
- •VOLUME 4
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •HYDROCEPHALUS, TOOLS FOR DIAGNOSIS AND TREATMENT OF
- •HYPERALIMENTATION.
- •HYPERBARIC MEDICINE
- •HYPERBARIC OXYGENATION
- •HYPERTENSION.
- •HYPERTHERMIA, INTERSTITIAL
- •HYPERTHERMIA, SYSTEMIC
- •HYPERTHERMIA, ULTRASONIC
- •HYPOTHERMIA.
- •IABP.
- •IMAGE INTENSIFIERS AND FLUOROSCOPY
- •IMAGING, CELLULAR.
- •IMAGING DEVICES
- •IMMUNOLOGICALLY SENSITIVE FIELD–EFFECT TRANSISTORS
- •IMMUNOTHERAPY
- •IMPEDANCE PLETHYSMOGRAPHY
- •IMPEDANCE SPECTROSCOPY
- •IMPLANT, COCHLEAR.
- •INCUBATORS, INFANTS
- •INFANT INCUBATORS.
- •INFUSION PUMPS.
- •INTEGRATED CIRCUIT TEMPERATURE SENSOR
- •INTERFERONS.
- •INTERSTITIAL HYPERTHERMIA.
- •INTRAAORTIC BALLOON PUMP
- •INTRACRANIAL PRESSURE MONITORING.
- •INTRAOCULAR LENSES.
- •INTRAOPERATIVE RADIOTHERAPY.
- •INTRAUTERINE DEVICES (IUDS).
- •INTRAUTERINE SURGICAL TECHNIQUES
- •ION-EXCHANGE CHROMATOGRAPHY.
- •IONIZING RADIATION, BIOLOGICAL EFFECTS OF
- •ION-PAIR CHROMATOGRAPHY.
- •ION–SENSITIVE FIELD-EFFECT TRANSISTORS
- •ISFET.
- •JOINTS, BIOMECHANICS OF
- •JOINT REPLACEMENT.
- •LAPARASCOPIC SURGERY.
- •LARYNGEAL PROSTHETIC DEVICES
- •LASER SURGERY.
- •LASERS, IN MEDICINE.
- •LENSES, CONTACT.
- •LENSES, INTRAOCULAR
- •LIFE SUPPORT.
- •LIGAMENT AND TENDON, PROPERTIES OF
- •LINEAR VARIABLE DIFFERENTIAL TRANSFORMERS
- •LITERATURE, MEDICAL PHYSICS.
- •LITHOTRIPSY
- •LIVER TRANSPLANTATION
- •LONG BONE FRACTURE.
- •LUNG MECHANICS.
- •LUNG PHYSIOLOGY.
- •LUNG SOUNDS
- •LVDT.
- •MAGNETIC RESONANCE IMAGING
- •MAGNETOCARDIOGRAPHY.
- •MANOMETRY, ANORECTAL.
- •MANOMETRY, ESOPHAGEAL.
- •MAMMOGRAPHY
- •MATERIALS, BIOCOMPATIBILITY OF.
- •MATERIALS, PHANTOM, IN RADIOLOGY.
- •MATERIALS, POLYMERIC.
- •MATERIALS, POROUS.
- •MEDICAL EDUCATION, COMPUTERS IN
- •MEDICAL ENGINEERING SOCIETIES AND ORGANIZATIONS
- •MEDICAL GAS ANALYZERS
- •MEDICAL PHOTOGRAPHY.
- •MEDICAL PHYSICS LITERATURE
- •MEDICAL RECORDS, COMPUTERS IN
- •MICROARRAYS
- •MICROBIAL DETECTION SYSTEMS
- •MICROBIOREACTORS
- •MICRODIALYSIS SAMPLING
- •MICROFLUIDICS
- •MICROPOWER FOR MEDICAL APPLICATIONS
- •MICROSCOPY AND SPECTROSCOPY, NEAR-FIELD
- •MICROSCOPY, CONFOCAL
- •MICROSCOPY, ELECTRON
- •MICROSCOPY, FLUORESCENCE
- •MICROSCOPY, SCANNING FORCE
- •MICROSCOPY, SCANNING TUNNELING
- •MICROSURGERY
- •MINIMALLY INVASIVE SURGICAL TECHNOLOGY
- •MOBILITY AIDS
- •MODELS, KINETIC.
- •MONITORING IN ANESTHESIA
- •MONITORING, AMBULATORY.
- •MONITORING, FETAL.
- •MONITORING, HEMODYNAMIC
- •MONITORING, INTRACRANIAL PRESSURE
- •MONITORING, NEONATAL.
- •MONITORING, UMBILICAL ARTERY AND VEIN
- •MONOCLONAL ANTIBODIES
- •MOSFET.
- •MUSCLE ELECTRICAL ACTIVITY.
- •MUSCLE TESTING, REHABILITATION AND.
- •MUSCULOSKELETAL DISABILITIES.
588MONITORING, UMBILICAL ARTERY AND VEIN
107.Piper IR, Chan KH, Whittle IR, Miller JD. An experimental study of cerebrovascular resistance, pressure transmission, and craniospinal compliance. Neurosurgery 1993;32:805–816.
108.Nichols JS, Beel JA, Munro LG. Detection of impaired cerebral autoregulation using spectral analysis of intracranial pressure waves. J Neurotrauma 1996;13:439–456.
109.Bruce DA, et al. Diffuse cerebral swelling following head injury in children: The syndrome of ‘‘malignant brain edema’’. J Neurosurg 1981;54:170–178.
110.Daley ML, Pasupathy H, Griffith M, Robertson JT, Leffler
C.Evaluation of autoregulation of cerebral blood flow by correlation of arterial and intracranial pressure signals. IEEE Trans Biomed Eng 1995;42:420–424.
111.Bruce DA, et al. Pathophysiology, treatment and outcome following severe head injury in children. Child’s Brain 1979;5:174–191.
112.Daley ML, Patterson S, Marmarou A, Leffler CW, Stidham
G.Pediatric traumatic brain injury: Correlation of intracranial and arterial pressure signals, Proc. 18th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society, Amsterdam, Netherlands, Nov. 1996.
113.Czosnyka M, Smielewski P, Kirkpatrick P, Laing R, Menon D, Pickard J. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 1997;41:11–19.
114.Czosnyka M, Smielewski P, Kirkpatrick P, Laing R, Menon D, Pickard J. Continuous assessment of the cerebral vasomotor reactivity in head injury. Acta Neurochirugica 1998;71:74–77.
