen MC, Hermansen MG. Intravascular catheter complications in the neonatal intensive care unit. Clin Perinatol; 2005;32(1):141–156.
40.Barrington KJ. Umbilical artery catheters in the newborn: Effects of position of the catheter tip. Cochrane Database Syst Rev CD000505, 2000.
41.Kempley ST, Bennett S, Loftus BG. Randomized trial of umbilical arterial catheter position: Clinical outcome. Acta Paediatr 1993;82:173–176.
42.Mokrohisky ST, Levine RL, Blumhagen JD. Low positioning of umbilical-artery catheters increases associated complications in newborn infants. N Engl J Med 1978;299:561–564.
43.Umbilical Artery Catheter Trial Study Group. Relationship of intraventricular hemorrhage or death with the level of umbilical artery catheter placement: A multicenter randomized clinical trial. Pediatrics 1992;90:881–887.
44.Haldeman S, Fowler GW, Ashwal S. Acute flaccid neonatal paraplegia: A case report. Neurology 1983;33:93–95.
45.Cumming WA, Burchfield DJ. Accidental catheterization of internal iliac artery branches: A serious complication of umbilical artery catheterization. J Perinatol 1994;14:304–309.
46.Klaus MH, Fanaroff AA. Care of the high-risk neonate, 5th ed. Philadelphia: WB Saunders; 2001.
47.Nowlen TT, Rosenthal GL, Johnson GL. Pericardial effusion and tamponade in infants with central catheters. Pediatrics 2002;110:137–142.
48.O’Grady NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Pediatrics 2002;110(5):e51.
49.Tudos AJ, Besselink GAJ, Schasfoort RBM. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab on a Chip: 2001;1(2):83–95.
50.Wolfbeis OS. Fiber-optic chemical sensors and biosensors. Anal Chem 2001;74(12):2663–2677.
51.Zhang XC. Terahertz wave imaging: Horizons and hurdles. Proceedings of the First International Conference on Biomedical Imaging and Sensing Applications of THz Technology, Physics in Medicine Biology 2002;47(21):3667–3677.
See also ARTERIES, ELASTIC PROPERTIES OF; BLOOD GAS MEASUREMENTS;
FIBER OPTICS IN MEDICINE; NEONATAL MONITORING; STRAIN GAGE.
MONOCLONAL ANTIBODIES
BRENDA H. LASTER
JACOB GOPAS
Ben Gurion University of the
Negev
Beer Sheva, Israel
INTRODUCTION
This article outlines the association of antibodies within the human immune system, the structural and binding characteristics of antibodies, and the development and production of monoclonal antibodies. Recent advancements in recombinant DNA techniques and genetic engineering are described, including the use of plants to increase the production capacity of Mabs. Their usefulness as biological and medical reagents is further elaborated in a description of the various instrumentation, techniques,
MONOCLONAL ANTIBODIES |
597 |
and assays employed in the diagnosis and treatment of diseases as well as their utility in the research laboratory.
THE IMMUNE SYSTEM AS IT APPLIES TO ANTIBODIES (1)
The immune system is normally directed at foreign molecules borne by pathogenic microorganisms. However, the immune system can also be induced to respond to simple nonliving molecules. Any substance that can elicit an immune response is said to be immunogenic and is called an immunogen. There is a clear operational distinction between an immunogen and an antigen. An antigen is defined as any substance that can bind to a specific antibody (see below), but is not necessarily able to elicit an immune response by itself.
Immunization
The deliberate induction of an immune response is known as immunization. To determine whether an immune response has occurred and to follow its course, the immunized individual is usually monitored for the appearance of antibodies directed at the specific antigen. Monitoring the antibody response usually involves the analysis of relatively crude preparations of sera. The serum is the fluid phase of clotted blood, which contains a variety of specific antibodies against the immunizing antigen as well as other soluble serum proteins.
Cells Participating in an Immune Response
B lymphocytes (or simply B cells) are one of the two major types of lymphocytes that enable the adaptive immune response. When activated, B cells differentiate into plasma cells that secrete antibodies. T lymphocytes or T cells consist of three main classes. One class differentiates upon activation into cytotoxic T cells, which may kill foreign tissues, cancer cells, and cells infected with virus. The second class of T lymphocytes is T helper cells that differentiate into cells that activate and enable the proper function of other cells, such as B cells. The third class is the T suppressor cells that limit the extent of the immune response.
Antigen Recognition
Both T and B lymphocytes bear receptor proteins on their surface that allow them to recognize antigen. Collectively, these receptors are highly diverse in their antigen specificity, but each individual lymphocyte is equipped with membrane-bound receptors that will recognize only one particular antigen. Each lymphocyte therefore recognizes a different antigen. Together, the receptors of all the different lymphocytes are capable of recognizing a very wide diversity of antigens, which encompass most of the different antigens an individual will meet in a lifetime. These include those antigens that are exclusively synthesized in the laboratory. The B cells do not secrete antibody until they have been stimulated by specific antigen. The B-cell antigen receptor (BCR) is a membrane-bound form of the same antibody that they will secrete when activated by antigen. Thus the antigen recognized by both the BCR and
598 MONOCLONAL ANTIBODIES
the secreted antibody present in the same B cell, are identical.
Antibodies
Antibody molecules as a class are now generally known as immunoglobulins (Ig), and the antigen receptor of B lymphocytes is known as surface immunoglobulin. The T cell antigen receptor (TCR) is related to immunoglobulins, but is quite distinct from it in structure and function.
Structure and Function of Antibodies
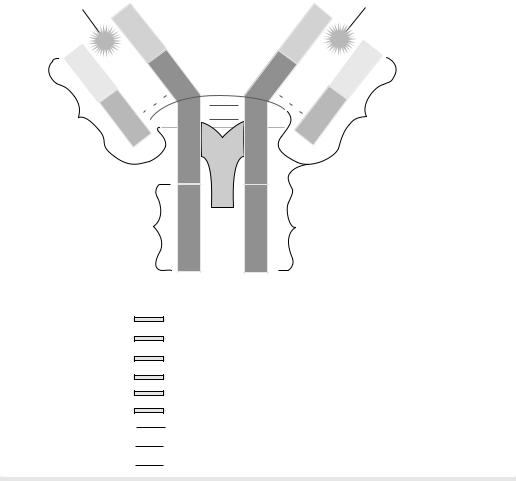
Antibodies are the antigen-specific products (proteins) secreted by B cells. The antibody molecule has two separate functions: one is to bind specifically to molecules from the immunogen (pathogen) that elicited the immune response; the other is to recruit various cells and molecules in order to remove and destroy the pathogen once the antibody is bound to it. These functions are structurally separated in the antibody molecule. One region of the antibody specifically recognizes antigen and the other engages the effector mechanisms that will dispose of it.
