
- •VOLUME 4
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •HYDROCEPHALUS, TOOLS FOR DIAGNOSIS AND TREATMENT OF
- •HYPERALIMENTATION.
- •HYPERBARIC MEDICINE
- •HYPERBARIC OXYGENATION
- •HYPERTENSION.
- •HYPERTHERMIA, INTERSTITIAL
- •HYPERTHERMIA, SYSTEMIC
- •HYPERTHERMIA, ULTRASONIC
- •HYPOTHERMIA.
- •IABP.
- •IMAGE INTENSIFIERS AND FLUOROSCOPY
- •IMAGING, CELLULAR.
- •IMAGING DEVICES
- •IMMUNOLOGICALLY SENSITIVE FIELD–EFFECT TRANSISTORS
- •IMMUNOTHERAPY
- •IMPEDANCE PLETHYSMOGRAPHY
- •IMPEDANCE SPECTROSCOPY
- •IMPLANT, COCHLEAR.
- •INCUBATORS, INFANTS
- •INFANT INCUBATORS.
- •INFUSION PUMPS.
- •INTEGRATED CIRCUIT TEMPERATURE SENSOR
- •INTERFERONS.
- •INTERSTITIAL HYPERTHERMIA.
- •INTRAAORTIC BALLOON PUMP
- •INTRACRANIAL PRESSURE MONITORING.
- •INTRAOCULAR LENSES.
- •INTRAOPERATIVE RADIOTHERAPY.
- •INTRAUTERINE DEVICES (IUDS).
- •INTRAUTERINE SURGICAL TECHNIQUES
- •ION-EXCHANGE CHROMATOGRAPHY.
- •IONIZING RADIATION, BIOLOGICAL EFFECTS OF
- •ION-PAIR CHROMATOGRAPHY.
- •ION–SENSITIVE FIELD-EFFECT TRANSISTORS
- •ISFET.
- •JOINTS, BIOMECHANICS OF
- •JOINT REPLACEMENT.
- •LAPARASCOPIC SURGERY.
- •LARYNGEAL PROSTHETIC DEVICES
- •LASER SURGERY.
- •LASERS, IN MEDICINE.
- •LENSES, CONTACT.
- •LENSES, INTRAOCULAR
- •LIFE SUPPORT.
- •LIGAMENT AND TENDON, PROPERTIES OF
- •LINEAR VARIABLE DIFFERENTIAL TRANSFORMERS
- •LITERATURE, MEDICAL PHYSICS.
- •LITHOTRIPSY
- •LIVER TRANSPLANTATION
- •LONG BONE FRACTURE.
- •LUNG MECHANICS.
- •LUNG PHYSIOLOGY.
- •LUNG SOUNDS
- •LVDT.
- •MAGNETIC RESONANCE IMAGING
- •MAGNETOCARDIOGRAPHY.
- •MANOMETRY, ANORECTAL.
- •MANOMETRY, ESOPHAGEAL.
- •MAMMOGRAPHY
- •MATERIALS, BIOCOMPATIBILITY OF.
- •MATERIALS, PHANTOM, IN RADIOLOGY.
- •MATERIALS, POLYMERIC.
- •MATERIALS, POROUS.
- •MEDICAL EDUCATION, COMPUTERS IN
- •MEDICAL ENGINEERING SOCIETIES AND ORGANIZATIONS
- •MEDICAL GAS ANALYZERS
- •MEDICAL PHOTOGRAPHY.
- •MEDICAL PHYSICS LITERATURE
- •MEDICAL RECORDS, COMPUTERS IN
- •MICROARRAYS
- •MICROBIAL DETECTION SYSTEMS
- •MICROBIOREACTORS
- •MICRODIALYSIS SAMPLING
- •MICROFLUIDICS
- •MICROPOWER FOR MEDICAL APPLICATIONS
- •MICROSCOPY AND SPECTROSCOPY, NEAR-FIELD
- •MICROSCOPY, CONFOCAL
- •MICROSCOPY, ELECTRON
- •MICROSCOPY, FLUORESCENCE
- •MICROSCOPY, SCANNING FORCE
- •MICROSCOPY, SCANNING TUNNELING
- •MICROSURGERY
- •MINIMALLY INVASIVE SURGICAL TECHNOLOGY
- •MOBILITY AIDS
- •MODELS, KINETIC.
- •MONITORING IN ANESTHESIA
- •MONITORING, AMBULATORY.
- •MONITORING, FETAL.
- •MONITORING, HEMODYNAMIC
- •MONITORING, INTRACRANIAL PRESSURE
- •MONITORING, NEONATAL.
- •MONITORING, UMBILICAL ARTERY AND VEIN
- •MONOCLONAL ANTIBODIES
- •MOSFET.
- •MUSCLE ELECTRICAL ACTIVITY.
- •MUSCLE TESTING, REHABILITATION AND.
- •MUSCULOSKELETAL DISABILITIES.
20.American National Standard for Wheelchairs, Volume 1, Requirements and Test Methods for Wheelchairs (Including Scooters). Virginia: Rehabilitation Engineering and Assistive Technology Society of North America; 1998.
21.Fuller GF. Falls in the Elderly Am Fam Physician 2000;61(7):2159–2168.
22.Rentschler AJ, et al. Intelligent walkers for the elderly: Performance and safety testing of the VA-PAMAID robotic walker. J Rehab R&D 2003;40(5):423–432.
23.Abel T, et al. Energy Expenditure in wheelchair racing and hand biking- a basis for prevention of cardiovascular diseases in those with disabilities. Eur J Cardio Prev Rehab 2003;10(5):371–376.
24.Franklin BA, Bonsheim K, Gordon S. Resistance training in cardiac rehabilitation. J Cardiopulm Rehab 1991;11:99–107.
25.Kenedy DW, Smith RW. A comparison of past and future leisure activity participation between spinal cord injured and non-disabled persons. Paraplegia 1990;28:130–136.
26.National wheelchair basketball association (2003–2004) official rules and case book retrieved from National Wheelchair Basketball Association. http://www.mwba.org. Accessed.
27.Vanlandewijck Y, Daily C, Theisen DM. Field test evaluation of aerobic, anaerobic, and wheelchair basketball skill performances. Int J Sports Med 1999;20(8):548–554.
28.Janssen TWJ, Dallmeijer AJ, Van der Woude LHC. Physical capacity and race performance of handcycle users. Jour Rehab R&D 2001;38(1):33–40.
29.Lapolla T. International rules for the sport of wheelchair rugby. http://quadrugby.com/rules.htm. Accessed 2000.
30.Wheelchair tennis handbook. International Tennis Federation. http://www.itfwheelchairtennis.com. Accessed 2004.
31.Kurtz M. Difference makers. Sports’ N Spokes 2002;28(2):10–14.
32.Russell JN, et al. Trends and Differential use of Assistive Technology Devices: United States, 1994. Adv Data 1997; 292:1–9.
33.Shaw G, Gillispie B. Appropriate portection for wheelchair riders on public transit buses. J Rehab R&D 2003;40(4):309– 320.
34.International Standards Organization, Wheeled mobility devices for use in motor vehicles. ISO 7176-19, Vol. 31. 2004.