115.Czosnyka M, Smielewski P, Piechnik S, Schmidt E, Al–Rawi PG, Kirkpatrick PJ, Pickard JD. Hemodynamic characterization of intracranial pressure plateau waves in head– injured patients. J Neurosurg 1999;92:11–19.
116.Steiner LA, Czosynka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Critical Care Med 2002;30: 733–738.
117.Steiner LA, Coles JP, Johnston AJ, Chatfield DA, Smielewske P, Fryer TD, Aigvirhio FI, Clark JC, Pickard JD, Menon DK, Czosynka M. Assessment of cerebrovascular autoregulation in head–injured patients. Stroke 2003;34: 2404–2409.
118.Oertel M, Kelly DF, Lee JH, Glenn TC, Vespa M, Martin NA. Is CPP therapy beneficial for all patients with high ICP. Acta Neurochir 2002;81:67–68.
119.Howells T, Elf K, Jones PA, Ronne–Engstrom E, Piper I, Nilsson P, Andrews P, Enbald P. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J Neurosurg 2005;102(2):311–317.
120.Marmarou A. A theoretical and experimental evaluation of the cerebrospinal flid system. Ph.D. Dissertation, Drexel University, 1973.
121.Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg 1975;43:523–534.
122.Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg 1978;48(3):332–344.
123.Ekstedt J. CSF hydrodynamic studies in man. 1. Method of constant pressure CSF infusion. J Neurol Neurosurg Psych 1977;40(2):105–119.
124.Agarwal GC, Berman BM, Stark L. A lumped parameter model of the cerebrospinal fluid system. IEEE Trans Biomed Eng 1969;16:45–53.
125.Ursino M. A mathematical study of human intracranial hydrodynamics. Part 1—the cerebrospinal fluid pulse pressure. Ann Biomed Eng 1988;16:379–401.
126.Ursino M. A mathematical study of human intracranial hydrodynamics. Part 2—Simulation of clinical tests. Ann Biomed Eng 1988;16:403–416.
127.Pasley RL, Leffler CW, Daley ML. Modeling modulation of intracranial pressure by variatrion of cerebral venous resistance induced by ventilation. Ann Biomed Eng 2003;31: 1238–1245.
128.Czosnyka M, Piechnik S, Richards HK, Kirkpatrick P, Smielewski P, Pickard JD. Contribution of mathematical modeling to the interpretation of bedside tests of cerebrovascular autoregulation. J Neurol Neurosurg Psych 1997;63: 721–731.
See also BIOTELEMETRY; HYDROCEPHALUS, TOOLS FOR DIAGNOSIS AND TREATMENT OF; NEONATAL MONITORING; NEUROLOGICAL MONITORS.
MONITORING, NEONATAL. See NEONATAL
MONITORING.
MONITORING, UMBILICAL ARTERY AND VEIN
AHMAD ELSHARYDAH
HAIBO WANG
RANDALL C. CORK
Louisiana State University
Health Center
Department of Anesthesiology
Shreveport, Louisiana
INTRODUCTION
In the neonatal intensive care unit (NICU), monitoring is an integral part of patient care. The primary goal of monitoring is to ensure that early and appropriate intervention can be initiated before to the onset of complications. Monitoring is also a means by which the effect of interventions and therapies may be recorded, evaluated, and controlled. The NICU staff have to deal with a full range of conditions that can arise in the preterm or critically ill neonate, including hypoxemia, hypoglycemia, hypotension, acidosis, and other serious problems. This has led to the evolution and development of several monitors and different sensor-based technologies for use in NICU monitoring including umbilical vessel monitoring. These sensors may provide more accurate and reliable monitoring of neonatal physiological and biochemical changes with a rapid response time. The umbilical vessels may be directly accessed in the first few days of life. An umbilical artery catheter (UAC) may be used for blood pressure monitoring, blood sampling, and fluid or drug infusion. An umbilical vein catheter (UVC) may be used for central venous pressure monitoring, blood sampling, and fluid or drug infusion. Different types of commercially available umbilical catheters are used for these purposes. These catheters differ in their length, size, number of ports, and their material (such as silicone and polyurethane). Blood pressure (BP) monitoring is an important part of neonatal intensive care both for the acutely ill and the convalescing neonate. The most accurate method of measuring BP is by direct intra-arterial recordings, which
usually use an umbilical catheter to access the umbilical artery. As blood gas measurement methods and monitors have progressed in adult critical medicine, most of the new techniques and sensors have been used in the NICU by using umbilical artery catheterization. This article addresses the potential benefits of umbilical vessel catheters and associated monitoring devices. It sheds light on the catheters and monitors available on the market and explains the complications and the risks of these catheters. Furthermore, this article looks at the direction of this technology in the future, and it tries to stimulate development of new technology for use in the monitoring of critically ill newborn infants (1–3).
Historical Aspects
In 1946, Louis K. Diamond, a pediatrician from Boston, and F. H. Allen, Jr. developed a technique that allowed blood transfusion to take place through the infant’s umbilical cord vein. Regular transfusions were difficult because of the small size of blood vessels in newborns, and there was a further complication due to the use of steel needles and rubber catheters. Diamond used plastic tubing on the umbilical vein, which was larger than average and remained open for several days after birth (4). By the 1960s, electronic monitors came into use and blood gases began to be measured. By the 1970s, the use of umbilical catheters and arterial pressure transducers was routine
(5). The first organized NICU opened its doors at Yale-New Haven Hospital in 1960. The first successful use of extracorporeal membrane oxygenation (ECMO) was in 1975. ECMO eventually reduced infant mortality from 80% to 25% for the critically ill infants with acute reversible respiratory and cardiac failure unresponsive to conventional therapy (6).