The antigen-binding region varies extensively among antibody molecules and is thus known as the variable region or V region, labeled as VH (heavy chain) and VL (light chain). It is this variability that allows each antibody molecule to recognize and bind a particular antigen. The total repertoire of antibodies made by a single individual is large enough to ensure that virtually any structure can be bound. The association between the antibody and the antigen depends on their steric conformation. That is, depending on the size and interatomic distance of these reacting molecules, a tight fit between the antibody combining sites and the antigenic determinant can occur.
The region of the antibody molecule that engages the effector functions of the immune system, but is not associated with antibody binding and does not vary in the same way is known as the constant region or C region. It is typified by the IgG antibody shown in Fig. 1 and is designated CL (light chain) and CH (heavy chain). It has five main forms, or isotypes, that are specialized for activating the different immune effector mechanisms.
The remarkable diversity of antibody molecules is the consequence of a highly specialized mechanism by which
Antigen binding
NH2
NH 2
Antigen
binding
NH |
2 |
VH |
|
|
|
|
|
||
|
|
VL |
CH1 |
|
|
|
|
||
|
|
s |
s |
|
Pepsin Cleavage |
|
|||
CL |
|
|||
F (ab')z fragment |
CH2 |
|||
|
||||
|
|
COOH |
||
lostype Determinate |
CH3 |
µ,γ,α,δ, or £ |
|
|
|
|
VH |
NH |
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
CH1 |
VL |
|
|
|
|
|
|
|
|
s |
s |
s |
|
Fab fragment |
Papain |
s |
|
Cleavage |
|||
s |
s |
|
CL |
|
|
CH2 |
|
|
|||
|
|
|
|
|
|
Carbohydrate |
|
COOH |
|
|
|
|
|
|
|
|
|
|
|
CH3 |
Fc fragment |
|
|
|
|
|
|
|
|
COOH COOH
Heavy chain constant region.
Heavy chain variable region.
Light chain constant region.
Light chain variable region.
Carbohydrate.
Antigen binding site. s s Disul fide bonds.
Papain cleavage site.
Pepsin cleavage site.
Figure 1. Basic immunoglobulin structure. [Courtesy of Sigma-Aldrich (www.sigmaaldrich.com/ img/assets/8181/AntibodyExp).]
the genes that code for antibody production and that are expressed in any given B cell are assembled by DNA rearrangements that join together two or three different segments to form a V region gene during the development of the B cell. Subsequent DNA rearrangement can attach the assembled V-region to any C-region gene and thus produce antibodies of any of the five isotypes.
Antibodies are roughly Y-shaped molecules. All antibodies are constructed in the same way from paired heavy and light polypeptide chains. In Fig. 1, one can observe that the innermost regions of the Y-shaped molecule are the heavy chains; the light chains are the outermost regions. Within this general category, however, five classes (isotypes) of immunoglobulin -IgM, IgD, IgG, IgA, and IgEcan be distinguished biochemically as well as functionally. The five classes are defined by the structure of their heavy chain. Their distinctive functional properties are conferred by differences in the amino acid sequences of the carboxyterminal part of the heavy chain (COOH in Fig. 1) in the region that is not associated with the light chain. IgG (Fig. 1) will be used to describe the general structural features of immunoglobulin molecules.
The IgG antibodies are large molecules ( 150 kDa) composed of two different polypeptide chains. One of these polypeptide chains, 50 kDa in size, is termed the heavy or H chain. The other, is 25 kDa in size, and is termed the light chain or L chain. The two chains are present in an equimolar ratio, and each IgG molecule contains two heavy chains and two light chains. The two heavy chains are linked to each other by disulfide bonds and each heavy chain is linked to a light chain by a disulfide bond. In any one immunoglobulin molecule, the two heavy chains and the two light chains are identical, enabling them to bind two identical antigenic determinants.
The amino-terminal sequences of both the heavy and light chains vary greatly among different antibodies. The variability in sequence is limited to approximately the first 110 amino acids on the chain, corresponding to the first domain, whereas the carboxy-terminal sequences are constant between immunoglobulin chains, either light or heavy, of the same isotype.
Fragmentation of Antibodies
The antibody molecule can be readily cleaved by different proteases into functionally distinct fragments. For example, Fab fragments, each of which consists of two identical fragments, each containing the antigen binding region. Additionally, an Fc fragment can be extracted that interacts with effector molecules and cells, or one F(ab0)2 fragment that contains both arms of the antigen binding region. Figure 1 shows the sites of the derivation of these fragments. Genetic engineering techniques now permit the construction of designed variations of the antibody molecule such as a truncated Fab that comprises only the V region of a heavy chain linked to a V region of a light chain. This is called a single–chain Fv. A broad range of genetically engineered molecules are now becoming valuable therapeutic agents because their smaller size readily permits their penetration into tissue. Useful antibodies from animal sources have been engineered in a process referred
MONOCLONAL ANTIBODIES |
599 |
to as ‘‘humanization’’. This avoids their recognition as foreign, and prevents their rapid clearance from the body. The process utilizes the variable region of a mouse antibody coupled to the Fc region from human antibodies. Antibody fragments may also be coupled to toxins, radioactive isotopes and protein domains that interact with effector molecules or cells.
MONOCLONAL ANTIBODIES
Antibody Heterogeneity
The antibodies generated in a natural immune response or after immunization in the laboratory are a mixture of molecules of different antigen specificities and affinities. Because of their multiple specificities they are termed polyclonal antibodies. Some of this heterogeneity results from the production of antibodies that bind numerous different antigenic determinants (epitopes) present on the immunizing antigen. However, even antibodies directed at a single antigenic determinant can be markedly heterogeneous. Antisera (serum containing antibodies against specified antigens) are valuable for many biological purposes, but they have certain inherent disadvantages that relate to the heterogeneity of the antibodies they contain. First, each antiserum is different from all other antisera, even when raised in a genetically identical animal while using the identical preparation of antigen and immunization protocol. Second, antisera can be produced in only limited volumes, and thus it is impossible to use the identical serological reagent in a long or complex series of experiments, or in clinical tests or therapy. Finally, even purified antibodies may include minor populations of antibodies that give unexpected cross-reactions that confound the analysis of experiments and can be harmful in therapy. To avoid these problems, and to harness the full potential of antibodies, it became necessary to develop a method for making an unlimited supply of antibody molecules of homogeneous structure and known specificity. This has been achieved through the production of monoclonal antibodies from hybrid antibody forming cells or, more recently, by genetic engineering.
