
Ghai Essential Pediatrics8th
.pdf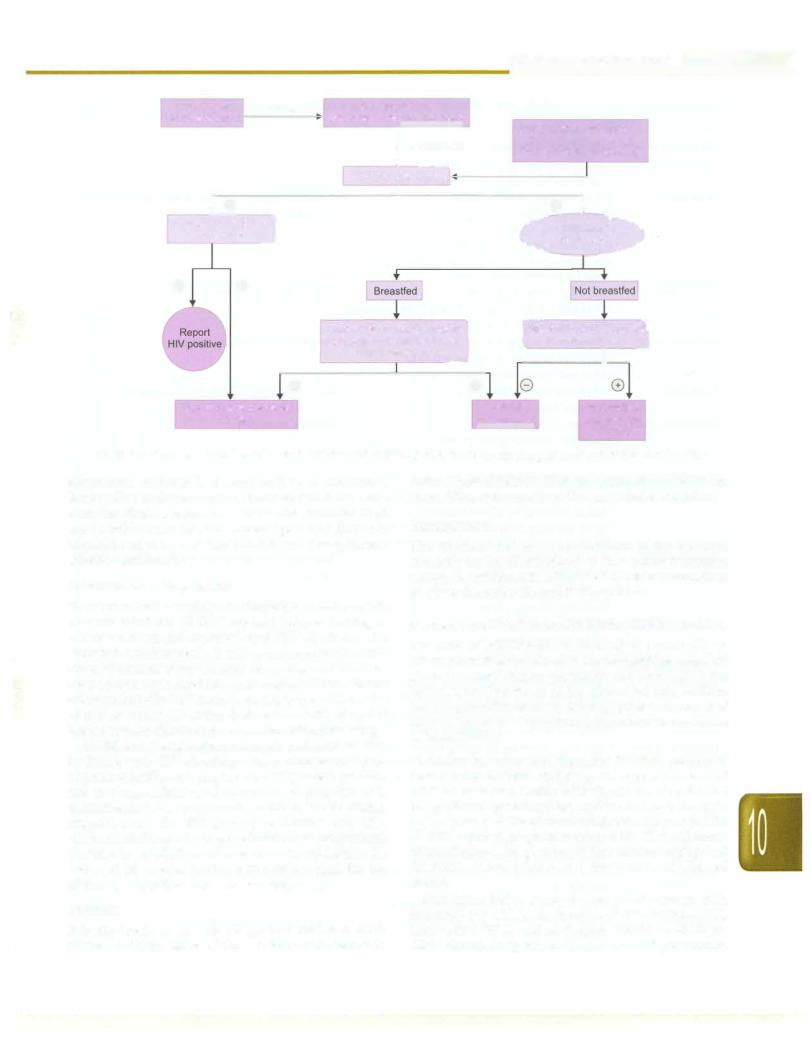
|
|
|
|
|
|
|
|
|
|
Infections and Infestations - |
||
HIV-infected |
Delivery |
HIV-exposed infant |
|
|
|
|||||||
pregnant woman |
|
|
(breastfed and |
nonbreastfed) |
|
Symptomatic HIV-exposed |
||||||
|
|
|||||||||||
|
|
|
||||||||||
|
|
|
|
|
|
J -:: · |
child <18 months of age |
|||||
|
|
|
|
|
|
(not previously diagnosed) |
||||||
|
|
|
|
|
|
First HIV DNA PCR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
I |
|
|
|
|||
Repeat |
|
ie |
|
|
|
|
|
8 l |
||||
|
|
|
|
|
|
--;;;;ative |
||||||
HIV DNA PCR] |
|
|
|
|
||||||||
to confirm |
_J |
|
|
|
|
C PCR tes |
||||||
G8
Second PCR after 6-8 weeks I of stopping breastfeeding or I earlier if symptomatic __J
l
l[°s;cond PCR at 6 monthsJ to confirm status
I
|
G |
8 |
Report |
Repeat test |
||
Repeat test and refer for |
|
|
||||
followup |
HIV negative |
and refer for |
||||
|
|
|
|
|
|
followup |
|
|
|
|
|
|
|
Fig. 10.11: Diagnosis of infection with HIV in children <18 months. If child is >18-month-old, adult testing strategies may be used
alternative regimen is a combination of stavudine, lamivudine and nevirapine. The details of the anti retroviral drugs are shown in Table 10.6. Pediatric fixed dose combinations have been developed, and these are administered using a weight-band based dosing system (NACO guidelines).