Anatomical and Physiological Aspects
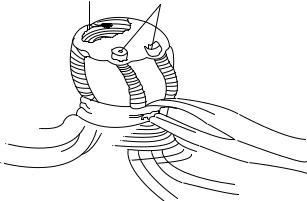
The umbilical cord is a cordlike structure about 56 cm long, extending from the abdominal wall of the fetus to the placenta. Its chief function is to carry nutrients and oxygen (O2) from the placenta to the fetus and return waste products and carbon dioxide (CO2) to the placenta from the fetus. It consists of a continuation of the membrane covering the fetus and encloses a mucoid jelly (Wharton’s jelly) with one vein and two arteries (7). Examination of the umbilical cord (after cut) normally reveals two umbilical arteries (UA) and one umbilical vein (UV) (Fig. 1). At skin level, the UV is usually in the 12 o’clock position and has a thinner wall and wider lumen than do the UAs (2). Before birth, blood from the placenta, about 80% saturated with O2, returns to the fetus by way of the UV. On approaching the liver, most blood flows through the ductus venous directly into the inferior vena cava (IVC), short-circuiting the liver. A smaller amount enters the liver sinusoids and mixes with blood from the portal circulation. After a short course in the IVC, it mixes with deoxygenated blood returning from the lower limbs before it enters the right atrium. The blood leaves the heart to the descending aorta, where it flows toward the placenta by way of the two UAs (7). The O2 saturation in the umbilical arteries is approximately 58%. Changes in the vascular system at birth are caused by
MONITORING, UMBILICAL ARTERY AND VEIN |
589 |
|
Umbilical vein |
Umbilical arteries |
|
Figure 1. Umbilical cord after it was cut.
cessation of placental blood flow and the beginning of respiration. These changes are summarized in closure of the umbilical arteries, closure of the umbilical vein and ductus venous, significant reduction in the pulmonary vascular resistance and right ventricle and right atrium pressures, and significant increase in the systemic vascular resistance, left ventricle, and left atrium pressures
(8). After birth, the blood volume of the neonate is about 300 mL, the cardiac output averages 500 mL/min, and the arterial blood pressure is about 70/50 during the first day, which increases slowly over the next several months. Arterial blood pressure of the neonate has been best correlated with birth weight. Moreover, systolic and diastolic pressures in the neonate are significantly correlated to blood pressure levels in the mother (9).
Indications and Contra-Indications for Umbilical Artery Catheterization
The primary indications for umbilical artery catheterization include frequent or continuous blood gas measurements, continuous monitoring of arterial blood pressure, and resuscitation (umbilical venous line may be the first choice) (10). Secondary indications include infusion of maintenance glucose-electrolyte solutions or medications, exchange transfusions, angiography, and a port for frequent blood sampling, especially in a very low-birth-weight neonate. These catheters should stay in place only as long as a primary indication exists, with the exception of the very low-birth-weight neonate who may need it for vital infusions and frequent blood sampling. Contraindications include evidence of local vascular compromise in lower limbs or buttock areas, peritonitis, necrotizing enterocolitis, omphalitis, gastroischisis, and omphalocele.
Indications and Contra-Indications for Umbilical Vein Catheterization
The most frequent indications for umbilical venous catheterization include emergency medication administration, exchange transfusion, and partial exchange transfusion (10). This catheter is also used for frequent blood sampling and central venous pressure monitoring. Contraindications for the this catheter include routine fluid infusion

590 MONITORING, UMBILICAL ARTERY AND VEIN
Figure 2. Commercially available umbilical catheters: (a) singlelumen catheter and (b) dual-lumen catheter.
(relative contraindication), omphalitis, omphalocele, gastroischisis, necrotizing enterocolitis, peritonitis, and extrophy of the bladder.
Description of Available Catheters (Design and Material)
Catheters must be made of nontoxic materials that are the least injurious to the vascular intima and least likely to cause thromboses and early atherosclerotic lesions (11). Commercially available umbilical catheters are usually latex-free, made of silicon or high-quality aliphatic polyurethane elastomer (Tecoflex). Silicone catheters are soft, not-irritating, usually not reactive to body tissues and body fluids, not supportive of bacterial growth, and less problematic with blood clotting. Tecoflex is an advanced medical formulation of polyurethane. Its physical characteristics are very close to silicone; however, it is slightly stiffer during insertion, which makes it easier to insert and better to conduct arterial pressure. Tecoflex is thermosensitive, softens at body temperature (12), and significantly reduces the trauma to the vascular intima. These catheters have a rounded tip, which makes them less likely to perforate through the vessel during insertion. They have depth markings at every centimeter for more accurate placement and encased radiopaque stripes to confirm placement by X ray after placement. Umbilical catheters are available with single-lumen, dual-lumen, and triple-lumen catheters (13) (Fig. 2,a,b) in different sizes ranging from 2.6 to 8.0 Fr. (Table 1) Umbilical artery catheter tips are designed in two different ways: end-hole and side-hole tips. The side-hole catheters use a special electrode to measure blood gases and biochemicals. A Cochrane review by Barrington (14) showed that end-hole catheters are associated with a much decreased risk of aortic thrombosis compared with side-hole catheters. Therefore, umbilical artery catheters designed with a side-hole should not be used routinely for umbilical artery catheterization in the newborn. Furthermore, manufactures have made sterile, ready- to-go umbilical catheterization trays. These trays contain
Table 1. Neonate Weight and Umbilical Catheter Size
Neonate Weight (g) |
UAC Size (Fr) |
UVC Size (Fr) |
|
|
|
<1500 g |
3.5 Fr |
3.5 Fr |
>1500 g |
5.0 Fr |
5.0 Fr |
|
|
|
everything needed for umbilical catheterization, including drapes, towels, suture, umbilical tape, skin preparation materials, needles, forceps, syringes, and other instruments.