35.Hobson D. Wheelchair transport safety - the evolving solutions. J Rehab R&D 2000;37(5).
36.Bertocci G, Manary M, Ha D. Wheelchair used as motor vehicle seats: Seat loading in frontal impact sled testing. Med Eng & Phys 2001;23:679–685.
37.Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic Biomechanics socket interface: a review. J Rehab R&D 2001;38(2):161–174.
38.Weir RF, Childress DS, Grahn EC. Development of Exter- nally-Powered Prostheses for Persons with Partial Hand Amputations. Proceedings of the Chicago 2000 World Congress on Medical Physics and Biomedical Engineering; 2000 July 23rd–28, Chicago (IL):.
39.van der Linde H. A systematic literature review of the effect of different prosthetic components on human functioning with a lower-limb prosthesis. J Rehab R&D 2004;41(4):555–570.
40.Macfarlane PA, et al. Transfemoral amputee physiological requirements: comparisons between SACH foot walking and flex-foot walking. J Prosthe & Ortho 1997;9(4):138–143.
41.Nielsen DH, Shurr DG, Golden JC, Meier K. Comparison of Energy Cost and Gait Efficiency During Ambulation in Below-Knee Amputees Using Different Prosthetic Feet - a Preliminary Report. J Prosthet Orthotics 1989;1(1):24–31.
42.Heller BW, Datta D, Howitt J. A pilot study comparing the cognitive demand of walking for transfemoral amputees using the Intelligent Prosthesis with that using conventionally damped knees. Clin Rehab 2000;14(5):518–522.
MONITORING IN ANESTHESIA |
555 |
43.Romo HD. Prosthetic knee. Phys Med Rehab Clin N Am 2000;11(3):595–607.
44.Taylor MB, Clark E, Offord EA, Baxter C. A comparison of energy expenditure by a high level trans-femoral amputee using the Intelligent Prosthesis and conventionally damped prosthetic limbs. Prosthet Ortho Int 1996;8:116–121.
45.Jia X, Zhang M, Lee WC. Load Transfer Mechanics Between Trans-Tibial Prosthetic Socket and Residual Limb - Dynamic Effects. J Biomech 2004;37(9):1371–1377.
46.Lee WC, Zhang M, Jia X, Cheung JT. Finite Element Modeling of the Contact Interface Between Trans-Tibial Residual Limb and Prosthetic Socket. Med Eng Phys 2004;26(8):655–662.
47.Winson CCL, Zhang M, Boone D, Contoyannis B. FiniteElement Analysis to Determine Effect of Monolimb Flexibility on Structural Strength and Interaction Between Residual Limb and Prosthetic. J Rehab R&D 2004;41(6a):775–786.
48.Phillips B, Zhao H. Predictors of Assistive Technology Abandonment. Assis Technol 5(1):1993; 178–184.
49.Scherer MJ. The change in emphasis from people to person: introduction to the special issue on assistive technology. Disabil Rehab 2002;24(1–3):1–4.
50.Marks LJ, Michael JW. Science, Medicine, and the Future: Artificial Limbs. BMJ 2001;323(7315):732–735.
51.Beil TL. Interface Pressures During Ambulation Using Suction and Vacuum-Assisted Prosthetic Sockets. J Rehab R&D 2002;39(6):693–700.
52.Michael JW. Modern Prosthetic Knee Mechanisms. Clin Orthop Relat Res 1999;361:39–47.
53.Buckley JG, Spence WD, Solomonidis SE. Energy Cost of Walking: Comparison of"Intelligent Prosthesis’’ With Conventional Mechanism. Arch Phys Med Rehabil 1997;78(3):330–333.
54.Twiste M, Rithalia SV, Kenney L. A Cam-Displacement Transducer Device for Measuring Small Two-Degree of Freedom Inter-Component Motion in a Prosthesis. Med Eng Phys 2004;26(4):335–340.
55.Lee WC, Zhang M, Jia X, Cheung JT. Finite Element Modeling of the Contact Interface Between Trans-Tibial Residual Limb and Prosthetic Socket. Med Eng Phys 2004;26(8):655– 662.
See also BLIND AND VISUALLY IMPAIRED, ASSISTIVE TECHNOLOGY FOR;
ENVIRONMENTAL CONTROL; LOCOMOTION MEASUREMENT, HUMAN; REHABILITATION AND MUSCLE TESTING.
MODELING OF PHYSIOLOGICAL
SYSTEMS. See PHYSIOLOGICAL SYSTEMS MODELING.
MODELS, KINETIC. See TRACER KINETICS.
MONITORING IN ANESTHESIA
TARMO LIPPING
VILLE Ja¨NTTI
ARVI YLI-HANKALA
Tampere University of
Technology
Pori, Finland
INTRODUCTION
Anesthesia is one of the most complex and mysterious phenomena in clinical work. The main feature of anesthesia
556 MONITORING IN ANESTHESIA
is the loss of consciousness, which suggests its relatedness to sleep, epilepsy, and various kinds of brain trauma. In the case of anesthesia and sedation, consciousness is manipulated deliberately to prevent the patient from being aware of their state and the medical procedures carried through.
Recent decades have seen significant advancements in mapping various psychological functions to corresponding brain areas, however, the knowledge of the formation of human consciousness is still based on uncertain hypothesis. This complexity makes anesthesia monitoring extremely challenging.
This article first addresses the problem of anesthesia monitoring from the clinical, as well as from the physiological, point of view. The main emphasis is on monitoring anesthetic depth as this is the most discussed topic in anesthesia monitoring today. It starts with clinical indicators of anesthetic depth, gives an overview on the methods used in commercially available depth-of-anesthesia monitors, and describes some new algorithms proposed and evaluated for anesthesia electrocardiograms (EEG) monitoring in recently published works. Finally, the feasibility of monitoring brain function is argued using neurophysiological parameters like EEG, Auditory Evoked Potentials (AEPs), and so on, in the Intensive Care Unit (ICU) and the Emergency Room (ER).
ANESTHESIA AS A PROCESS AND A PROCEDURE
Anesthesia can be seen from the clinical point of view as a procedure, carried out according to certain protocol. From the physiological point of view anesthesia is a process evolving in the nervous system as the dose of an anesthetic agent increases.
Anesthesia as a Procedure
The goal of general anesthesia in the operating room (OR) is to render the patient unaware so that they do not feel pain during the surgery or recall the events afterward. It is also important that the patient does not react to surgical stimuli by movement. In the ICU, the goal of sedation is to keep the patient calm and painless. Too deep anesthesia causes prolonged awakening times after surgery in OR and longer treatment times in the ICU. The goals of anesthesia and sedation can be achieved by hypnotics (unconsciousness producing drugs), analgesics (antinociceptive drugs), and neuromuscular blocking agents. The choice of drugs is mainly made based on experience and clinical signs during the treatment.
Although general anesthesia is considered a safe procedure, various complications like postoperative nausea, vomiting, and pain are relatively frequent. The incidence of recall of events and awareness during anesthesia is rare ( 0.1%), however, the consequences may be traumatic for the patient (1). Anesthesia-related mortality has decreased significantly during past decades having recently been estimated at 1 death per 200,000–300,000 cases of anesthesia (2–4).