Cotrimoxazole Prophylaxis
In resource-limited settings, cotrimoxazole prophylaxis is recommended for all HIV exposed infants starting at 4-6 weeks of age and continued until HIV infectioncan be excluded. Cotrimoxazole is also recommended for HIV exposed breastfeeding children of any age and cotrimo xazoleprophylaxis should be continued untilHIVinfection canbeexcluded byHIVantibodytesting(beyond 18months of age) or virological testing (before 18 months of age) at least six weeks after complete cessation of breastfeeding.
All children younger than one yr of age documented to be living with HIV should receive cotrimoxazole pro phylaxisregardlessofsymptomsor CD4percentage. After one yr of age, initiation of cotrimoxazole prophylaxis is recommended for symptomatic children (WHO clinical stages 2, 3 or 4 for HIV disease) or children with CD4 <25%. All children who begin cotrimoxazole prophylaxis (irrespective of whether cotrimoxazole wasinitiatedin the first yr of life or after that) should continue until the age of five yr, when they can be reassessed.
Nutrition
It is important to provide adequate nutrition to HIV infected children. Many of these children have failure to
thrive. These children will need nutritional rehabilitation. In addition, micronutrients like zinc may be useful.
Immunization
The vaccines that are recommended in the national schedule can be administered to HIV infected children except thatsymptomaticHIVinfectedchildrenshould not be given the oral polio and BCG vaccines.
Prevention of Mother to Child Transmission (PMTCT)
The risk of MTCT can be reduced to under 2% by interventions thatinclude antiretroviral (ARV) prophylaxis given to women during pregnancy and labor and to the infant in the first weeks of life, obstetrical interventions including elective cesarean delivery (prior to the onset of labor and rupture of membranes)and complete avoidance of breastfeeding.
Antiretroviral drug regimens for treating pregnant women For HIV-infected pregnant women in need of
ART for their own health, ART should be administered irrespective of gestational age and is continued through out pregnancy, delivery and thereafter (recommendedfor all HIV-infected pregnant women with CD4 cell count <350 cells/mm3, irrespective of WHO clinicalstaging;and for WHO clinical stage 3 or 4, irrespective of CD4 cell count).
Recommended regimen for pregnant women with indication for ART is combination of zidovudine (AZT), lamivudine (3TC) and nevirapine (NVP) or efavirenz (EFV) during antepartum, intrapartum and postpartum

--E•s•s•e•n.ti•a•I.P.ed•i•a.tr.ic•s------------- |
------------------- |
|
|
Table 10.6: Commonly used antiretroviral drugs in children |
|
Drug |
Dose |
Side effects |
Nucleoside reverse transcriptase inhibitors |
|
|
Abacavir (ABC) |
3 mo-13 yr: 8 mg/kg/dose q 12 hr |
|
|
>13 yr: 300 mg/dose q 12 hr (max 300 mg/dose) |
Hypersensitivity |