Production of Monoclonal Antibodies
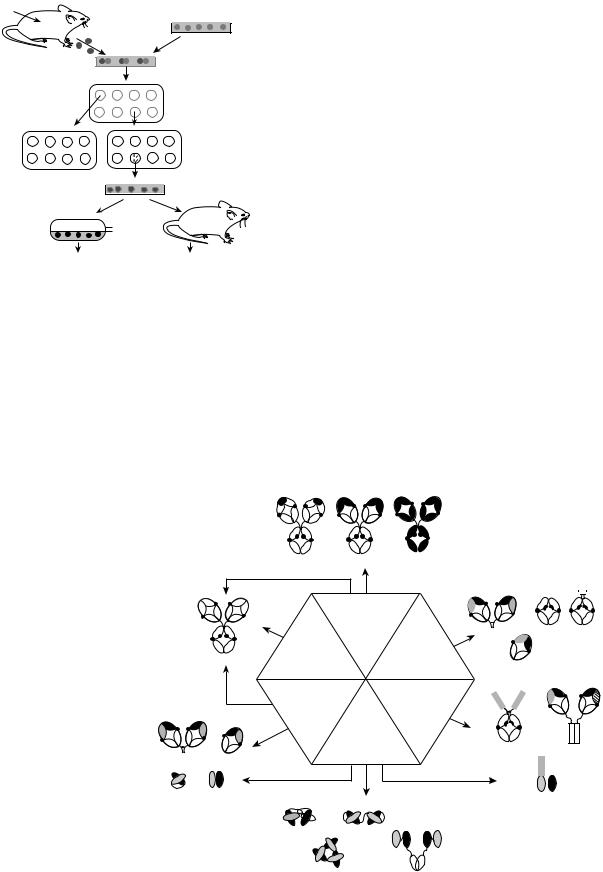
In 1975, using cell culture techniques, Kohler and Milstein
(2) found a way to grow an immortal B-cell-like lymphocyte that continuously produced an antibody with a predetermined specificity. The procedure required the immunization of a mouse in order to produce a large population of B-cells in the spleen that would secrete a specific antibody. However, in cell culture, the life span of spleen cells is only a few days. To produce a continuous source of antibody, the B cells had to grow continuously. This was achieved by fusing the spleen cells that contained a particular gene, the hypoxanthine-guanine phosphoribosyl transferase (HGPRT) gene with immortal myeloma cells (cancerous plasma cells). In general, plasma cells are mature-antibody secreting B cells. The myeloma cells were preselected to ensure three specific properties. First, immortality in cell culture; second, that they were sufficiently altered so that
600 MONOCLONAL ANTIBODIES
Antigen
|
|
Myeloma cells |
Spleen cells |
|
(HGPRT-) |
|
|
Fusion |
|
|
1.Culture in |
|
|
HAT medium |
3. Clone each |
|
2.Test each |
|
supernatant |
|
positive culture |
|
for antibodies |
|
|
4. Test each |
|
|
supernatant |
|
|
for antibodies |
|
5. Expand positive clones |
|
|
propagate |
|
in vitro |
or |
in vivo |
|
|
|
Harvest monoclonal antibodies
Figure 2. Schematic of hybridoma protocol. [Courtesy of Prof. John Kimball (http://users.rcn.com/jkimball.ma.ultranet/ Biology Pages/M/Monoclonals.html).]
they did not secrete antibody, and third, that they would not flourish in a particular growth medium containing hypoxanthine, aminopterin and thymidine (HAT). The HAT medium was previously shown to be a highly selective medium for those specific hybrid cells that lack the gene for the enzyme, HGPRT. Consequently, all unfused myeloma and spleen cells would not survive in the HAT medium. The HGPRT gene that was contributed by the spleen cell
permitted only hybrid cells to survive in the HAT medium, because only hybrid cells would be able to grow in the culture due to the conferral of immortality by the myeloma cells. Therefore, the immune spleen cells conferred both antibody specificity and the HGPRT gene to the hybrid cell, while the myeloma cell conferred immortality to the spleen cell and they were able to survive indefinitely in culture. This is the method that is used today to produce individual hybridomas (the hybrid cells) that are then screened for antibody production. Single antibody-producing cells that produce an antibody with the desired specificity are cloned. These cloned hybridoma cells are grown in bulk culture to produce large amounts of antibody that are used in a variety of ways. Since each hybridoma is descended from a single B cell, all cells of a particular hybridoma cell line produce the same hybridoma molecule. This is the monoclonal antibody (Mab). The procedure is shown as a schematic outline in Fig. 2.
Recombinant Genetic Engineering
The field of antibody engineering endeavors to improve the target specificity and effector function of the antibodies by altering their construction, while retaining their binding characteristics. This is achieved by using new recombinant engineering technologies to redesign the molecular architecture of the antibodies. Examples of this are shown in Fig. 3, where in a process called genetic engineering, recombinant DNA (spliced DNA that is formed from two or more sources and then rejoined) is produced by putting genes from one species into the cells of an individual organism of another species. The foreign DNA becomes
Figure 3. Antibody engineering: examples of antibody-based constructs and respective routes for their generation. Hybridoma technology provides a way of producing mouse monoclonal antibodies. Genetic engineering has encouraged the creation of chimeric, humanized, and human antibodies, antibody fragments (traditionally obtained by partial digestion of immunoglobulins with proteases), multimeric antibody fragments, fusion (immunoadhesins and immunotoxins) and bispecific antibodies. Multimeric antibody fragments (diabody, triabody, and tetrabody) are represented as multivalent structures, although they can also be engineered to be multispecific. The minibody depicted is a dimer that can be linked to the CH3 fragment via a LD linker or a flexible linker (FlexMinibody). Bispecific F(ab)2 is shown as a Fab dimer linked noncovalently via interaction of amphipathic helices. (Courtesy of American Chemical Society and American Institute of Chemical Engineers, Copyright # 2004.) (See Ref. 3.)
|
|
CDR-Grafted |
Chimeric |
Mouse |
|
|
|
|
|
|
Mouse |
|
|
|
|
|
|
|
Hybridoma |
|
|
|
|
|
|
Transgenic |
Enzyme |
|
F(ab')2 |
pFc' Fc |
|
|
|
digestion |
|
|
|
||
|
Human |
mouse |
|
|
|
|
|
|
(papain, pepsin) |
Fab |
|
||||
|
|
|
|
|
|
|
|
|
|
In vitro |
|
Chemical |
|
|
|
|
|
Ab |
|
conjugation |
|
|
|
|
|
libraries |
|
|
|
|
|
|
|
|
Recombinant |
Immunoadhesin |
Bispecific F(ab)2 |
||
F(ab')2 |
Fab |
|
systems |
|
|||
|
|
|
|
|
|
||
scFv |
Fv |
|
|
|
|
Immunotoxin |
|
|
|
|
|
|
|
||
Diabody scFv2
Triabody Minibody
part of the host’s genome (its genetic content) and is replicated in subsequent generations descended from that host. An alternative technique for producing antibody-like molecules is the Phage Display Libraries for Antibody V-region Production (4). In this approach, gene segments that encode the antigen-binding variable region of antibodies are fused to genes that encode the coat protein (outside surface) of a bacteriophage (viruses that infect bacteria). In essence, mRNA from primed human B-cells is converted to cDNA. The large variety of diverse antibody genes are expanded by the polymerase chain reaction (PCR) to generate a highly diverse library of antibody genes. Bacteriophage containing such gene fusions are then used to infect bacteria, resulting in phage particles that have outer surfaces that express the both antibody-like fusion protein, and the same antigen-binding domain displayed on the outside of the bacteriophage. The collection of such recombinant phages, each displaying a different antigen-binding domain on its surface, is known as a phage display library. A particular phage can be isolated from the mixture and can be used to infect fresh bacteria. Each phage isolated in this way produces a monoclonal antigen-binding particle analogous to a monoclonal antibody. A complete antibody molecule can then be produced by fusing the V region of a particular phage with the invariant part of the immunoglobulin gene. These reconstructed antibody genes can be introduced (transfected) into a suitable host cell line. These genes will become part of the cell’s genome and will secrete antibodies akin to hybridomas.