Anesthetic agents can be divided into inhalation anesthetics (e.g., halothane and isoflurane) and intravenous
anesthetics (e.g., thiopental and propofol). Intravenous drugs are becoming more popular as they are short acting, do not cause gas pollution, are easy to administer, and do not cause airway irritation. Desirable properties of anesthetic agents include rapid, smooth and safe induction of and emergence from anesthesia, no accumulation in the body, minimal effects on cardiovascular functions, no irritation to tissues and veins, low potential to hypersensitivity reactions.
Anesthesia as a Process
During the last decades, it has become obvious that different anesthetic and sedative agents produce their effects with different mechanisms, and therefore from the physiological point of view depth of anesthesia is a vague notion (5). It is more meaningful to talk about components forming the state we usually call anesthesia. These include amnesia, unconsciousness (hypnosis), antinociception, and neuromuscular blockade (paralysis). Different neurophysiological modalities should be used in order to assess these components. In the operating room, the patient is said to be anesthetized while the term sedation is used in the ICU. Some drugs, like propofol, are useful in producing both anesthesia and sedation at different concentrations, while some others are most useful as anesthetics or sedatives. There is evidence that anesthesia and sedation can be produced via different structures in the brainstem. Hypnosis and sedation cause similar changes in the EEG signal (described in the section changes in Neurophysiological Variables During Anesthesia). As all the available depth-of-anesthesia monitors are either fully or partly based on the EEG, the terms depth of hypnosis and depth of sedation are used depending on the corresponding clinical situation and are, in the context of available monitoring devices, roughly equivalent.
The action of anesthetics can be studied at various levels of neuronal function (6). The models underlying these studies can be divided into those operating at the molecular– cellular level and those explaining anesthetic processes at higher levels (generator models). State-of-the-art knowledge on the molecular and neuronal substrates for general anesthesia has recently been reviewed in Ref. 7. The model proposed by Flohr describes the action of anesthetics as disruption of computational processes dependent on the NMDA receptors (8). The activation state of these receptors in cortical neurons determines the complexity of representational structures that can be built-up in the brain and thus the level of consciousness. Steyn-Ross et al. performed numerical simulations of a single-macrocolumn model of the cerebral cortex and found that the effects of anesthetics can be modeled by cortical phase transition (9). Their simulations explain several trends in the EEG caused by anesthetic actions and predict the decrease in spectral entropy of the EEG signal with deepening anesthesia. This model has supported the development of the Entropy module, described in the section Recently Developed Methods, Applied in Commercial Anesthesia Monitors. Great cautiousness must be taken, however, in interpretation of the models operating at molecular level, because they include only a small part of the neurophysiological functions known to be involved in consciousness and generation of anesthesia-induced EEG patterns.
REGULATION OF MEAN FREQUENCY
|
[5] Uncouple |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[6] Depress PFC |
|
||||||||||||||
|
|
|
|
Cortico-cortical α / β / γ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
parietal-PFC |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
interactions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
∆ |
|
Cortical-subcortical |
|
α |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
[2] Block interaction with DLPFC |
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
to |
prevent memory transfer |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
CT-TC |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cingulate |
θ |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
γ loop |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N. Reticularis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
[4] |
Block |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
gamma |
|
|
[3] Close |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
loops |
|
|
thalamic |
|
|
|
|
Hippocampus |
|
|
|
|
|
||||||||||||
|
Dorsal striatum |
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
gates |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
Thalamus |
α |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amygdala |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
S. Nigra |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Septal nuclei |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Entorhinal CX |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Ventral |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Sensory |
|
|
|
tegmental area |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
inputs |
|
|
|
|
|
Reticular |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
formation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
[1] Depression of ARAS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
GENERATION OF THETA |
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
MONITORING IN ANESTHESIA |
557 |
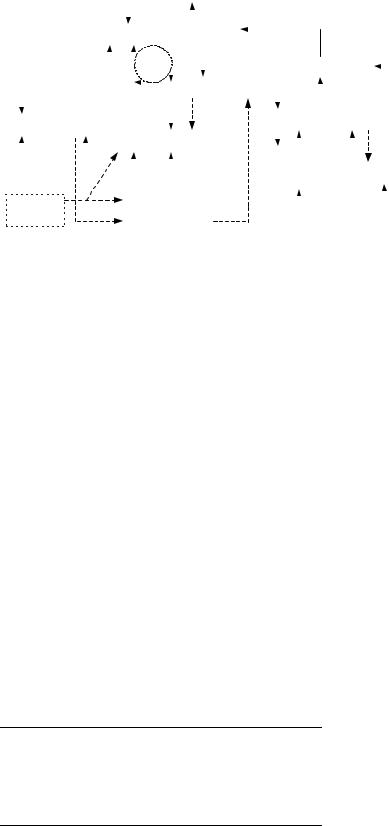
Figure 1. The Anesthetic Cascade model (redrawn with permission from: E. R. John and L. S. Prichep, ‘‘The anesthetic cascade: A theory on how anesthesia suppresses consciousness,’’ Anesthesiology, Vol. 102, Fig. 11 (p. 468), 2005).
John et al. developed a higher level model based on a complex neuroanatomical system described in (10). This model, described and thoroughly discussed in volume 10 of Ref. 11, incorporates and explains an extensive bulk of results obtained from EEG, evoked potential and magnetic resonance imaging (MRI) image analysis, as well as laboratory studies. Loss of consciousness is described as the following cascade of events, called the Anesthetic Cascade by the authors, involving various brain regions (Fig. 1) (6): (1) depression of the brainstem; (2) depression of mesolim- bic-dorsolateral prefrontal cortex interactions leading to blockade of memory storage; (3) inhibition of the nucleus reticularis of the thalamus, resulting in closure of thalamic gates (seen as increasing u rhythm in the EEG); (4) blocking of thalamocortical reverberations (g loop) and perception; (5) uncoupling of parietal-frontal transactions (coherence in g frequency band decreases); (6) depression of prefrontal cortex to reduce awareness (increase in frontal u and d rhythm).
Definitions of the EEG rhythms used in the description of the Anesthetic Cascade are given in Table 1.
The model by John et al. underlies the Patient State Index for depth-of-anesthesia monitoring, described in the section Recently Developed Methods, Applied in Commercial Anesthesia Monitory. Although the Anesthetic Cascade model covers a large set of neurophysiological functions, it does not explain patterns like burst suppression, for example.
Table 1. Definition of the EEG Rhythmsa
EEG Rhythm |
Frequency Range, Hz |
|
|
Delta (d) |
< 4 |
Theta (u) |
4–8 |
Alpha (a) |
8–12 |
Beta (b) |
12–25 |
Gamma (g) |
25–50 |
aExact frequencies may vary slightly from source to source.
MONITORING ADEQUACY OF ANESTHESIA
Clinical Indicators and Measures of Anesthetic Depth
The verb monitor originally means to check systematically or to keep watch. Thus, monitoring actually does not necessarily involve medical equipment, but refers also to clinical inspection. As clinical indicators of anesthetic depth are often used as a reference for automated depth- of-anesthesia monitoring methods, they are shortly described here.