Didanosine |
0-3 mo: 50 mg/m2/dose q 12 hr |
Peripheralneuropathy, pancreatitis, abdominalpain, |
|
3 mo-13 yr: 90-150 mg/m2 q 12 hr |
diarrhea |
|
(max 200 mg/dose) |
|
|
>13 yr and <60 kg: 125 mg tablets q 12 hr |
|
|
>13 yr and >60 kg: 200 mg tablet q 12 hr (higher |
|
|
dose for powder preparations) |
|
Lamivudine (3TC) |
1 mo-13 yr: 4 mg/kg q 12 hr |
Pancreatitis, neuropathy, neutropenia |
|
>13 yr and <50 kg: 4 mg/kg/dose q 12 hr |
|
|
>13 yr and >50 kg: 150 mg/dose q 12 hr |
|
Stavudine (d4T) |
1 mo-13 yr: 1 mg/kg q 12 hr |
Headache, GI upset, neuropathy |
|
>13 yr and 30-60 kg: 30 mg/dose q 12 hr |
|
|
>13 yr and >60 kg: 40 mg/dose q 12 hr |
|
Zalcitabine |
<13 yr: 0.01 mg/kg/dose q 8 hr |
Rash, peripheral neuropathy, pancreatitis |
|
>13 yr: 0.75 mg q 8 hr |
|
Zidovudine (AZT) |
Neonates: 4 mg/kg BD |
Anemia, myopathy |
|
3 mo-13 yr: 90-180 mg/m2 q 6----8 hr |
|
|
>13 yr: 300 mg q 12 hr |
|
Non-nucleoside reverse transcriptase inhibitors |
|
|
Nevirapine (NVP) |
2 mo-13 yr: 120 mg/m2 (max 200 mg) q 24 hr for |
Skin rash, Stevens-Johnson syndrome |
|
14 days, followed by 150-200 mg/m2 q 12 hr |
|
|
>13 yr: 200 mg q 24 hr for 14 days, then increase to |
|
|
200 mg q 12 hr if no rash or other side effects |
|
Efavirenz (EFV) |
>3 yr: 10-14.9 kg: 200 mg q 24 hr |
Skin rash, CNS symptoms, increased transaminase |
|
15-19.9 kg: 250 mg q 24 hr |
levels |
|
20-24.9kg: 300 mg q 24 hr |
|
|
25-32.4 kg: 350 mg q 24 hr |
|
|
32.5-39.9 kg: 400 mg q 24 hr |
|
|
40 kg: 600 mg q 24 hr |
|
Protease inhibitors |
|
|
Amprenavir |
4-16 yr and <50 kg: 22.5 mg/kg q 12 hr (oral |
|
|
solution) or 20 mg/kg q 12 hr (capsules) |
|
|
>13 yr and >50 kg: 1200 mg q 12 hr (capsules) |
|
Indinavir |
500 mg/m2 q 8 hr; >13 yr: 800 mg q 8 hr |
Hyperbilirubinemia, nephrolithiasis |
Lopinavir/(LPV) |
6 mo-12 yr: 7-<15 kg: 12 mg/kg lopinavir/ |
Diarrhea, fatigue, headache, nausea; increased |
ritonavir |
3 mg/kg ritonavir q 12 hr with food; 15-40 kg: |
cholesterol and triglycerides |
|
10 mg/kg lopinavir/2.5 mg/kg ritonavir q |
|
|
12 hr with food |
|
|
>12 yr: 400 mg lopinavir/100 mg ritonavir |
|
|
q 12 hr with food |
|
Nelfinavir |
<13 yr: 50-55 mg/kg q 12 hr |
Diarrhea, abdominal pain |
|
>13 yr: 1250 mg q 12 hr (max 2000 mg) |
|
Ritonavir |
<13 yr: 350-400 mg/m2 q 12 hr (starting dose: |
Bad taste, vomiting, nausea, diarrhea, rarely, |
|
250 mg/m2) |
hepatitis |
|
>13 yr: 600 mg q 12 hr (starting with 300 mg) |
|
Saquinavir |
50 mg/kg q 8 hr; >13 yr: soft gel capsules- |
Diarrhea, headache, skin rash |
|
1200 mg q 8 hr |
|

period;EFV-basedregimensshould not be newly-initiated during the first trimester of pregnancy.
Recommended regimen for pregnant women who are not eligible for ART for their own health, but for preventing MTCT is to start ART as early as 14 weeks gestation or as soon as possible when women present late in pregnancy, in labor or at delivery.