Monoclonal Antibodies Produced in Plants, Plantibodies
After undergoing genetic engineering techniques, plant cells are capable of assembling and producing unlimited quantities of antibodies, referred to as plantibodies (5). This trademark name for human antibodies manufactured in plants has functionally limitless production capacity and lower costs than those associated with the yeast fermentation process that is currently being used to produce mass quantities of human antibodies. This fairly recent finding might prove to be of benefit in the medical, consumer, and industrial applications of monoclonals. For example, it has been postulated that the development of plantibodies with a capability of sequestering heavy metals or radioactive compounds might have a very positive impact on the environment, particularly because their production is very inexpensive and large supplies are easily produced. Because the corn crop is so readily available worldwide, and its kernel stores natural plantibodies, these can be purified as needed by standard milling procedures. Potato and tomato crops are also being used. The first clinical use of the effectiveness of a plantibody was against the bacterium, Streptococcus mutans. This organism produces lactic acid that erodes tooth enamel. The plantibody was brushed onto human teeth for 3 weeks and tooth decay was prevented for up to 4 months. The action of the antibody was to prevent the bacterium from binding to the tooth surface. Plantibody-containing gels are being developed to prevent genital herpes infections and to protect newborn babies during delivery against transmission of the Herpes virus from infected mothers. Plantibodies against human immu-
MONOCLONAL ANTIBODIES |
601 |
nodeficiency verus (HIV) and the production of sperm are also being developed. Concerns have been expressed about the use of genetically engineered food crops because of the potential dangers of their getting into the wrong hands, or disturbing the ecological balance.
The aforementioned technologies have revolutionized the use of antibodies by providing a limitless supply of antibodies with single and known specificity. Monoclonal antibodies are now used in most serological assays, as diagnostic probes and as therapeutic agents.
TECHNIQUES FOR USING MONOCLONAL ANTIBODIES AS SEROLOGICAL AND DIAGNOSTIC PROBES
Monoclonal antibodies can serve as tools for diagnosing and treating disease and are valuable agents in the research laboratory. Their utility required the development of procedures that would permit them to be viewed at the particular region of interest. Some of the most widely used techniques are described in the following sections (1).
Immunofluorescence
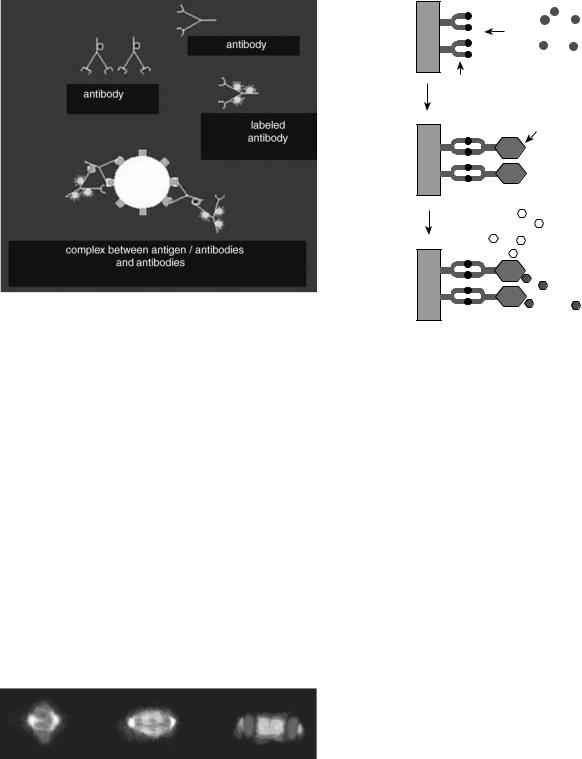
Since antibodies bind stably and specifically to their corresponding antigen, they are invaluable as probes for identifying a particular molecule in cells, tissues, or biological fluids. Monoclonal antibody molecules (Mabs) can be used to locate their target molecules accurately in single cells or tissue sections by a variety of different labeling techniques. When either the antibody itself, or the antiMab that is used to detect it, is labeled with a fluorescent dye the technique is known as immunofluorescence. As in all serological techniques, the antibody binds stably to its antigen, allowing any unbound antibody to be removed by thorough washing. The fluorescent dye can be covalently attached directly to the specific antibody, but more commonly, the bound antibody is detected by a secondary fluorescent anti-immunoglobulin; that is, the first antibody binds to the antigen and a fluorescent secondary antibody (antibody) is targeted to the primary antibody–antigen complex. The technique is known as indirect immunofluorescence, which is demonstrated in Fig. 4, where the binding of the first antibody to the antigen is followed by the binding of the antibody. The dyes chosen for immunofluorescence are excited by light of a particular wavelength, and emit light of a different wavelength in the visible spectrum. By using selective filters that can permit only certain wavelengths of light to pass, only that light coming from the dye or fluorochrome used is detected in the fluorescence microscope. Therefore, the antibody can be located by virtue of its emission of fluorescent light. The recently developed confocal fluorescent microscope considerably enhances the resolution of the technique. If different dyes are attached to different antibodies, the distribution of two or more Mabs can be determined in the same cell or tissue section. Differentiating between the antibodies occurs because either the dye or the fluorochrome will excite at different wavelengths or because they will emit their fluorescence at different wavelengths. An example of the immunofluorescence technique is shown in Fig. 4, whereby, through the use of monoclonal antibodies targeted to
602 MONOCLONAL ANTIBODIES
Solid
Add antigen
Antibody
Add antibody-enzyme conjugate
Enzyme
Figure 4. Indirect immunofluorescence. The primary antibody binds to the antigen and the fluorescent antiantibody binds to the primary thereby increasing the signal. [Courtesy of Prof. v. Sengbusch (http://www.biologie.uni-hamburg.de/b-online/d00/ copyrig.htm).]
intracellular proteins, certain structures within the cell become visible. The structure shown in Fig. 5 is that of the spindle, which appears during cell division. During certain phases of cell division, the chromosomes arrange themselves in the equatorial plane of the spindle. The spindle is made up of microtubules that, in turn, are composed of proteins. Monoclonal antibodies that would bind to two specific proteins, a and g-tubulin, of the microtubule were synthesized and labeled with two different fluorochromes. During cell division, the cells were exposed to the fluorescent-labeled Mabs that formed a complex with the proteins and permitted visualization of the spindle.