In the case of inhalation anesthetics, drug concentration can be monitored by measuring the partial pressure of the anesthetic in exhaled air (end-tidal concentration). Due to the variation of the potency of anesthetic agents, a universal unit of minimum alveolar concentration (MAC) has been applied. The value 1 MAC is the partial pressure of an inhaled anesthetic at which 50% of the unparalyzed subjects cease to express protective movement reaction to skin incision. The primary rationale behind the development of the term MAC was the need to compare the potency of different volatile anesthetics, not the effort to monitor the anesthetic state of an individual patient.
For intravenous anesthetics, no such direct measure can be derived and the effect of anesthetics can be estimated using pharmacokinetic models (effect-site concentration). In this case, the accuracy of the estimate depends on the adequacy of the model. If all subjects would react to anesthetics in exactly identical ways these concentration measures would provide a perfect indicator of adequacy of anesthesia. However, there is an intersubject variability in the effect of anesthetics, and therefore other indicators are needed.
Clinical indicators of the adequacy of surgical anesthesia can be divided into those measuring hypnosis and those measuring nociceptive–antinociceptive balance. The indicators measuring hypnosis include pupillary light reflex, tested by allocating a flashlight to one eye and observing both pupils for constriction; corneal reflex,

558 MONITORING IN ANESTHESIA
Table 2. Ramsay Score for Assessment of Level of Sedationa
Score |
Clinical Status |
|
|
1 |
Patient anxious and/or agitated |
2 |
Patient cooperative |
3 |
Patient responds to commands only |
4 |
Brisk response |
5 |
Sluggish response |
6 |
No response to loud auditory stimulus |
aSee Ref. 13.
tested by applying a wisp of cotton wool to the cornea or by electrical stimulation using special electrodes (12); Eyelash reflex, tested by brushing the eyelashes with a moving object or by electrical stimulation; loss of counting, tested by letting the subject count slowly as long as they can from the onset of infusion–injection; syringe dropping, tested by letting the subject hold a syringe between their thumb and forefinger as long as they can; loss of obeying verbal commands.
The indicators measuring nociceptive–antinociceptive balance include avoidance reaction to nociception. This is mainly a spinal reflex, however, it correlates well with the concentration of most anesthetics; electrical tetanic stimulation, applied using needle electrodes or adhesive skin electrodes to the upper or lower limb; autonomic nervous system—mediated reactions or motor reactions to laryngoscopy and endotracheal intubation. This is a natural stimulus in many clinical situations in the operating room.
These indicators test the functioning of different neural pathways and their applicability depends on the anesthetic used. For example, ketamine leaves corneal and pupillary light reflexes intact.
For more graded and standardized clinical assessment of sedation and hypnosis, several scoring systems have been developed. Probably the most widely used such systems are the Ramsay score (Table 2) and the Observer’s Assessment of Alertness and Sedation Scale (OAAS; Table 3). These scoring systems are developed for use in the ICU as they include scores for agitated states and cover mainly lighter levels of anesthesia. Therefore, they do not necessarily indicate the adequacy of anesthesia for surgical procedures. Also, the assessment obtained using these scoring systems is subjective.
Table 3. OAAS Score for Assessment of Level of Sedationa
Score |
Clinical Status |
|
|
5 |
Responds readily to command |
|
spoken in normal tone |
4 |
Lethargic response to command |
|
spoken in normal tone |
3 |
Lethargic response to command |
|
spoken loudly and repeatedly |
2 |
Appropriate response to loud tone |
|
and mildly painful stimulus |
1 |
Appropriate response to loud tone |
|
and moderately painful stimulus |
0 |
No response |
aSee Ref. 14.
Changes in Neurophysiological Variables with Deepening Anesthesia
All the commercial monitors of hypnosis employ the EEG signal. Although different anesthetic agents induce specific features and patterns in the EEG, certain common trends in signal properties with deepening anesthesia can be seen. At subanesthetic levels, several agents produce oscillations at beta frequency range, sometimes called beta buzz. This activity is seen dominantly in the frontal brain areas. With increasing anesthetic concentrations, the activity becomes more widespread, decreases in frequency and increases in amplitude. Around concentrations, causing the subjects to stop responding to stimuli (1 MAC for inhalation anesthetics), the EEG activity slows further and high amplitude theta and delta waves occur. With further increasing concentration, the burst-suppression (BS) pattern occurs, finally turning into continuous suppression. The dynamics of this pattern, as well as the waveforms of bursts, varies for different anesthetic agents (Fig. 2). Several anesthetic agents tend to induce epileptiform seizure activity in patients with a prior history of seizures and even in subjects with no previous history of seizures (15,16).
In addition to the EEG signal, AEPs have been used for anesthesia monitoring. The latency of early cortical responses Pa and Nb increases and the amplitude decreases with deepening anesthesia (17). Also, late cortical responses to auditory stimuli, specifically the amplitude and latency of the N100 peak have been found to improve the assessment of the level of consciousness in ICU patients (18). A commercially available brain monitor, the AEP Monitor/2 by Danmeter A/S, combines AEPs with EEG parameters to calculate the cAAI index (see the next section).
In most commercially available monitoring devices, the EEG signal is obtained from the electrodes placed at the forehead. This makes the recording procedure easier in clinical situations. The electrodes tend to pick up frontal EMG, which is an artifact from the point of view of the EEG signal but may be used as a valuable indicator of nociception in light anesthesia (19). The EMG component of the measurement is either explicitly or implicitly incorporated into most of the available monitoring devices (see the section Discussion).
Another neurophysiological variable proposed for anesthesia monitoring is the respiratory sinus arrhythmia (RSA) component of the heart rate (HR) signal (20). Although potentially valuable addition to the assessment of the level of consciousness, this variable has not made its way to anesthesia monitoring devices to date.
Short History of Brain Monitoring in Anesthesia
Since the first measurements of human electroencephalogram, performed by Hans Berger in 1920s, this modality has been applied to studying the effects of various drugs, including anesthetics. The emergence of microprocessors and digital techniques for signal analysis opened new perspectives for anesthesia monitoring.
The first commercial brain monitoring device based on digital signal analysis was the Cerebral Function Analyzing Monitor (CFAM1), developed in 1975 by Prior and

MONITORING IN ANESTHESIA |
559 |
(a)100 V
2 s
Fz
(b)100 V
7 s
z |
28 |
|
Figure 2. Samples of BS pattern in EEG during deep propofol (a) and sevoflurane (b) anesthesia. Detection of BS suppression and calculation of BS ratio is an important part of all modern depth-of- hypnosis monitors. The pattern varies significantly among anesthetic drugs. In the case of propofol anesthesia, spindles can be observed (marked by boxes in the figure). Note that the scale of the time axes is different for upper and lower curves.
Maynard (21). This device used the Motorola 6808 8-bit microprocessor. The display of the CFAM1 was divided into two sections, one showing the 10th and 90th percentile as well as the mean of the EEG amplitude distribution while the other showing the percentage of weighted (prewhitened) EEG activity per herz in the beta, alpha, theta, and delta frequency bands (Fig. 3). In addition, muscle activity, EEG suppression ratio, and electrode impedance were displayed. An important feature of the CFAM1 was the possibility of monitoring averaged evoked potentials. Since the introduction of CFAM1, the CFAM family of brain monitors has been continuously developed with the recently introduced CFAM4 being the latest member of this product family. Comprehensive list of publications referring to the CFAM family can be found at www.cfams.- com/references/a4a.htm.