Umbilical Vessel Catheterization Procedure
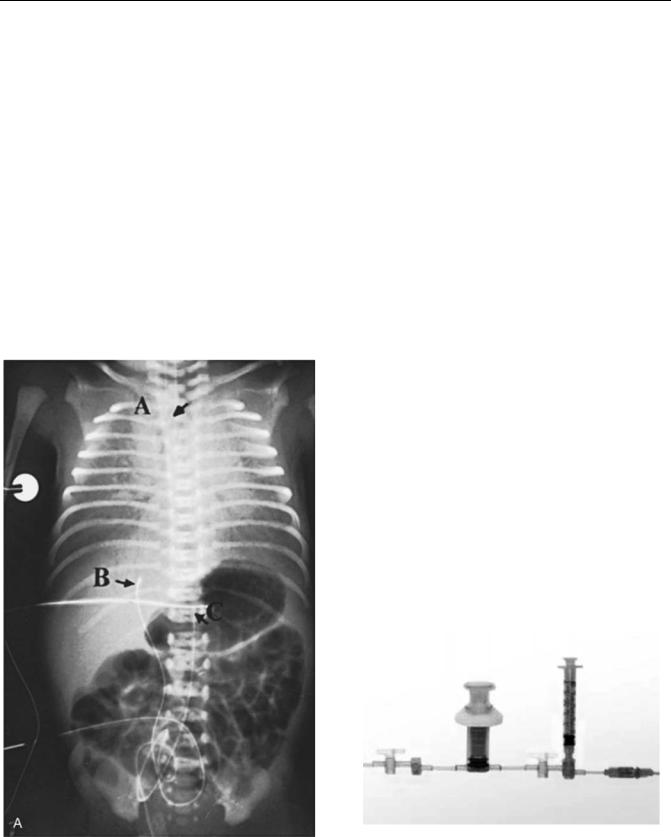
The infant must be supine and restrained (2). A field around the umbilicus is sterilized and draped, and a silk suture is looped around the base of the umbilical stump. The distal end of the stump is cut off, leaving 2 cm of stump, and the vessels are occluded to prevent blood loss. For the umbilical artery catheterization (15), the stump is firmly grasped with the gloved fingers of one hand, and one of the two thick-walled umbilical arteries is dilated with a curved iris forceps; then the umbilical artery catheter is inserted into the artery. Some resistance may be encountered when the catheter has been advanced 3 to 5 cm into the vessel, but this resistance can usually be overcome by applying steady downward pressure on the catheter. If the catheter cannot advance, a second catheter can be inserted into the other artery while leaving the first catheter in place. This maneuver often causes one or the other vessel to relax and permits one catheter to be advanced into the aorta. Advancement of an UAC should place the tip above the celiac axis but below the ductus arteriosis. All air should be removed from the system. The accidental injection of small amounts of air (<0.1 mL) may obstruct blood flow to the legs for several hours. The catheter should be attached to a pressure transducer and the arterial pressure measured. For the umbilical vein catheterization, the single, large, thin-walled umbilical vein is grasped with an iris forceps, and the air-free catheter, which is connected to a closed stopcock, is inserted 3 to 5 cm into the vessel with a twisting motion. The UVC tip should lie a few centimeters into the umbilical vein or inferior vena cava. The stopcock must be closed to prevent aspiration of air through the catheter should the patient take a deep breath. It is imperative that no air be injected through venous catheter, because the air may enter the systemic circulation through the foramen ovale and occlude a coronary or cerebral artery. If it does, the neonate may die or suffer central nervous system damage. If the catheter ‘‘tickles’’ the atrial septum, the neonate may suffer arrhythmias. Withdrawal of the cathetera short distance can solve the problem. Plain radiographs should be taken to confirm placement (Fig. 3). ‘‘High’’ placementoftheUACisdefinedinonemajorreviewoftheliterature as one with ‘‘the tip in the descending aorta above the level of the diaphragm and below the left subclavian artery’’ and ‘‘low’’ placement of the UAC as one with ‘‘the tip above the aortic bifurcation and below the renal arteries’’ (16).
Neonatal Blood Gas and Biochemical Measurement
The blood gas measurement is the most widely used clinical method for assessing pulmonary function in the neonate. It forms the basis for diagnosis and management of neonates with cardiorespiratory disease (17). The physiology of blood gases is discussed in other parts of this Encyclopedia. We will discuss in this section some issues related to the neonatal blood gas measurement. The dissociation curve of fetal hemoglobin (as compared with adult) is shifted to
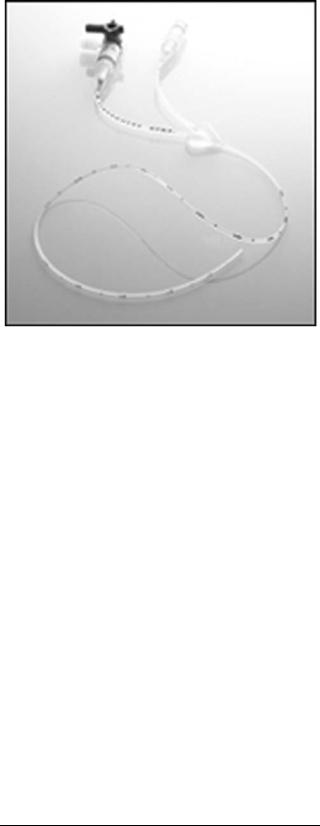
Figure 3. Anteroposterior roentgenogram shows the position of umbilical artery and vein catheters. Lateral roentgenogram is needed to distinguish the umbilical artery from the umbilical vein catheter and to determine the appropriate level of insertion. A ¼endotracheal tube; B ¼umbilical venous catheter. C ¼umbilical artery catheter passed up the aorta to T12.
the left, and at any arterial O2 partial pressure (PaO2) below 100 mm Hg (13332.2 Pa) fetal blood binds more O2. This shift seems to be the result of the lower affinity of fetal hemoglobin for 2,3-diphosphoglycerate (DPG). Shunting is a common occurrence in the neonate, such as in congenital cyanotic heart disease, persistent fetal circulation, or atelectasis. O2 supplementation does not prevent the hypoxia produced by such a shunt. Arterial carbon dioxide partial pressure (PaCO2) is an important measure of pulmonary function in neonatal respiratory disease. The initiation of ventilation with the first breath after normal delivery results in a rapid fall in PaCO2 within minutes of birth. PO2 rises rapidly to levels of 60 to 90 mm Hg (17). Immaturity of the kidney in the newborn affects the basal acid–base status and the response to additional acid and alkali loads (18). The blood bicarbonate (HCO3 ) concentration is typically lower than in the adult (18–21 mEq/L). However, the blood pH (7.35 –7.43) is only marginally decreased because of the compensatory increase in the neonatal respiratory rate. Table 2 lists the normal values of pH, PaCO2, and total CO2 in the adult and in preterm and term neonates (19). The transition from fetal to neonatal life, which is associated with rapid changes in fluid
Table 2. Acid–base Parameters in Neonates and Adults
(mean SD)
|
Preterm |
Term |
Adult |
|||
pH |
7.40 |
0.08 |
7.40 |
0.06 |
7.40 |
0.03 |
PCO2 |
34.0 |
9.0 |
33.5 |
3.6 |
39.0 |
2.6 |
Total CO2 |
21.0 |
2.0 |
21.0 |
1.8 |
25.2 |
2.8 |
MONITORING, UMBILICAL ARTERY AND VEIN |
591 |
and electrolyte balance, and the neonate’s small size make the electrolytes and glucose assessment difficult and complicated (20), especially in the first week of life. A physiologic decrease in extracellular water volume, as well as a transient increase in serum potassium and transient decreases in plasma glucose and total plasma ionized calcium concentrations, must be taken into account when monitoring neonatal electrolytes and glucose. Frequent and even continuous monitoring of physiological parameters is indicated in some cases, including glucose and calcium monitoring in the premature newborn (limited hepatic glycogen storage) and in newborns of diabetic mothers (21). Before birth, fetal glucose is slightly higher than maternal glucose. With cord clamping, neonatal plasma level plummets over the first 60–90 min of life (23), (23). Neonatal hormonal changes later leads to an increase in endogenous glucose production and stabilization of its level.