Immunohistochemistry
An alternative to immunofluorescence for detecting a protein in tissue sections is immunohistochemistry, in which the specific Mab is chemically coupled to an enzyme that converts a colorless substrate into a colored reaction pro-
Figure 5. Dividing cells (mitosis: metaphase, anaphase, and telophase) were stained with monoclonal antibodies against two intracellular proteins, a-tubulin in green, and g-tubulin in red. Because these proteins constitute the spindle, the intracellular structure upon which chromosomes line up during mitosis, the structure is visualized by virtue of the difference in the fluorochromes tagged to the Mabs that were bound to the proteins. Chromosomes were stained with a blue dye. (www.img.cas.cz/dbc/ gallery.htm.)
Substrate
(colorless)
Add substrate
Product
Figure 6. Schematic of ELISA assay protocol. [Courtesy of Prof. John Kimball (http://users.rcn.com/jkimball.ma.ultranet/Biology Pages/E/Elisa.html).]
duct in situ. The Enzyme-Linked ImmunoSorbent Assay (ELISA) is a technique that detects and quantifies specific antigens from a mixture. It is widely used in procedures that screen blood for viral or bacterial contamination, to detect infections, toxins, illegal drugs, or allergens, and in measuring hormone levels, such as in pregnancy or thyroid function. The assay involves the binding of an antibody to a solid surface and exposing it to the antigens. A second complex, consisting of the same antibody, but additionally tagged with a particular enzyme, is exposed to the initial antibody–antigen conjugate and binds. After washing the surface to remove excess unbound antigen, a colorless substrate is added that permits the antigen to be converted into a colored product that can be read and measured using absorption spectrometry. The intensity of color is proportional to the concentration of bound antigen. A schematic of the ELISA assay is shown in Fig. 6. A more detailed description of the Elisa procedure can be found in (Kimball’s Biology Pages http://biology-pages.info). Horseradish peroxidase and alkaline phosphatase are also used as enzymes in immunochemistry assays. An example of the utility of these enzymes for protein detection is shown in Fig. 7.
Immunoelectron Microscopy
Antibodies can be used to detect the intracellular location of structures or particular molecules by electron microscopy, a technique known as immunoelectron microscopy. After labeling Mabs with gold particles and targeting them to samples, they can then be examined in the transmission electron microscope. Since electrons do not penetrate through gold particles, the regions in which the antibodies bind appear as dark dots.

MONOCLONAL ANTIBODIES |
603 |
Figure 7. Detection of the measles virus (MV) P-protein by Western Blot in MV infected and noninfected cells. Whole-cell lysates were prepared from MV that was either persistently infected (NS20-MV) or notinfected (NS20) mouse neuroblastoma cells. The proteins in the lysates were separated by SDS–PAGE and blotted onto nitrocellulose paper. The blot was incubated with a Mab against the MV P-protein, followed by a secondary antimouse immunoglobulin antibody linked to horseradish peroxidase. The P-protein band was detected when a substrate was added that was modified by peroxidase on the blot and caused light to be released. Light was detected on a specific band after exposure to film. The results show that only the measlesinfected cells express the viral protein. (Courtesy of Jacob Gopas.)
Blotting Techniques
Immunoblotting or Western blotting is used to identify the presence of a given protein in a cell lysate. Cells are placed in detergent to solubilize all cell proteins and the lysate (the material resulting from the ruptured cells) is run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), which enables protein migration and separation by size. Further resolution is achieved if the proteins are initially separated by charge according to their isoelectric point and then by size. This technique is referred to as two-dimensional (2D) gel electrophoresis. The proteins are then transferred (blotted) from the gel to a stable support, such as a nitrocellulose membrane for easier handling. Specific proteins of interest in the lysate’s mixture are detected by incubating the membrane with a Mab that can react with a defined protein on the membrane. An example of the technique is shown in Fig. 7. The proteins
bound to the antibodies are revealed byenzyme-labeled, antiimmunoglobulin antibodies. By this technique the presence or absence, as well as the amounts of specific proteins, can be monitored following a variety cell treatments. Specific DNA labeled with antigen (hapten)-bound nucleotides can be blotted onto a membrane and detected with Mabs against the hapten. This allows the detection of viral or bacterial DNA in tissues or body fluids, as generated by PCR.
Purification Techniques
Affinity chromatography and immunoprecipitation are techniques that enable purification of molecules and their characterization. A mixture of molecules can be incubated with a Mab, which is chemically attached to a solid support. The bound antibody–antigen complex is washed from unbound molecules by centrifugation, and then the molecule of interest is eluted for further characterization. These techniques are useful for protein purification, for determining its molecular weight, its abundance, distribution, and whether it undergoes chemical modifications as a result of processing within the cell.
Immunoelectrophoresis
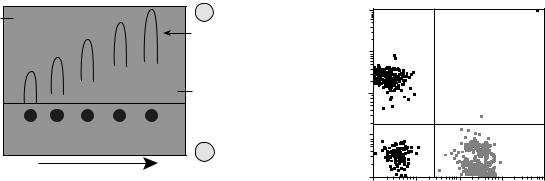
Two-dimensional electrophoresis is used to separate different antigens that might be present in one solution. The antigens are separated on the basis of their electrophoretic mobility. The currents are run at right angles to each other, driving the antigens into the antiserum (containing Mabs). Peaks are obtained when the antigen forms a complex with the antibody; the area under the peaks gives the concentration of antigen as shown in Fig. 8. Rocket electrophoresis is a similar technique. Here, after a current is applied, the antigens are separated based upon their ionic charge by their differential migration through a gel that contains antibody. As shown in Fig. 9, concentration is determined by the migration distance. In countercurrent electrophoresis, the greater internal osmotic pressure drives the antibody backwards into a gel after a current is applied. An antigen that is negatively charged will form a complex with the antibody in the gel in a pH-dependent process.