In 1982 Datex-Ohmeda (Helsinki, Finland) introduced its first EEG monitor for anesthesia, the Anesthesia Brain Monitor (ABM). Like in most of the later monitoring devices, the location of the EEG electrodes in the ABM monitor was on the forehead. The monitor displayed the root-mean squared (rms) value of the EMG and the RMS, as well as the zero-crossing frequency of the EEG signal. The EMG and EEG signals were obtained from the same electrodes–bandpass filter of 65–300 Hz was applied to obtain the EMG while frequencies 1,5–25 Hz were used to obtain the EEG. The ABM monitor is described in (22).
At the beginning of 1990s Thomsen et al. took a different approach to anesthesia monitoring in their Advanced Depth of Anesthesia Monitor (ADAM) (23). They divided the signal into consecutive 2 s segments, applied a prewhitening filter, and derived 11 parameters: the rms value and 10 correlation coefficients from each segment. Either the values of the first 10 autocorrelation lags or the coefficients of the 10th-order autoregressive model were suggested as features. To create a set of reference classes, an unsupervised repetitive hierarchical cluster analysis was applied to the data bank of preannotated recordings of
halothane and isoflurane anesthesia. Six clusters were defined, corresponding to anesthetic levels from drowsiness to very deep anesthesia. The classification was adjusted according to the anesthetic agent used. Burstsuppression was detected separately and the suppression ratio in 2 s segments was incorporated into classification. Anesthetic depth was displayed as the class probability histogram: A plot where each line represented the clusters
Figure 3. Layout of the screen of the CFAM1 monitor (with permission from D. Maynard).

560 MONITORING IN ANESTHESIA
obtained for 10 s period of the recording. The clusters were color coded. In spite of its advanced approach, ADAM was never implemented in a commercial anesthesia monitoring device.
Recently Developed Methods, Applied in Commercial
Anesthesia Monitors
The Bispectral Index Score (BIS), developed by Aspect Medical Systems Inc. in 1997, marked a breakthrough in anesthesia monitoring. The output of the BIS monitor is a single number between 0 and 100 achieved by combining in a nonlinear fashion from the following parameters (24):
relative beta ratio calculated in spectral domain as
log P30 47 , where P30 47 and P11 20 denote signal power
P11 20
in frequency ranges 30–47 and 11–20 Hz, respectively;
SynchFastSlow measure calculated in bispectral domain
as log |
B0:5 47:0 |
, where B |
0:5 47:0 |
and B |
40:0 47:0 |
denote the sum |
|
P40:0 47:0 |
|
|
|||
of magnitudes of the bispectrum values in the corresponding frequency ranges; BS ratio. Bispectrum (the third-order spectrum), is defined as the two-dimensional (2D) Fourier transform (FT) of the third-order cumulant sequence c3(k1, k2) of the signal:
Bðv1; v2Þ FT$ c3ðk1; k2Þ |
ð1Þ |
If the direct current (dc) component of the signal has been removed (as is usually the case), c3(k1, k2) equals to the third order moment sequence m3(k1, k2), defined as:
m3ðk1; k2Þ ¼ efsðnÞsðn þ k1Þsðn þ k2Þg |
ð2Þ |
where e{ } denotes expected value. Overview on the estimation of higher order spectra can be found in (25).
The weighting of the three parameters forming the BIS depends on signal properties and is not disclosed. In light anesthesia, relative beta ratio is dominating while SynchFastSlow measure becomes more important with deepening anesthesia. The function combining the parameters was developed empirically, based on thousands of EEG records. An important part of BIS is its careful artifact rejection scheme, dealing with heartbeat artifacts, eyeblinks, wandering baseline, muscle artifacts, and so on BIS has become very popular among anesthesiologists; the bulk of literature dealing with the behavior of BIS in various clinical situations, discussing its advantages as well as disadvantages, incorporates more than 1000 papers. Comprehensive bibliography can be found on the web-pages of Aspect Medical Systems Inc.
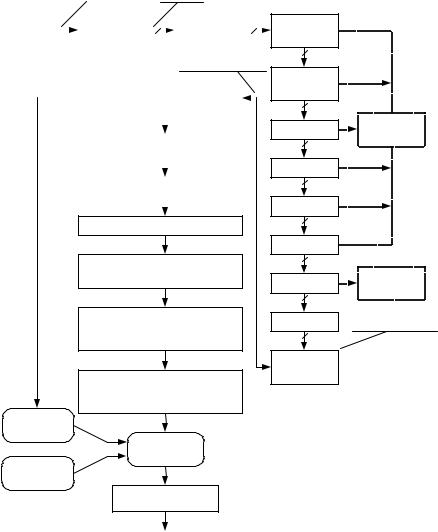
At the beginning of this decade Physiometrix Inc. brought to market the PSA 4000 depth-of-hypnosis monitor, based on the Patient State Index (PSI) (26). The development of the PSI was based on a library of 20,000 cases of EEG records. In addition, a library of surgical cases, a library of artifacts and results from volunteer studies (for calibration), were used. In PSI, the EEG signal is measured from four electrodes: Fp1, Fpz, Cz, and Pz, with the reference at linked ear electrodes. Signal analysis is based on power in standard EEG frequency bands (see Table 1) and incorporates the calculation of the following parameters: absolute power gradient between Fp1 and Cz leads in the
gamma frequency band (25–50 Hz); absolute power changes between Fpz and Cz leads in beta (12–25 Hz) and between Fpz and Pz; leads in alpha (8–12 Hz) frequency bands; total spectral power (0.5–50 Hz) at the Fp1 lead; mean frequency of the total spectrum at Fpz lead; absolute power in delta frequency band (0.5–4 Hz) at Cz; relative power at Pz lead in slow delta frequency band;
The calculated parameters go through a mathematical transformation that guarantees their Gaussian distribution in order to be rescaled into the Z-score (Fig. 4). The Z-score sets the calculated parameters into relation with the parameter values obtained for reference population giving the percentage of the reference population that lies more standard deviations away from the mean than the calculated parameter (6). The Z-scored parameters are fed into discriminant analysis with adaptive discriminant functions. EEG suppression is detected separately: The suppression ratio is included in the discriminant analysis. The discriminant analysis yields the PSI index: a scalar between 0 and 100 with higher level of hypnosis corresponding to lower PSI value.