Technical Aspects of Neonatal Blood Gas
and Biochemical Measurement
Technologic innovations in the development of biosensors and microprocessors have led to development of bedside small and accurate point-of-care (POC) devices (24). POC devices have been used widely in critical care units, including the NICU. These devices are divided into two groups: ‘‘Analyzers,’’ which are not attached to the patient blood source and require blood sampling, and ‘‘monitors,’’ which are continuous or near-continuous patient-attached POC monitors (25). Neonatal biochemical measurement may include several important blood parameters, such as sodium, potassium, calcium, glucose, and even lactate Table 3.
Intermittent Blood Gas and Biochemical Measurement (Sampling). This is the most common technique used in the NICU for invasive neonatal blood gas and biochemical measurement. Usually a small blood sample is withdrawn from a blood vessel, such as an umbilical vessel through an umbilical catheter. This sample is analyzed by using a bedside point-of-care analyzer or sent to a small satellite laboratory unit in the NICU or to the central laboratory. Blood gas analyzers are discussed in other articles in this Encyclopedia. These analyzers use the principles of Clark’s electrode for PO2 measurement and Severinghaus’s electrode for PCO2 measurement. Some blood-sample collecting systems are commercially available, such as the Edward VAMP Jr. system manufactured by Edward (Fig. 4), which are manufactured specifically for neonate and small children use. This system is latex-free, disposable, closed, with small volume systems. Such systems are designed to the decrease the risks of blood loss, infection, and air bubbles (26).
Continuous Intravascular Neonatal Blood Gas and Biochemical Sensors. There are several drawbacks for using frequent arterial blood gas (ABG) sampling in the neonate (27). This method may result in blood loss that can necessitate blood transfusion. Moreover, in this method, rapid changes in blood gas values may be missed, especially
592 MONITORING, UMBILICAL ARTERY AND VEIN
Table 3. The Main Blood-Chemistry Parameters Monitored in the Neonatal Care Unit, With Typical Sensing Principles and Transducers
Parameter |
Sensing Principle(s) |
Transducer(s) |
|
|
|
Invasive Blood Pressure |
Electrical, impedance |
Strain gauge, piezoresistor |
|
Optical, reflection |
Photodetector and emitter |
PO2 |
Optical, fluorescent |
Photomultipler tube |
|
Electrochemical, amperometric |
Clark oxygen electrode |
PCO2 |
Optical, fluorescent |
Photomultipler tube |
|
Electrochemical, potentiometric |
Ion-sensitive electrode |
Glucose |
Optical, colorimetric |
Photodetector |
|
Electrochemical, amperometric |
Enzyme modified biosensor |
Lactate |
Optical, colorimetric |
Photodetector |
|
Electrochemical, amperometric |
Enzyme modified biosensor |
Electrolytes (K, Na, Ca, Cl) |
Optical, colorimetric |
Photodetector |
|
Electrochemical, potentiometric |
Ion-selective electrode (ISE) |
pH |
Optical, colorimetric |
Photodetector |
|
Electrochemical, potentiometric |
Ion-sensitive electrode |
Hemoglobin |
Optical, absorption |
Photodetector and emitters |
|
|
|
in conditions needing quick and close ABG monitoring, such as after surfactant administration (28) and during high-frequency ventilation (29). These drawbacks dictate the need for a more efficient real-time way to monitor ABGs (30). For the last two decades, intra-arterial PaO2 monitoring has been available with the use of a Clark electrode (31)
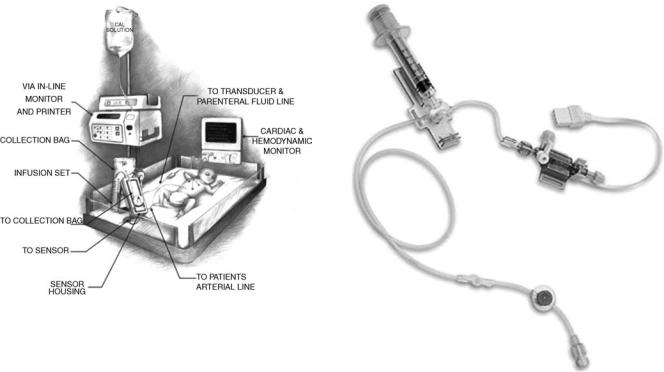
or a multiparameter sensor with an umbilical artery catheter (32). New fiber-optic continuous blood gas monitoring sensors have been validated and used in the neonate with an UAC, such as Neotrend. These devices promise to be safe, easy to use, and accurate in newborns. However, the cost-effectiveness of these devices is still not well established (33) (refer to the blood gas measurement article in this Encyclopedia for details about Neotrend). The ex vivo in-line VIA Low Volume Mode blood gas and chemistry monitoring system (VIA LVM Monitor; Metracor Technologies, Inc., San Diego, CA) is an in-line, low-volume POC monitor for neonates and children. Studies have shown promising results in using this monitor in the neonate. However, its cost-effectiveness has not been established yet (25,34). This device measures pH, PaCO2, PO2, Naþ, Kþ, and hematocrit (Hct) by automatically drawing blood (almost 1.5 mL) from a patient’s arterial catheter, analyzing it, and reinfusing the blood sample back into the patient. Results are usually displayed in 1–2 min. The operator performs an initial calibration, and then the device performs self-calibration after each sample and at least every 30 min. This machine is compatible with all sizes of UACs and peripheral arterial catheters. Figure 5 shows a diagram of the VIA LVM monitor and its components at the neonate bedside.
|
Figure 5. Diagram of the VIA LVM in-line ex vivo monitor and |
Figure 4. Edward VAMP Jr. blood-sample collecting system. |
its components at the neonate bedside. |
Neonatal Hemodynamic Monitoring
Direct arterial blood pressure monitoring is the most accurate technique for determining arterial pressure in the neonate
(9). This method is best done by using umbilical artery catheterization. It is an easy and quick procedure in comparison with other neonatal artery catheterizations, such as radial and femoral artery catheterization. After the umbilical artery catheterization is done as described above, it is connected to a pressure transducer and a continuous flow device, as well as stopcock and manometer tubing.
Basic Concepts. Hemodynamic pressure monitoring requires several basic components to accurately measure the physiologic pressures. These components are: (1) an intravascular catheter, (2) connecting tubing and stopcocks to connect that catheter and the patient’s blood vessels to the monitoring system, (3) a pressure transducer to convert the mechanical impulse of a pressure wave into an electrical signal through movement of a displaceable sensing diaphragm, (4) a continuous flush device that fills the pressure tubing with fluid and helps prevent blood from clotting in the catheter, (5) an amplifier that increases the low-voltage signal from the pressure transducer to a signal that can be displayed on a display device, (6) an oscilloscope to display waveforms and a digital readout to display numerical data, and (7) a processor or microcomputer that is used to calculate various hemodynamic parameters based on the measured variables.