Instrumentation
An immensely powerful tool for defining and enumerating and isolating cells is the use of the fluorescence-activated
Precipitin arc
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
Antibody in gel |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Figure 8. Two-dimensional immuno- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
- |
|
|
|
|
|
electrophoresis. Antigens are sepa- |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
- |
rated on the basis of electrophoretic |
|||
|
Ag2 |
Ag1 |
Starting |
|
|
|
mobility. [Courtesy of the Natural |
|||||
|
|
|
|
Toxins Research Center at Texas |
||||||||
|
|
|
|
well |
|
|
|
|
|
|||
|
|
First run |
|
|
|
|
Second run |
|
|
A&M University – Kingsville (http:// |
||
|
|
|
|
|
|
|
|
ntri.tamuk.edu/).] |
||||
604 MONOCLONAL ANTIBODIES
Antibody |
+ |
|
|
||
in agarose |
Precipitin |
|
gel |
||
arcs |
||
|
||
|
(rockets) |
pH 8.6
Antigen 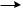 wells
wells
–
Increasing antigen concentration
Figure 9. Rocket electrophoresis. Antigen is electrophoresed into gel containing antibody. The distance from the starting well to the front of the rocket shaped arc is related to antigen concentration. [Courtesy of the Natural Toxins Research Center at Texas A&M University – Kingsville (http://ntri.tamuk.edu/).]
cell sorter (FACS). This instrument is used to study the properties of cell subsets identified using Mabs to cell surface proteins. Individual cells are first tagged by treatment with specific fluorescent Mabs. The mixture of labeled cells is then forced with a much larger volume of fluid through a nozzle, creating a fine stream of liquid containing cells spaced singly at intervals. As each cell passes through a laser beam it scatters the laser light, and any dye molecules bound to the cell will be excited and fluoresce. Sensitive photomultiplier tubes detect both the scattered light, which gives information on the size and granularity of the cell, and the fluorescence emission, provide quantification of the binding of the labeled Mabs, and on the expression of cell-surface proteins by each cell. In the cell sorter, the signals passed back to the computer are used to generate an electric charge, which is passed from the nozzle through the liquid stream. Droplets containing a charge can then be deflected from the main stream as they pass between plates of opposite charge. In this way a specific population of cells, distinguished by the binding of the labeled antibody and its defined electrical charge, can be extracted and purified from a mixed population of cells. Alternatively, to deplete a population of cells, a labeled antibody directed at marker proteins expressed by undesired cells will direct the cells to a waste channel, retaining only the unlabeled cells. Several Mabs labeled with different fluorochromes can be used simultaneously. FACS analysis can give quantitative data on the percentage of cells bearing different molecules, and the relative abundance of the particular molecules in the cell population, 10,000 cells in a typical experiment demonstrates the retrieval of data after FACS analysis. An example of data output from FACS is shown in Fig. 10.
Mabs as Molecular Probes
Mabs can also be used to determine the function of molecules. Some antibodies are able to act as agonists, when the binding of the Mab to the molecule mimics the binding of the natural ligand (antigen) and activates its function. For example, antibodies to the CD3 antigen present on mature human T cells have been used to stimulate the T cells. This
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
PE |
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
CD19 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
10 |
101 |
102 |
103 |
104 |
100 |
||||
|
|
CD3 FITC |
|
|
Figure 10. FACS analysis. Characterizing cells at different stages of development through the use of fluorescent labeled monoclonal antibodies against cell surface markers is one of the most common applications of flow cytometry. Changes in the relative numbers, absolute counts, or in the ratio of cell types can provide valuable information as to the status of the immune system in human disorders or animal models. Different cell types can be detected and quantified from a mixed population by the use of monoclonal antibodies labeled with different fluorescent dyes that have nonoverlapping emission spectra. In this example experiment, blood lymphocytes were incubated with two different Mabs, CD19, and CD3. CD19 was labeled with the fluorochrome phycoerythrin (PE) and binds a cell membrane molecule specific for B-lymphocytes. The CD3 was labeled with fluorecein isothyocyanate (FITC) that detects a cell membrane protein specific for T-lymphocytes. Three populations of cells were detected in this experiment according to the antibody bound to the cells. The logarithmic x and y axis represent relative amounts of fluorescence detected on cells labeled with FITC or PE, respectively. The blue dots represent cells unstained by either of the antibodies, the red dots represent B-lymphocytes that were detected by CD19 and the green dots represent T-lymphocytes, CD3 positive cells. No cells were detected that bound both antibodies (top right quadrant).
occurs because CD3 is associated with the T-cell receptor and is responsible for signal transduction of the receptor. Conversely, Mabs can function as antagonists, inhibiting the binding of the natural ligand and thus blocking its function. For example, antibodies that block the epidermal growth factor receptor (a growth stimulating protein) function as antagonists.
THE USE OF MONOCLONAL ANTIBODIES
AS THERAPEUTIC AGENTS
Mabs against cell-surface molecules have been used to remove specific lymphocyte subsets or to inhibit cell function in vitro. Cytotoxic drugs kill proliferating cells indiscriminately. In contrast, antibodies can interfere with immune responses in a nontoxic and much more specific manner. For example, Mabs can be used to remove undesirable lymphocytes from donor bone marrow cells prior to transplantation. This treatment selectively removes lymphocytes that recognize the host tissues as ‘‘foreign’’ and induce a potentially fatal condition known as Graft versus Host reaction (6).
Mabs are being tested experimentally to inhibit transplant rejection, to alleviate and suppress autoimmune disease and in cancer detection and treatment. The major impediment to therapy with monoclonal antibodies in humans is that these antibodies are mostly of mouse origin, and humans rapidly develop antibody responses to mouse antibodies. This not only blocks the actions of the mouse antibodies, but leads to allergic reactions. If this occurs, future treatment of the same patient with any mouse Mab is unacceptable. In principle, the problem can be avoided by producing antibodies that are not recognized as foreign by the human immune system. Several strategies are being explored for their construction. One approach is to clone human V regions into a phage display library (see above) and select for its ability to bind human cells. With this method, Mabs that are entirely human in origin can be obtained. Second, mice that lack endogenous immunoglobulin genes can be made ‘‘transgenic’’ (chimeric). That is, they can have human genes put into their genome through recombinant DNA techniques. When this occurs, they will then express human immunoglobulin heavy and light genes and eventually antibody molecules. A third approach is to graft the variable region of a mouse Mab into the rest of the human immunoglobulin molecule, in a process known as humanization. These recombinant antibodies are far less immunogenic in humans than the Mabs of the parent mouse, therefore, they can be used for more efficient and repeated treatment of humans with far less risks. In some cases even humanized antibodies may evoke an immune response and must be administered with immunosuppressive drugs.
THE USE OF MONOCLONAL ANTIBODIES IN THE DETECTION, FOLLOW-UP, AND TREATMENT OF CANCER
Tumor-Specific Antigens
For the greater part of the twentieth century, it was assumed that any antigens present on the cell surface of tumor cells would also be present in normal cells; therefore, few investigations were undertaken to elicit any autoimmune response against cancer cells. However, once inbred mouse strains bearing transplanted syngeneic (genetically identical) tumors became available, research studies validated that immune reactions against these tumors could be induced with no toxic effects on normal tissues, and scientists began to pursue the identification of ‘‘tumor specific antigens’’. Shared tumor antigens were found in many of the same types of cancers in different patients, and unique antigens were isolated that were specific for a particular cancer in a particular patient. The SEREX database lists the antigens that have been isolated from humans (7). These antigens have the ability to generate an immune response when introduced into a patient.