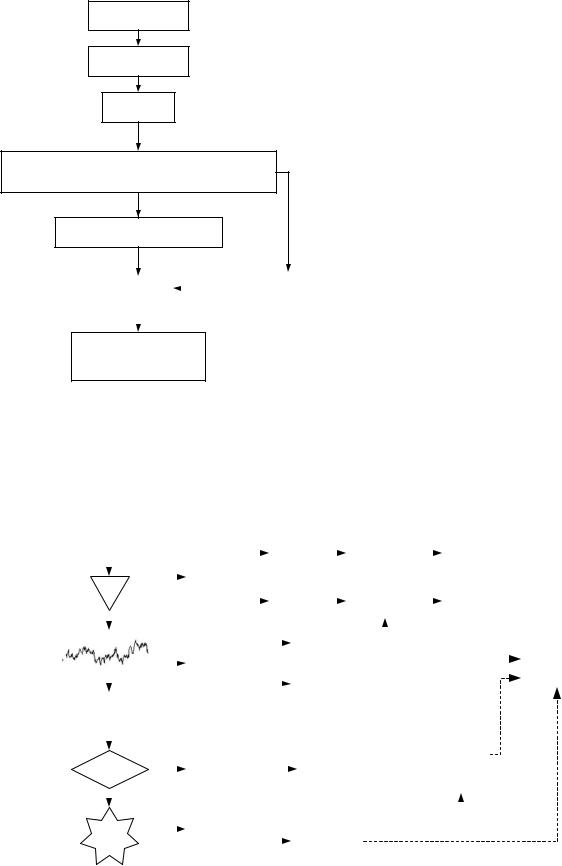
The Narcotrend anesthesia monitoring system was developed by a German group and first introduced in 2000 (27,28). This system has its roots in sleep analysis: A five-stage sleep scoring system was further developed into a system of 6 stages and 14 substages for level- of-hypnosis monitoring. These stages are mapped to a scale of 0–100 in the Narcotrend algorithm. The EEG signal is derived from one or two electrodes; the most common electrode location is on the forehead, however, according to the authors other electrode locations are possible. The signal is sampled at 128 Hz and prefiltered using lower and upper cutoff frequencies of 0.5 and 45 Hz, respectively. The principal idea underlying the method is similar to that of the PSI: Several variables calculated from the EEG signal are fed to discriminant analysis with separate detection of BS (Fig. 5). The variables are classified as timeand frequencydomain ones and contain signal power, autoregressive coefficients, relative power in standard EEG frequency bands, median frequency (the frequency dividing the signal spectrum into two parts of equal energy), spectral edge frequency (SEF95, the frequency below which 95% of signal energy is contained), and spectral entropy. The algorithm also contains plausibility check to ensure that the signal segment is actually similar to a typical EEG sample of corresponding stage and to detect patterns in the EEG signal untypical for general anesthesia (e.g., epileptic activity). The detailed algorithm of the Narcotrend index is proprietary.
Another EEG-based depth-of-anesthesia monitoring device is the recently introduced M-Entropy module for the Datex-Ohmeda S/5 anesthesia monitor. As the name indicates, the method is based on the idea that the entropy of the EEG signal decreases with deepening anesthesia. Signal entropy can be defined and calculated in many different ways (see also the next section) of which spectral entropy is employed in the M-Entropy module. Spectral entropy in the frequency range f1–f2 is expressed as
|
|
f2 |
|
1 |
|
|
X |
|
|||
Sð f1; f2Þ ¼ |
fi |
¼ |
f1Pnð fiÞlog |
Pnð fiÞ |
ð3Þ |
|
|
|
|
|
|
|
|
|
Fs = 2500 |
|
Fs = 250 |
||||||||
FP1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
FPZ' |
|
|
|
Filter/ |
|
|
|
|
Mains |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
PZ |
|
|
|
decimation |
4 |
|
|
|
notch filter |
4 |
|||
|
|
|
|
|
|
||||||||
CZ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Power spectrums |
||||||
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
QEEG measures; |
|
|
|
|||||
|
|
|
|
|
Power spectrum; sub-banding |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
QEEG measures epoch buffer |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compute mean of measures |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Log transform measures
Z-Score components [mean, standard deviation]
Compute wake/sedated
Discriminant functions based on
Z-Scored components
Compute discriminant PSI based on ratio of
wake to wake + Sedated
Arousal observer
Discriminant
observer
Suppression observer
Filter & decimate
Nonlinearity
4
EOG evaluation
4
Magnitude
4
Stationarity
4
Slew rate
4
Cautery
4
Suppression
4
Data editor
4
Frequency
transform
PSI
where Pn(fi) is the normalized power spectrum of the signal. S(f1, f2) is again normalized by log N(f1, f2), where N(f1, f2) is the number of frequency components in the range f1–f2, to give a value between 0 and 1. In the original version of the device, the analysis was performed on a single EEG channel measured from the forehead. In this derivation, muscle activity dominates over the EEG at frequencies higher than 30 Hz. The algorithm of the M-Entropy module, like that of the early ABM-monitor by Datex-Ohmeda, employs these high frequency components to detect the early response of the patient to nociceptive stimuli. This is done by calculating spectral entropy over two frequency ranges: 0.8–32 Hz (called state entropy) and 0.8–47 Hz (called response entropy). The difference between these two entropies indicates the contribution of the EMG component to the response entropy. As in the other described monitors, BS is detected separately. The details of the algorithm (variable window length, obtaining the output value in the case of BS, etc.) are described in (29).
The Danmeter AEP Monitor/2 (further development of the A-Line monitor) employs the composite AAI Index,
MONITORING IN ANESTHESIA |
561 |
Artifact
index
Suppression
ratio
2.5 s Artifact free EEG
Figure 4. Schematic of the calculation of the PSI index. (Redrawn with permission from D. R. Drover et al., ‘‘Patient State Index: Titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia,’’ Anesthesiology, Vol. 97, Fig. 3 (p. 88), 2002).
combining the middle latency auditory evoked potentials in 20–80 ms latency range, calculated from the 25–65 Hz bandpass filtered signal, with spontaneous EEG. The purpose of combining the two modalities is to get a better response to the lightening of hypnosis due to, for example surgical stimuli (achieved by usind AEPs) while retaining sensitivity during deep anesthesia (achieved by using the EEG). The schematic of the cAAI index calculation is presented in Fig. 6. Using evoked potentials poses a problem in on-line monitoring due to the long delay needed for obtaining the averaged response. This problem has been solved in the cAAI calculation by applying the autoregressive model with exogenous input (the ARX model). The ARX model enables to calculate the response to stimuli based on the average of 18 sweeps using the average of 256 sweeps as a reference. The algorithm is described in detail in (30) and compared with conventional evoked potential averaging techniques in Ref. 31. In addition to AEPs, the cAAI index incorporates logarithmic EEG power ratio ½logðP30 47=P10 20Þ& and the burst suppression ratio. The EMG is extracted and monitored
562 MONITORING IN ANESTHESIA
EEG
Filters
Artefact detection
Time-domain analysis |
Frequency-domain analysis: |
- amplitude measures |
- fast fourier transform |
- autoregressive parameters |
- spectral parameters |
Suppression detection / discriminant functions
|
|
|
|
"Background" |
Plausibility |
|
|
||
|
|
parameters |
||
checks |
|
|
||
|
|
|
|
|
|
|
|
|
|
Narcotrend stage (A-F)
and Narcotrend index (100-0)
Figure 5. Schematic of the calculation of the Narcotrend index (with permission from B. Schultz).
separately based on the 65–85 Hz bandpass filtered signal.