Two options are available
Option 1. Daily AZT in antepartum period, combination of single dose of NVP at onset of labor and dose of AZT and 3TC during labor followed by combination of AZT and 3TC for 7 days in postpartum period.
Option 2. Triple antiretroviral drugs starting as early as 14 week of gestation until one week after all exposure to breastmilk has ended (AZT + 3TC + LPV or AZT + 3TC + ABC or AZT + 3TC + EFV) where ABC abacavir, LPV lopinavir.
Omission of the single dose-NVP and AZT+3TC intra and postpartum may be considered for women who received at least four week of AZT before delivery. If a woman received a three-drug regimen during pregnancy, a continued regimen of triple therapy is recommended for mother through the end of the breastfeeding period.
Regimens for Infants Born to HIV Positive Mothers
(a) If mother received only AZT during antenatal period:
For breastfeeding infants. Daily NVP from birth until one wk after all exposure to breast milk has ended. The dose of nevirapine is 10 mg/dayPO for infants <2.5 kg; 15 mg/ day PO for infants more than 2.5 kg.
For nonbreastfeeding infants. Daily AZT or NVP from birth until 6 wk of age. The dose of AZT is 4 mg/kg PO per dose twice a day.
(b) If mother received triple drug ART during pregnancy and entire breastfeeding: Daily AZT or NVP from birth until 6 weeks of age irrespective of feeding
lntrapartum lnteNentions
Avoid artificial rupture of membranes (ARMs) unless medically indicated. Delivery by elective cesareansection at 38 weeks before onset of labor and rupture of membranes should be considered. Avoid procedures increasing risk of exposure ofchildto maternal blood and secretions like use of scalp electrodes.
Breastfeeding
Breastfeeding is an important modality of transmission of HIV infection in developing countries. The risk of HIV infection via breastfeeding is highest in the early months of breastfeeding. Factors that increase the likelihood of transmission include detectable levels of HIV in breast milk, the presence of mastitis and low maternal CD4+ T cell count. Exclusive breastfeeding has been reported to carry
Infections and Infestations -
a lower risk ofHIVtransmission than mixed feeding. Mothers known to be HIV-infected should only give commercial infant formula milk as a replacement feed when specific conditions are met (referred to earlier as affordable, feasible, acceptable, sustainable and safe (AFASS) in the 2006 WHO recommendations onHIV and infantfeeding). Otherwise exclusive breastfeeding is recommended during the first 6 months of life. WHO recommends that the transition between exclusivebreastfeeding and early cessation of breastfeeding should be gradual and not an "early and abrupt cessation". Replacement feeding should be given by katori spoon.
Conclusion
HIV infection in children is a serious problem in many developing countries. The severe manifestations of HIV infection, conditions resulting from severe immuno supressionanddrug toxicities may require intensive care. Development of a vaccine to prevent HIV infection is the high priority area. There is also need to find have more efficacious antiretroviral drugs that have fewer adverse effects. Making available antiretroviral therapy at an affordable cost remains a big challenge. On short-term there is a need to find effective ways to control vertical transmission from mother to child. It may help in sub stantial reduction in childhood HIV infection load.
Suggested Reading
Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. WHO, 2006
IAP, NACO. Guidelines for HIV Care and Treatment in Infants and Children. November 2006
UNAIDS. UNAIDS Report on the Global AIDS Epidemic, 2012
Influenza
The influenza virus is capable of causing disease in humans, birds and animals. In the industrialized world morbidity, absenteeism, economic burden and mortality due to influenza is well quantified and significant. Influenza has recently gained more prominence owing to the 2009 novel HlNl pandemic.