Pressure Transducers. Pressure transducers are divided in two groups: (1) External transducers located away from the intravascular catheter and connected to that catheter via fluid-filled pressure tubing, and (2) catheter-tip transducers. The external transducers use three types of sensing elements: (1) strain gauges. These consist of an electrically conductive elastic material that responds reversibly to deformation by a change in electrical resistance. The resistance is converted into a voltage signal by connecting the elements to form a Wheatstone bridge circuit. The output voltage is proportional to the applied pressure and the excitation voltage. Strain gauges are the most common method of pressure transduction. (2) Silicon strain gauges. These are thin slices of silicon crystal bonded onto the back of a diaphragm. The movement of the diaphragm causes a change in the resistance of the crystal, which can be converted into an output signal. Silicon strain gauges are more sensitive than standard strain gauges, but they are affected by temperature and are non-linear. (3) Optical sensors: These are also diaphragms, but in this case, the movement of the diaphragm is sensed by reflecting a beam of light off the silver back of the diaphragm onto a photoelectric cell. The intensity of light sensed by the photoelectric cell changes with the diaphragm position, causing a decrease in its electrical output.
The Pressure Measurement System. The arterial waveform can be characterized as a complex sine wave, which is the summation of a series of simple sine waves of different amplitude and frequencies. The fundamental frequency (or first harmonic) is equal to the heart rate. The first 10
MONITORING, UMBILICAL ARTERY AND VEIN |
593 |
harmonics of the fundamental frequency contribute to the waveform. Any measurement system responds to a restricted range of frequencies only. Within this range, the system may respond more sensitively to some frequencies than to others. The response of the system plotted against the signal frequency is the frequency response of the system. However, the measurement system may possess natural frequencies or resonances determined by the inertial and compliant elements in a mechanical system. These resonances can distort the output signals. Therefore, it is essential that the natural frequencies do not lie in the operating frequency range of the instrument. Moreover, the output signal of a measurement may differ from the input signal created by the bloodstream because of the inertial components, frictional effects of movement, viscous forces of fluids, and electrical resistance. The property that determines these effects is called the damping of the system (35).
Technical Management of Pressure Monitoring System.
These are some significant practical points in operating this system:
1.Removal of all air bubbles from system: Air is more compressible than fluid, and it tends to act as a ‘‘shock absorber’’ within the pressure monitoring system, leading to an overdamped waveform, which may lead to false readings of the blood pressure. Moreover, air bubbles may cause serious air embolism, especially in neonates and small children.
2.Zeroing the transducer: The accuracy of invasive pressure measurements is dependent on the establishment of an accurate reference point (Zeroing). This is done by opening the stopcock to atmospheric pressure and zeroing the measurement system to eliminate the effect of the atmospheric pressure, and by leveling the transducer to the level of the upper portion of the right atrium (the patient’s ‘‘midaxillary line’’ or ‘‘phlebostatic axis’’) to eliminate the effect of the blood hydrostatic pressure.
3.Fast-flush technique: A ‘‘fast-flush’’ or ‘‘square wave test’’ is performed by opening the valve of the continuous flush device, which leads to an acute increase in the fluid flow rate through the catheter-tubing system from the usual 1–3 mL/h to 30 mL/h. This generates an acute rise in pressure within the system such that a square wave is generated on the monitor. With closure of the valve, a sinusoidal pressure wave of a given frequency and progressively decreasing amplitude is generated. A system with appropriate dynamic response characteristics will return to the baseline pressure waveform within one to two oscillations. If the fast-flush technique produces dynamic response characteristics that are inadequate, the clinician should troubleshoot the system (i.e., remove all air bubbles, minimize tubing length and stopcocks, etc.) until an acceptable dynamic response is achieved. The above-explained basic concepts in hemodynamic monitoring are used in pressure monitoring in neonates as well as in adults. Because of
594 MONITORING, UMBILICAL ARTERY AND VEIN
Figure 6. Neonatal/Pediatric Deltran pressure tubing with needleless blood collection system.
the small size of a neonate (or premature newborn) and because of the different indications and expected complications from adults and older children, these monitors have been modified to fit these requirements. These modifications and changes include (1) using a more simple tubing system with fewer stopcocks to reduce the risk of air embolism and infection,
(2) using a smaller volume tubing system with small syringes to decrease blood loss and the need for blood transfusion, and (3) using a special constant-low-rate flush system to decrease the risk of fluid overloading. After correct placement of the umbilical artery catheter, a stopcock (free of air bubbles) is connected to its distal end. Then a fluid-filled, well-flushed pediatric/ neonatal blood pressure tubing is connected to that stopcock (or directly to the catheter). The transducer is zeroed and leveled to the midaxillary level. There are several commercially available neonatal/pediatric pressure transducers and tubing systems. Figure 6 shows a disposable, latex-free neonatal/pediatric Deltran blood pressure monitoring and needleless blood collection system (36). For central venous pressure (CVP) monitoring, a dual-lumen or triple-lumen umbilical vein catheter may be used. Figs. 2 and 7 show some of the currently available catheters. Neonatal/pediatric pulmonary artery catheters (PACs) have been used through the umbilical vein. However, they are difficult to place and have numerous complications and risks associated with their placement. Other noninvasive cardiac monitors, such as echocardiography, can assess cardiac output and other physiological cardiac parameters. Thus, use of the neonatal PAC has decreased significantly. The most common indications for pediatric PACs are for cardiogenic shock, for severe distributive shock, for the use of very high ventilator pressures to achieve ade-
Figure 7. Catheter.
quate oxygenation, and for the perioperative management of patients who have undergone complex cardiac or other major surgeries (37). The smallest thermodilution catheter available is 5 Fr in size, although a single-lumen 4-Fr catheter exists and is useful for measuring pulmonary artery pressure (PAP) or obtaining mixed venous saturation (MvO2). Potential complications of pulmonary artery catheterization include pulmonary artery erosion or infarction, dysrhythmia, damage to the pulmonic valve, coiling in the right ventricle, and cardiac perforation (38).