A somewhat different concept of anesthesia monitoring hasbeen used inthe CerebralState Monitor(CSM; Danmeter A/S, Odense, Denmark) and the SNAP monitor (Everest
Biomedical InstrumentsInc).Thesemonitoringdevicescome in the form of a handheld wireless PDA-type tool, convenient to use in a clinical situation. The CSM displays the Cerebral State Index, calculated based on the 6–42 Hz bandpass filtered EEG, the EMG component calculated from the same signal, but in 75–85 Hz frequency range, as well as the burst suppression ratio. The algorithm of the second version of the SNAP index is described in (32). Two variables, the low frequency variable LF (0.1–40 Hz) and the high frequency variable HF (80–420 Hz) are derived from a single frontal EEG channel. The HF and LF are scaled to fit into the intervals 0.0–1.0 and 0.0–100, respectively. The SNAP index is expressed as SI ¼ 100—(HF LF); thus the index can be thought of as the reversed version of HF-modulated LF.
New Parameters Proposed for Monitoring Anesthetic Depth
In spite of the large selection of available methods, new parameters for quantifying depth of hypnosis are being proposed continuously. This is mostly due to the following reasons: the variety of procedures and combinations of drugs in surgical anesthesia is wide. No method performs well in all cases; monitoring in anesthesia is closely related to monitoring brain dysfunction and detection of brain ischemia and hypoxia: important tasks faced in cerebral function monitoring in the ICU and emergency room (see the section Monitoring Outside the Operating Theater). The available depth-of-hypnosis monitors are generally not suitable for these applications; the neurophysiological basis of consciousness is still an unsolved problem: applying modern signal analysis tools to neurophysiological measurements during anesthesia can hopefully offer new insight to the problem.
Several groups have recently published studies on the behavior of various complexity–entropy measures during
A-Line Electrodes |
|
|
|
|
|
|
|
|
MTA256 |
|
|
|
|
If SNR low |
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
sweeps |
|
|
|
smooth signal |
|
|
|
ARX |
|
|
||||||||||
|
|
|
|
|
|
|
|
|
BP filter |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AMP |
|
|
|
|
|
|
AEP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MODEL |
|
|
|
|
||
|
|
|
|
|
|
|
25-65 Hz |
|
|
|
|
|
|
MTA18 |
|
|
|
|
If SNR low |
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
sweeps |
|
|
|
smooth signal |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Smooth |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
Estimate |
|
|
|
|
|
|
|
factor |
|
|
|
|
|
|
|
|
|
|
|
|
AAI |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
SNR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Show SNR |
|
|
|
|
|
|
|
|
|
|
calculation |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
bar/symbols |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
A/D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Converter |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EEG |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Signal |
Yes |
|
|
|
Bandpass filter |
|
|
|
|
|
|
EMG |
|
|
|
|
|
|
LF / HF |
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
EMG |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
OK? |
|
|
|
|
|
|
|
|
|
|
|
|
calculation |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
65-85 Hz |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
No |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bandpass filter |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Reject |
|
|
|
|
|
|
Burst Suppr. |
|
|
|
|
|
|
BS % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
1-35 Hz |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
calculation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 6. Schematic of the calculation of cAAI index (with permission from E. W. Jensen).

anesthesia and sedation. These measures come from different signal analysis frameworks.
Correlation dimension is a measure for quantifying the behavior of chaotic signals in the phase space (33). The signal s of finite length N is divided into N mþ1 time series: sm(i) ¼ {s(i), s(i þ 1), . . . , s(i þ m 1)}, where m is the embedding dimension. After that, for each i the quantity Cim(r) is calculated:
Cm r |
number of such j that d½SmðiÞ; Smð jÞ& r |
4 |
|
|||||
i ð Þ ¼ |
N |
|
m |
þ |
1 |
|
ð |
Þ |
|
|
|
|
|
|
|
||
where the distance d between the phase space vectors sm(i) and sm(j) is defined as
½ mðiÞ; Smð jÞ& ¼ k 1;2;...;mðjsði þ k 1Þ sð j þ k 1ÞjÞ |
ð5Þ |
|||||
d S |
|
max |
|
|
|
|
|
|
¼ |
|
|
|
|
Correlation dimension D can be estimated as |
|
|||||
|
|
D |
|
d logðCmðrÞÞ |
6 |
|
|
|
|
¼ |
ð |
Þ |
ð Þ |
|
|
|
d log r |
|
||
where CmðrÞ ¼ |
i CimðrÞ=N m þ 1: Although EEG can- |
|||||
not be |
considered strictly chaotic, except in the case of some |
|||||
|
P |
|
|
|
|
|
abnormal conditions, this measure has, for example, been found to have good correlation with the end-site concentration of sevoflurane (34).
Probably the most intensively studied complexity/ entropy measure for the assessment of depth of hypnosis is Approximate entropy (ApEn). In general, entropy measures information-richness, regularity and randomness of a signal. The intuitive idea behind anesthesia monitoring using signal entropy is that with deepening anesthesia EEG becomes more regular and its entropy decreases. Approximate entropy, like correlation dimension, is calculated in the phase space. First, fm(r) is defined based on Cmi(r) in Eq. 4 as
1 |
N mþ1 |
|
|
|
FmðrÞ ¼ |
|
|
logCimðrÞ |
ð7Þ |
N m þ 1 |
i 1 |
|||
|
|
¼ |
|
|
|
|
X |
|
|
Approximate entropy is then defined as |
|
|||
A pEnðm; rÞ ¼ FmðrÞ Fmþ1ðrÞ |
ð8Þ |
|||
Approximate entropy has been studied and compared to other methods as an indicator of anesthetic depth in (35–37).
The classical entropy measure, introduced for information theory by Claude Shannon in 1948 (38), the Shannon
entropy |
(ShEn), is calculated as ShEn ¼ |
i pi log pi, |
|
where p |
|
amplitude obtains |
|
|
i is the probability that signal |
|
P |
the range of values ai. In practice, ShEn can be estimated based on the histogram of the values of signal samples, and therefore long signal segments are needed to achieve smooth histograms. An important property of Shannon entropy is that signal samples are considered as independent trials of some experiment, taking no notice on the time order of the samples. Signals having equal probability for all possible amplitude values have the highest Shannon entropy. In Ref. 39, it has been found that Shannon entropy of the EEG recorded between frontopolar electrodes increases with increasing concentration of desflurane: A behavior opposite to other entropy measures.
MONITORING IN ANESTHESIA |
563 |
Other measures of the EEG, found to correlate well with depth of hypnosis, include Lempel–Ziv complexity and Higuchi fractal dimension (35,40). Lempel–Ziv complexity is calculated by transforming the signal into symbols and calculating the reoccurrence rate of these symbols (41). Higuchi fractal dimension is calculated as the average rate of increase in the difference of signal amplitude values as the separation between the samples increases in logarithmic scales (42).
These studies demonstrate that although different measures of entropy or complexity quantify different phenomena, many of them may correlate with concentrations of selected anesthetics when electrode positions and signal bandwidth are selected properly.