Etiology and Epidemiology
Influenza A and B are RNA viruses that cause human disease. Influenza A is further classified into subtypes based on the two surface proteins hemagglutinin (H) and neuraminidase (N). Influenza B is classified into two distinct lineages Yamagata and Victoria but not into subtypes. Influenza has a highly segmented genome that is prone to frequent mutations and reassortrnent. This leads to frequent antigenic "drifts" when there is minor change in antigenicity and "shifts" where there is major change in antigenicity. These phenomena of antigenic change leads to evolution of new viruses to which there is littlepopulationimmunity andcausesannual outbreaks and occasionally pandemics. The novel HlNl pandemic

e_s_s_e_n_u_a_,.P.ed_,_·a.tr.ics________________________________
__
occurreddue toemergenceofa new swine origin influenza virus HlNlwhichwas pathogenic to humans andcapable ofrapid human to human transmission andto which there was no preexisting immunity. Avian H5Nl commonly referred as bird flu is a highly pathogenic strain of influenza virus that infects and kills humans in close contactwithdiseasedbirds but has notacquiredpandemic potential due to limited human to human transmissibility. The currently circulating influenza virus strains are H3N2, pandemic HlNl and influenza B.
Influenza is transmitted from person-to-person through airborne droplet spread or through contact. The portal of entry is the respiratory tract and the virus attaches itself to the respiratory epithelium through the hemagglutinin which is the main virulence factor. The incubation period is 1-3 days and the period of infectivity is usually 7 days after illness onset and sometimes longer in those with severe disease. The attack rates are highest in children and youngadults. In temperateclimatesthere is a cleardefined influenza season in fall and winters but in tropical countries like India it occurs throughout the year.
Clinical Features
Inmostindividualsinfluenzaisaminorillnesscharacterized by a combination offever, runny nose, sore throat, cough, bodyache, headache, abdominal pain, diarrhea and vomiting. The illness may have a biphasic course. Recovery usually occurs within a week. It is sometimes difficultto differentiatefromcommoncold. Asymptomatic and subclinical infections are also very common.
A small proportion of individuals (less than 1%) can have complications and severe disease. The risk of complications is higherat extremes ofage(children below 2 and the elderly), pregnant women and those who have just delivered, those with underlying comorbidities such as any chronic neurologic, metabolic, cardiac, pulmonary or renal disease,those who areimmunocompromisedand those with severe asthma. In the novel HlNl epidemic the elderly were spared due to pre-existent immunity and morbid obesity emerged as an important risk factor.
The most dreaded complication of influenza is pneumonia with acute respiratory distress syndrome, respiratoryfailureand sometimesshockandrenalfailure. As many as 30% of these patients have bacterial co infection with S. pneumoniae and S. aureus. Progression is very rapid and most patients require ventilator support over the next 24 hr. Occasionally othercomplicationssuch as encephalitis, seizures, quadriparesis and myocarditis have been reported. Transplacental transmission to newborn and neonatal complications have also been reported. Complications usually set in by day 4 or 5 of illness. The redflag symptoms are persistent high fever ofmore
than 3 days duration, reappearance of fever after initial defervescence, breathlessness, dyspnea, tachypnea, hemoptysis in older children and adolescents, extreme weakness, poor oral intake and altered sensorium.
It has been estimated that the novel HlNl pandemic caused 18,000 deaths globally with case fatality rates ranging from 0.0004% to 1.5 % (0.83% in India) and one third of those who died had no underlying risk factor.
Diagnosis
Influenza is primarily a clinical diagnosis. The complete bloodcountmay show leukopenia and thrombocytopenia; the liver enzymes and CPK may be mildly elevated. Diagnosis may be confirmed by antigen detection or PCR on throat/nasopharyngealswabs. Antibody tests in blood are not useful. Specific diagnostic tests such as PCR are not useful in routine clinical practice. They are expensive and have a turnaround time of 24-48 hr. Hence if specific therapy has to be administered, it has to be started before results become available. If the test is negative therapy cannot be discontinued as the sensitivity is only 60-70% and even lower if the sample is not properly collected. Thus the test does not help in the clinical decision of either starting orstoppingtherapy. In many instances,thereport of the throat swab isreceived when thepatienthas already recovered. Henceforth molecular diagnosis of influenza should be restricted to hospitalized patients with severe disease when a definitive diagnosis helps in tracking the severity of the outbreak.