Complications and Risks
Umbilical Artery Catheter. Many complications and risks are associated with UAC placement (39). Therefore, these catheters should not be used solely for fluid and medication administration. If an infant does not require frequent arterial blood sampling or continuous blood pressure monitoring, there is almost no justification for leaving a UAC inserted. The advantages and disadvantages of ‘‘high’’ versus ‘‘low’’ UAC are still debated. Recent studies (40–43) have found that ‘‘high’’ catheters are associated with a decreased incidence of complications without a statistically significant increase in any adverse sequelae. Therefore, one major review of the literature has concluded that ‘‘there appears to be no evidence to support the use of low placed umbilical artery catheters. High catheters should be used exclusively’’ (40). It is clear that UACs that are located between the ‘‘high’’ and ‘‘low’’ positions are never appropriate. Catheters in these positions have been associated with refractory hypoglycemia (infusion into the celiac axis), paraplegia (infusion into the artery of Adamkievicz)

MONITORING, UMBILICAL ARTERY AND VEIN |
595 |
Table 4. Care of Indwelling Umbilical Catheter
Change all tubings and connections daily.
Secure and label all tubing, and connections should be secured and labeled appropriately.
Use only appropriate filters.
Maintain catheter, connections, and tubing free of blood to prevent clot formation, or inadvertent flushing of preexisting clots into the
neonate.
Flush catheter with 0.5 mL of flush solution each time blood sample is drawn.
Chart fluids infused in intake/output record.
Infuse heparinized parenteral solution continuously through catheter, interrupting only to obtain blood samples.
(44), and thromboses that affect the kidneys (infusion into the renal arteries) or the gut (infusion into the mesenteric arteries). A catheter that is found in this intermediate position should be pulled to a ‘‘low’’ position or removed. Similarly, catheters should not be placed below the level of L5 because of the risk of gluteal skin necrosis (45) and sciatic nerve damage (45). Catheters that are placed below the level of L5 should be removed promptly. Other complications may include catheter occlusion, infection, air embolism, breaks or transection of the catheter, electrical hazards, intravascular knots in the catheters, bladder injury, peritoneal perforation, Wharton’s jelly embolus, and others.
Umbilical Venous Catheter. Thromboembolic events and infections are common complications of UVC use. These complications are similar to those of other central catheters, although UVCs are associated with an increased risk of localized infections of the liver and heart. Complications that are specific to UVC commonly are the result of malposition of the catheter. Nearly all experts recommend placement of the catheter outside the heart in the IVC (46). Complications occur as a result of placement of the catheter in the right side of the heart or the left side (via the foramen ovale). Cardiac arrhythmias are common complications, but these arrhythmias usually resolve after catheter withdrawal from the heart. Cardiac perforation with subsequent pericardial effusion and cardiac tamponade has been reported (47) Quick diagnosis (high index of suspicion, chest radiograph, and ultrasonography) and prompt treatment with pericardiocentesis decrease mortality significantly in these neonates. Placement of the catheter in the portal system can result in serious hepatic injury. Hepatic necrosis can occur from thrombosis of the hepatic veins or infusion of hypertonic or vasospastic solutions into the liver. Necrotizing enterocolitis and perforation of the colon also have been reported after positioning of the catheter in the portal system. Other complications of umbilical venous catheters have been reported and include perforation of the peritoneum, electrical hazards, and digital ischemia.
Umbilical Catheter Removal and Maintenance
According to the American Academy of Pediatrics (AAP) guidelines (48), umbilical artery or venous catheters should be removed and not replaced if there is any sign of catheter-related bloodstream infections, vascular insufficiency, or thrombosis. Moreover, umbilical catheters must be removed as soon as possible when no longer needed or when any sign of vascular insufficiency to the lower extremities is observed. An umbilical artery catheter should not be left in place more than 5 days. However, an umbilical
venous catheter can be used up to 14 days if managed aseptically. Umbilical venous catheters may replaced only if the catheter malfunctions. Table 4 lists the important tasks for daily care of umbilical indwelling catheters.
FUTURE TRENDS IN INVASIVE NEONATAL MONITORING
Providing real-time, accurate, reliable, compact, at the bedside, and safe neonatal monitoring devices is the future goal of researchers and manufactures involved with neonatal critical care (3). However, the cost issue is still outstanding and is going to be a major factor in directing this industry. The market demand for integrated critical care units and the clinical demand for continuous and rapid biochemical monitoring at the bedside, especially in critically ill patients, will lead to a closer integration between the vital signs monitors and POC analyzers. The monitor and POC analyzer manufacturers have been separate companies. This will slowly change through the formation of strategic alliances and mergers. Therefore, new devices will combine continuous vital signs, blood gases, and blood chemistry in the critical care units, including the NICU. The demand for continuous monitoring of biochemical parameters is at the same time bounded by the requirement for low-blood-loss systems, especially in the NICU. This is where the in-line and indwelling analyzers can offer a major advantage over the POC analyzers. Fortunately, technology is also moving in the right direction to minimize iatrogenic blood loss and decrease the risk of infection through the advances made in sampling, preparation, and handling of liquids using microfluidic techniques and closed systems (49). It is likely that in-line monitors that interface to umbilical artery catheters will become more widespread and will require decreasing sam- ple-volumes. The continuing application and refinement of established optical assay methods, such as absorption and fluorescence spectroscopy, onto fibre-optic cables will enable the detection of increasing numbers of analytes by indwelling probes encased within the umbilical catheters (50,51).
SUMMARY
The use of umbilical vessel catheterization and associated monitoring techniques and devices has been advanced dramatically since Dr. Louis K. Diamond of Boston used plastic tubing on the umbilical vein for blood transfusion in 1946. Advances in invasive neonatal monitoring and neonatal intravascular access have led to a significant reduction in the incidence of complications and have increased the number of indications for umbilical catheters. Umbilical vessel catheterization is now a routine and safe procedure
596 MONITORING, UMBILICAL ARTERY AND VEIN
in the NICU. Moreover, with the increase in the survival rate of lowand very low-birth-weight neonates, the need for these catheters has increased. Sometimes these catheters are the only intravascular access that can be established in this new group of patients. The innovation and development of medical applications of silicone and polyurethane enable the manufacturers to make soft, small catheters with adequate lumen size. These catheters cause minimal adverse reaction to the newborn body. To decrease the risk of blood loss and infection, new blood sampling devices have been developed. These systems are closed and have small volume tubing. New technologies for continuous monitoring of blood gases and neonatal chemistries in-line have been integrated with the umbilical catheters by using special sensors encased in the tip of the catheters. The future trend is to use smaller, compact, real-time, bedside monitors combined with pulse oximetry and other vital signs monitors. However, the issue of the cost-effectiveness of these machines has yet to be determined.