The advent of monoclonal antibodies suggested the possibility of targeting and destroying tumors by making antibodies against tumor-specific antigens. However, this relies upon the identification of a tumor-specific antigen that is located on the surface of cancer cells. Because of their ability to differentiate between normal and malignant tissues and to exact a variety of antitumor responses,
MONOCLONAL ANTIBODIES |
605 |
Mabs offer a significant advantage to conventional forms of therapy. Several monoclonal antibodies have already been proven to be relatively well tolerated and effective for the treatment of many different malignant diseases.
Approaches to Cancer Immunotherapy
Approaches to cancer immunotherapy can be either active or passive. For example, in the active category, tumor vaccines that immunize against particularly defined tumor antigens, can be used. In the passive category is the use of monoclonal antibodies that are either conjugated, unconjugated, or radiolabeled. These same approaches can also be categorized as specific, wherein antigens are directly targeted, or nonspecific, where immune cells are used to directly target tumor cells. Other approaches are taken that elicit antitumor effects with different mechanisms, such as using antibodies to block growth factors or receptors on cells; targeting specific tissue components of the tumor or its blood vessels; interfering with cell signals; or with apoptosis (programmed cell death) (8).
Magic Bullets
While such Mab-based therapies offer a high potential to fulfill the promise of ‘‘magic bullets’’ for the treatment of malignant disease, successful application of these therapies is often impaired by several impediments. Factors inhibiting the therapeutic benefit of Mabs may include low or heterogeneous expression of target antigens by tumor cells, high background expression of antigen on normal cells, host antibody immune responses to the Mabs themselves, insufficient anti-tumor response after Mab binding, as well as physical obstructions preventing antibody binding, such as crossing to and from blood vessels as well as tissue barriers en route to the solid tumor mass (9). These factors influence the ability of the Mabs to penetrate to the tumor.
IMAGING TUMORS WITH MONOCLONAL ANTIBODIES
Mabs in Nuclear Medicine
The presence of malignant tumors can be detected through the use of monoclonal antibodies radiolabeled most frequently with the isotopes technetium-99m (99mTc) or indium-111 (111In). The particular label selected depends upon the size of the antibody. For example, large fragments or whole antibodies require a longer half-life isotope, such as 111In (T1/2 ¼ 2.8 days), whereas smaller Fab fragments, that are cleared from the body more quickly, can be labeled with 99mTc (T1/2 ¼6 h). Imaging is performed by a Single Photon Emission Computed Tomography (SPECT) camera whose detectors scan the body and register the radioactive counts. The counts are then mathematically transformed into an image that displays the sites of radioactivity. The nuclear medicine procedure that utilizes this procedure is known as Tumor-Specific Monoclonal Antibody Radioscintigraphy. Because of occasional difficulties with these techniques, such as inadequate tumor perfusion, inadequate amounts of antigen on the surface of the tumor cells, antigen heterogeneity, and nonspecific uptake, new
606 MONOCLONAL ANTIBODIES
approaches are being investigated. However, due to limited clinical experience, it is too early to predict whether they will improve imaging performance (10). Among these methods is the use of other imaging techniques, such as bone scans or computed tomography (CT), in conjunction with SPECT. In other approaches, attempts are being made to augment surface tumor cell antigens by prestimulation with growth factors, such as cytokines (11).
TREATMENT OF HEMATOLOGICAL MALIGNANCIES
Blood-Cell Cancers
Surface antigens on B- and T-cell lymphocytes are also useful targets for the treatment of blood cell (hematopoietic) malignancies, such as leukemias and lymphomas. These antigens are also expressed at high levels on the surface of various populations of malignant cells, but not on normal tissues. With few barriers present to impede Mab binding, hematologic malignancies are well suited to Mab-based therapy. In recent years, several promising Mab-based therapies for the treatment of hematologic malignancies have been developed and either have already received U.S. Food and Drug Administration (FDA) approval or are in the advanced phases of clinical testing (12). The chimeric antibody, rituxan (rituximAb, Genentech, San Francisco, CA) was among the first Mabs awarded Food and Drug Administration approval for the treatment of non-Hodg- kin’s lymphoma (13,14). This chimeric (human–mouse) antibody binds CD20, a cell surface antigen expressed on mature B lymphocytes and over 90% of non-Hodgkin’s lymphoma cells, but not on hematopoetic progenitor or stem cells. Rituxan has proven to be well tolerated and effective in the treatment of non-Hodgkin’s lymphoma either by itself, or in combination with traditional chemotherapy, particularly in patients who are refractory to other types of therapy (15). Campath-1 (alemtuzumAb, Ilex Oncology, San Antonio, TX) is another antibody that has also received FDA approval for the treatment of patients suffering from chronic lymphocytic leukemia. A third Mab to receive FDA approval for the treatment of hematologic malignancies is the chimeric Mab, mylotarg (gemtuzumAb ozogamicin, Wyeth-Ayerst Laboratories, Philadelphia, PA). This antibody targets the CD33 antigen expressed on myeloid (white cells) precursors and leukemic cells, and is absent from normal tissues and pluripotent hematopoetic (blood-cell producing) stem cells.
TREATMENT OF SOLID TUMORS
In comparison to the management of hematologic malignancies, successful treatment of solid tumors with Mabs has proven more elusive; however, some significant therapeutic benefits have been achieved. Herceptin (trastuzumAb, Genentech) is a humanized antibody that has received FDA approval for the treatment of metastatic breast cancer. This Mab recognizes an extracellular domain of the HER-2 protein. Clinical trials with herceptin have shown it to be well tolerated both as a single agent for second or third line therapy, or in combination with chemotherapeutic agents as
a first line of therapy. Combination therapy resulted in a 25% improvement of overall survival in patients with tumors that overexpress HER-2, and that are refractory to other forms of treatment (16).
The antiepithelial cellular adhesion tumors Mab molecule, Panorex (eclrecolomAb, GlaxoSmith-Kline, United Kingdom), is another Mab -based therapy that is currently being used for the treatment of colorectal cancer. Panorex has shown tangible benefit for cancer patients and has received approval in Germany for the treatment of advanced colorectal cancer. Like other Mabs used for the treatment of solid tumors, Panorex has proven more efficacious in the treatment of micrometastatic lesions and minimal residual disease in comparison to bulky tumor masses (17).
The failure of Mabs in the treatment of bulky lesions is primarily attributable to the low level of injected Mabs that actually reaches its target within a sizable solid-tumor mass. Studies using radiolabeled Mabs suggested that only a very small percentage of the original injected antibody dose, 0.01–0.1/g of tumor tissue, will ever reach target antigens within a solid tumor (18). This low level of binding is due to the series of barriers confronted by an administered Mab en route to antigens expressed on the surface of tumor cells.