MONITORING OUTSIDE THE OPERATING THEATER
Development of digital EEG equipment, increase in processing speed and memory capacity, and advancements in telecommunication technology have made cerebral function monitoring feasible in ICU and ER. Brain monitoring in ICU and ER has much in common with monitoring in anesthesia as the changes in the EEG caused by intoxication, metabolic disturbances and brain ischaemia are similar to those induced by general Anesthesia. Also, in the ICU the assessment of depth of sedation is desirable. The advantages offered by EEG monitoring in the ICU are based on the following findings (43): EEG is tightly linked to cerebral metabolism; EEG is sensitive to brain ischemia and hypoxia; EEG detects neuronal dysfunction at a reversible stage; EEG detects neuronal recovery when clinical examination cannot; continuous EEG provides dynamic information; EEG provides useful information about cerebral topography.
However, from the monitoring point of view, the situation in ICU and ER is a lot more complicated compared to that of OR. The patients may need various medication having effect on the EEG signal and misleading automated EEG analysis, the clinical situation of the patients is often complex, and the surrounding is hostile for interference-sensitive equipment. In ICU, recordings often need to last for several days and nights without disturbing the normal care of the patient. In ER, the EEG recording equipment needs to be extremely flexible and easy-to-use. In both situations the interpretation of the recordings poses a problem as no experienced EEG readers are usually around. The solution to the last problem is the usage of telecommunication protocols to transfer the data for interpretation.
Although the above described depth-of-anesthesia monitoring methods are sometimes applied to sedation monitoring and even to the detection of brain dysfunction in ICU, their performance in this situation is questionable. It is difficult to differentiate between the effects of hypoxia, ischemia and sedative drugs. The importance of having the underlying raw EEG signal available for review to confirm the significance of any trends and changes suggested by automatic analysis methods, especially in complex situations like ICU, has been repeatedly emphasized (44,45). A comprehensive brain monitor for ICU, especially for neuroscience ICU, should also be able to detect epileptic
564 MONITORING IN ANESTHESIA
patterns in EEG and desirably have the option for synchronous video recording (46). All this suggests that an adequate brain monitor for ICU or ER should be a much more complex device than today’s depth-of-anesthesia monitors.
DISCUSSION
Several considerations are appropriate concerning the available commercial depth-of-anesthesia monitors. First, different modalities should be used to assess the different components of anesthesia (see the section Anesthesia as a Process). Selecting EEG and AEPs as the basis for the assessment, the primary component of anesthesia considered would be hypnosis.
But even in this case there still remain other physiologically separate end-points like subcortically controlled reactions to nociceptive input (e.g., autonomic reactions), increased muscle tone, and movement response to surgery. Adding the fact that there are many anesthetic agents of different cell-level actions and that the interplay of hypnotic and antinociceptive medication modulates the anesthetic state (47), we are left with a complex situation that makes the comparison of the available algorithms for anesthesia monitoring a real challenge.
Another difficulty in comparing the results obtained with different methods is posed by the frequency band used for the calculation. All the commercial methods operate at least partly in the frequency domain although BIS applies third-order spectrum and in the Entropy module a nonlinear transformation follows the calculation of the power spectrum. For anesthesia, monitoring frequency domain can roughly be divided into the following physiologically meaningful areas: d (and partly u) frequencies, indicative of pre-BS deep anesthesia ( 0.5–6 Hz); a and b frequencies; the EMG component, overlapping with the EEG and extending to > 100 Hz.
The devices differ in the usage of d frequencies and in the way the EMG component is incorporated. While most of the methods employ frequency band starting from 0.1 Hz (SNAP)–0.8 Hz (Entropy), the Cerebral State Index and cAAI by Danmeter do not make use of d rhythms. Several devices like the A-2000 monitor by Aspect Medical Systems Inc., the AEP Monitor/2 and the Cerebral State Monitor by Danmeter as well as the PSA 4000 monitor by Physiometrix Inc. display the EMG power separately from their corresponding depth-of-anesthesia indices. The frequency band the EMG component is obtained from varies from device to device, falling into the range from 65 to 110 Hz. The SNAP index, the Entropy module and the Narcotrend index incorporate the information on EMG activity into their depth-of anesthesia indexes in different ways. In SNAP, the high frequency band used is 80–420 Hz, while the other two monitors use frequencies up to 47 Hz. The various entropy–complexity measures proposed for the assessment of anesthetic depth are sensitive to the prefilter settings as well (40). Thus it can be concluded that while comparing the performance of various algorithms, the following matters should be considered: the properties of the algorithm itself, the frequency band of the EEG signal it employs, and the location of the EEG electrodes.
In the future, it seems to be inevitable that brain monitoring becomes more common in ICU and emergency room. There is a compromise between the simplicity of the presentation of the output and versatility of the method. Monitoring such complex phenomenon as anesthesia by a single number is clearly an oversimplification. On the other hand, a device requiring sophisticated configuration and displaying a lot of parameters difficult to interpret gets easily rejected by clinicians. Connecting the algorithms to physiological models would certainly help the interpretation of the monitor output. Future will show if any of the new approaches such as measures of signal complexity find their way into the commercial devices. Operating in the frequency domain has the advantage of long-term experience in EEG analysis by means of frequency domain methods. Another advantage is the solid signal processing theory of frequency analysis. On the other hand, the theory of nonlinear systems is developing rapidly having made itsway to physiological signal analysis in various applications.
BIBLIOGRAPHY
1.Myles PS, et al. Patient satisfaction after anesthesia and surgery: Results of a prospective survey of 10811 patients. Br J Anaesth 2002;84:6–10.
2.Committee on Quality of Health Care in America IoM. In: Kohn L, Corrigan J, Donaldson M, editors. To Err Is Human: Building a Safer Health System. Washington: National Academy Press 1999;241.
3.Chopra V, Bovill J, Spierdijk J. Accidents, near accidents and
¨
complications. Br J Anaesth 1978;50(10):1041U6 36.
4.Lagasse RS. Anesthesia safety: Model or myth? A review of the published literature and analysis of current original data. Anesthesiology 2002;97:1609–1617.
5.Kissin I. General anesthetic action: An obsolete notion? Anesthol Analg 1993;76:215–218.
6.John ER, Prichep LS. The anesthetic cascade: A theory on how anesthesia suppresses consciousness. Anesthesiology 2005; 102:447–471.
7.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature Rev Neurosci 2004;5:709– 720.
8.Flohr H, Glade U, Motzko D. The role of the NMDA synapse in general anesthesia. Toxicol Lett 1998;100-101:23–29.
9.Steyn-Ross DA, Steyn-Ross ML, Wilcocks LC, Sleigh JW. Toward a theory of the general-anesthetic-induced 24 phase transition of the cerebral cortex. II. Numerical simulations, spectral entropy, and correlation times. Phys Rev E 2001; 64:011918 (12 p).
10.Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiat Clin Neurosci 1999;11:190–208.
11.Volume 10: Conciousness and Cognition 2001.
12.Mourisse J, et al. Electromyographic assessment of blink and corneal reflexes during midazolam administration: Useful methods for assessing depth of anesthesia? Acta Anaesthesiol Scand 2003;47:593–600.
13.Ramsay M, Savege T, Simpson B. Controlled sedation with alphaxolene/alphadalone. Br J Med 1974;2:656–659.
14.Chernik D, et al. Validity and reliability of the observer’s assessment of alertness/sedation scale: Study with intravenous midazolam. J Clin Psychopharmacol 1990;10:244–251.