BIBLIOGRAPHY
1.Walsh-Sukys MC, Fanaroff AA. Perinatal services and resources. In: Fanaroff AA, Martin RJ, editors. NeonatalPerinatal Medicine, Diseases of the Fetus and Infant. 6th ed. St. Louis: Mosby; 1999.
2.Stovroff M, Teague WG. Pediatric surgery for the primary care pediatrician, Part II: Intravenous access in infants and children. Pediatric Clin North Am 1998;45(6).
3.Murkov´ıc I, Steinberg MD, Murkov´ıc B. Sensors in neonatal monitoring: Current practice and future trends. Technol Health Care 2003;11:399–412.
4.Diamond LK, Allen Jr FH, Thomas Jr WO, Erythroblastosis fetalis. VII. Treatment with exchange transfusion. N Engl J Med 1951;244:39–49.
5.Klaus MH, Fanaroff AA. Care of the High-Risk Neonate, 5th ed. Philadelphia: WB Saunders; 2001
6.Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: A prospective randomized study. Pediatrics 1985;76:479–497.
7.Sadler TW. Langman’s Medical Embryology: Cardiovascular System, 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
8.Guyton AC, Hall JE. Textbook of Medical Physiology: Fetal and Neonatal Physiology, 10th ed. Philadelphia: W.B. Saunders; 2000.
9.Zahka KG. Principles of neonatal cardiovascular hemodynamics. In: Fanaroff AA, Martin RJ. editors, Neonatal-Peri- natal Medicine, Diseases of the Fetus and Infant. 6th ed. St. Louis: Mosby; 1999.
10.Grady M, Procedures. In: Gunn VL. Nechyba C, editors, Harriet Lane Handbook: A Manual for Pediatric House Officers, 16th ed. St. Louis: Mosby; 2002.
11.Brown EG, Krouskop RW. Monitoring, umbilical artery and vein. In: Webster JG, editor. Encyclopedia of Medical Devices and Instrumentation, New York: Wiley; 1988
12.Chidi CC, King DR, Boles Jr ET, An ultrastructural study of the intimal injury induced by an indwelling umbilical artery catheter. J Pediatr Surg 1983;18:109–115.
13.http://www.utahmed.com/umbili-c.htm.
14.Barrington KJ. Umbilical artery catheters: Catheter design (CochraneReview).In: TheCochraneLibraryIssue4.Oxford;1997.
15.Gregory GA. Resuscitation of the newborn. In: Miller RD, editor. Miller’s Anesthesia. 6th ed. New York: Elsevier; 2005.
16.Barrington KJ. Umbilical artery catheters in the newborn: effects of position of the catheter tip. Cochrane Database Syst Rev CD000505, 2000.
17.Carlo WA. Assessment of pulmonary function. In: Fanaroff AA, Martin RJ, editors. Neonatal-Perinatal Medicine, Diseases of the Fetus and Infant. 6th ed. St Louis: Mosby; 1999.
18.Stork JE, Stork EK. Acid-base physiology and disorders in the neonate. In: Fanaroff AA, Martin RJ. editors, NeonatalPerinatal Medicine, Diseases of the Fetus and Infant. 6th ed. St. Louis: Mosby; 1999.
19.Lorenz JM, Kleiman LI, Kotagal UR, Reller MD. Water balance in very low birth infants: Relationship to water and sodium intake and effect on outcome J Pediatrics 1982;101: 423–432.
20.Lorenz JM. Assessing fluid and electrolyte status in the newborn, Nat Acad of Clin Biochem. Clin Chem 1997;43(1):205–210.
21.Heck LI, Erenberg A. Serum glucose values during the first 48 h of life. J Pediatr 1978;110:119–122.
22.Srinivasan G, Pildes RS, Cattamanchi G, Voora S, Lillian LD. Plasma glucose values in normal neonates: A new look. J Pediatr 1984;105:114–119.
23.Tsang RC, Chen IW, Freidman MA, Chen I. Neonatal parathyroid function: role of gestational and postnatal age. J Pediatr 1973;83:728–730.
24.Ehrmeyer SS, Laessig RH, Leinweber JE, Oryall JJ. Medicare/CLIA final rules for proficiency testing: Minimum intralaboratory performance characteristics (CV and bias) needed to pass. Clin Chem 1990;36:1736–1740.
25.Billman GF, et al. Clinical performance of an in-line, ex vivo point-of-care monitor: A multicenter study. Clin Chem 2002;48(11): 2030–2043.
26.http://www.edwards.com/Products/PressureMonitoring/ Vamp Jr.htm
27.Meyers PA, Worwa C, Trusty R, Mammel MC. Clinical validation of a continuous intravascular neonatal blood gas sensor introduced through an umbilical artery catheter. Respir Care 2002;47(6):682–687.
28.Kresch MJ, Lin WH, Thrall RS. Surfactant replacement therapy. Thorax 1996;51(11):1137–1154.
29.Nelle M, Zilow EP, Linderkamp O. Effects of high-frequency oscillatoryventilationoncirculationinneonateswithpulmonary interstitialemphysemaofRDS.IntenCareMed1997;23(6):671– 676.
30.Goddard P, et al. Use of continuously recording intravascular oxygen electrode in the newborn. Arch Dis Child 1974;49(11): 853–860.
31.Weiss IK, Fink S, Harrison R, Feldman JD, Brill JE. Clinical use of continuous arterial blood gas monitoring in the pediatric intensive care unit. Pediatrics 1999;103:440–445.
32.Morgan C, et al. Continuous neonatal blood gas monitoring using a multiparameter intra-arterial sensor. Arch Dis Child Fetal Neonatal Ed 80(2):F93–F98.
33.Rais-Bahrami K, Rivera O, Mikesell GT, Short BL. Continuous blood gas monitoring using in-dwelling optode method: Comparison to intermittent arterial blood gas sampling in ECMO patients. J Perinatol 2002;22(6):472–474.
34.Widness JA, et al. Clinical performance of an in-line point- of-care monitor in neonates. Pediatrics 2000;106(3): 497– 504.
35.Gardner RM. Invasive pressure monitoring. In: Civetta JM, Taylor RW, Kirby RR. editors Critical Care, 3rd ed. Philadelphia: Lippincott-Raven; 1997. pp 839–845.
36.http://www.utahmed.com/deltran.htm.
37.Ewert P, Nagdyman N, Fischer T, Gortner L, Lange PE. Continuous monitoring of cardiac output in neonates using an intra-aortic Doppler probe. Cardiol Young 1999;9(1): 42–48.