ELICITING ANTITUMOR RESPONSES
After successfully negotiating the gauntlet of obstacles obstructing access to the target cells within a tumor, a therapeutic Mab must still be capable of eliciting a potent antitumor response. Although it is often ambiguous as to the exact mechanisms by which a particular Mab may mediate an antitumor response, both direct and indirect mechanisms can potentially be involved.
Antibodies of the IgG1 and IgG3 isotypes can support effector functions of both antibody-dependent cellmediated cytotoxicity and complement-dependent cytotoxicity. Antibody-dependent cell-mediated cytotoxicity is triggered by interaction between the Fc region of a cell-bound antibody and Fc receptors on immune effector cells such as neutrophils, macrophages, and natural killer cells. This mechanism is critical for the antitumor effects of several therapeutic Mabs.
Many early studies showed that murine Mabs had limited potential to elicit a potent antitumor response, because the murine Fc regions are less efficient at recruiting human effector cells than their human counterparts. This problem has been largely alleviated by the use of chimeric and humanized antibodies. Genetic engineering techniques have also been used to improve the immunologic effects of therapeutic Mabs by altering antibody shape and size, increasing the valency (bonds of affinity) of Mabs, and creating bifunctional antibodies with two antigenic receptors, one to a tumor antigen and another to an effector cell to increase efficiency of antibody-dependent cellmediated cytotoxicity (19).
In addition to immunologic effects, Mabs can induce antitumor effects by a variety of direct mechanisms, including the induction of apoptosis (programmed cell
death) (20), or the prevention of soluble growth factors from binding their cognate receptors, such as epidermal growth factor (EGF-R) (21) and HER-2 (22). Additionally, Mabs can also be engineered to deliver a cytotoxic agent directly to the tumor. This offers the potential to combine the biological effects of Mabs with the additional effect of a targeted cytotoxic response. The anti-CD33 Mabs, mylotarg, is one such antibody. Combined with the cytotoxic agent, calichaemicin, mylotarg has been reported to be relatively well tolerated, and effective in the treatment of chronic lymphocytic leukemia (23). Antibodies can also be engineered to deliver ionizing radiation directly to tumor cells. Mabs have been conjugated to both a- and b-particle emitting radionuclides (24). Clinical trials in humans also portend the promise of radiolabeled Mabs for the treatment of cancer. In a recent phase III randomized study, patients with relapsed or refractory nonHodgkin’s lymphoma were treated with yttrium-90 and iodine-131 labeled Mabs targeting the CD20 antigen (ibrituximomAb, tiuxetan, and tositumomAb, respectively). Patients treated with these radiolabeled Mabs showed a statistically significant increase in overall response compared with those treated with an unlabeled version of the Mab (rituximAb) (25).
OTHER USES FOR MONOCLONAL ANTIBODIES
Proteomics
After having sequenced the entire human genome, the current task is to understand the ‘‘proteome’’ by identification and quantification of all proteins in a given sample. So far, DNA microarrays have been employed to detect the transcription level [production of messenger ribonucleic acid (mRNA)] of genes in cells. However, it has been found that there is no stringent correlation between transcription level and protein abundance. Furthermore, the status of a protein in terms of its modification and structure cannot be determined by DNA microarrays. To solve this problem, antibody microarrays are envisioned to replace DNA microarrays in proteome research. These arrays consist of a multitude of different antibodies that are immobilized on a solid support and allow characterization of the protein repertoire of a given sample. However, the production of such antibody microarrays and its application require the provision of highly specific and stable antibodies, possessing high affinity and showing no cross-reactivity. Protein and antibody microarrays can be made to encompass as many as 10,000 samples on a chip within the dimensions of a microscope slide (26).
Monoclonal Antibodies in the Food Industry
Monoclonal antibodies are being used in the wine industry. Odors sometimes observed in spoiled food or corked wines are often the result of microbes present in the wood packaging materials. However, this phenomenon has also been observed in bottled water, suggesting that there may be secondary contaminants, such as residues of pesticides that can affect the quality of any packaged food or beverage. To further the quality assurance of products in the
MONOCLONAL ANTIBODIES |
607 |
wine industry, a project is being carried out to raise antibodies against a TCA molecule (2,4,6-trichcholoanisole) that is thought to be present in cork stoppers and is responsible for the musty taste in wine. The ELISA assay will be employed to detect trace amounts of the contaminating molecule. Also an immunosensor will be used to electrochemically detect the antibody levels present (27). Monoclonal antibodies have also been developed against the vegetative cells and spores of Bacillus cereus (28). This bacterium seems to be implicated in food poisoning and is also responsible for food spoilage. It is impossible for the food industry to exclude B. cereus from its products because B. cereus cells can survive heat processing and can grow in foods kept at refrigerated storage conditions. Two different antibodies were developed. One was used as a specific capture antibody to destroy the bacterium; the other as a detector antibody that would simply identify the presence of B. cereus. The ELISA assay was used to detect and quantify the vegetative cells of this pathogenic organism.
Potato cyst nematodes are pests that destroy the potato food crops. Monoclonal antibodies are being used to assist in the development of the plant’s resistance to the nematode (29). Recombinant plant monoclonal antibodies have been engineered to protect poultry against coccidosis infections (30).
Monoclonal Antibodies and Bioterrorism
The same plant biotechnology described above is being developed to create strategic reserves of vaccines and antibodies for infectious agents that could be used in biowarfare. Multiple genes can be engineered in plants intended to provide prolonged immunity against new strains of pathogens that have different mechanisms of action. With this technology, every plant cell will produce the signature protein of a particular biowarfare agent. That protein, in turn, will trigger an immune response in a person who consumes the plant material in an unprocessed or lightly processed form, but it will not cause the disease. These antibodies can prevent infection on surface areas, including nasal passages; clear infectious organisms from the body; identify foreign organisms for destruction; and neutralize and remove toxins. Among the diseasecausing substances are several potential bioterrorism agents, such as the botulism toxin, anthrax, Ebola virus, plague, and ricin, a poisonous protein found in the seeds of the castor oil plant. Vaccines for anthrax (Bacillus anthracis) and bubonic and pneumonic plague (Yersinia pestis), two potentially deadly diseases that can be delivered as airborne agents, are being developed. Preliminary data predicts success in using these plant-derived vaccines (31).
CONCLUDING REMARKS
Antibodies, monoclonal antibodies and antibody derivatives constitute 20 % of biopharmaceutical products currently in development. Antibodies represent an important and growing class of biotherapeutics. Progress in antibody engineering has allowed the manipulation of the basic antibody structure into its minimal essential functions, and multiple methodologies have emerged for raising