Treatment
Definitive treatment of influenza is with M2 inhibitors (amantadine/rimantidine) or neuraminidase inhibitor drugs (oseltamivir and zanamivir). These drugs reduce duration of symptoms, risk of complications and death. Though they are most effective if given within the first 48 hr of illness; therapy is useful even if given later at any time point of a severe illness. The pandemic HlNl strain and most current seasonal flu strains are resistant to the M2 inhibitors. Hence as per current recommendations oseltamivir is the first line drug and zanamivir should be used in those with oseltamivir resistant virus. The therapeutic dose of oseltamivir is 30 mg twice daily in those with weight less than 15 kg, 45 mg twice daily for 15-24 kg, 60 mg twice daily for 25-34 kg and 75 mg twice daily for those 35 kg and above. Oseltamivir though formally not approved for infants, is generally safe and may be used in a dose of 2-3 mg/kg twice daily. The duration of therapy is 5days. In patients withvery severe disease double the recommended dose for 10 days may be used. Oseltamivir is well tolerated with occasional GI and neurological side effects.
For any patient presenting with influenza like illness (ILI), the treatment strategy depends on two factors: the severity of illness and the likelihood of complications. In patients with mild disease who are not at risk for complications, only symptomatic treatment is indicated. Antibiotics and antivirals should not be prescribed. Patients should be counseled about the red flag signs and asked to seek medical care in the event these occur. These

patients should be asked to stay at home till they are afebrile to prevent disease transmission to others.
Patients with ILi who are at high-risk for complications should be started on antiviral therapy irrespective of the severity of disease. The use of antivirals in all children below the age of 5 with flu like illness is however deba table.
For patients who present with symptoms of severe illnessorwhohavecomplications, antiviral treatment with oseltamivir should be started without delay. An effort should be made to rule out other illnesses with similar symptomatology. In patients with signs of lower respiratory involvement, antibiotics like coamoxiclav or cephalosporins (cefuroxime, ceftriaxone or cefpodoxime) should also be used as bacterial coinfections are common. Supportive and intensive care is as for pneumonia or ARDS.
Prevention
Vaccination is the most effective preventive strategy and is discussed further in Chapter 9. Chemoprophylaxis with oseltamivir is also effective in preventing influenza. The dose is 30-75 mg of oseltamivir (as per weight) to be taken once daily for 10 days. It must be remembered that chemoprophylaxis is the biggest risk factor for drug resistance. Chemoprophylaxis should be considered only for very high-risk household contacts like pregnant women and the severely immunocompromised.
Household transmission can be reduced by good ventilation in the room, proper hand hygiene and adherence to cough etiquettes. Nosocomial transmission to other patients and health care workers is a concern. Vaccination for all health care workers especially during outbreaks should be considered. All patients with suspected ILi should be isolated in single rooms or cohorted inoneward. A distance of at least 1 m should be kept between patients as the droplets can travel for this distance only. Regular disinfection of all surfaces should be carried out. The staff caring for these patients should use a well fitting surgical mask that should be changed every 4 hr. They should use hand hygiene both before and after patient contact. Negative pressure rooms, N 95 masks, gowns though ideal are possible only in high resource settings.
School children show one of the highest infection rates and outbreaks are common in school. For reducing transmission, the classrooms should be well ventilated, children should be trained in hand hygiene and cough etiquettes and sick children should be prohibited from attending school till afebrile. Temporary school closure may be considered during a pandemic.
Suggested Reading
Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (HlNl) 2009 Influenza. Clinical Aspects of Pandemic 2009 Influenza A (HlNl) Virus Infection. NEJM 2010;362:1708-19
I 239
Emerging Viruses
This section deals briefly with some of the new emerging viral diseases seen in India.
Crimean-Congo hemorrhagic fever virus is a RNA virus of the Bunyaviridae family normally infecting cattle and occasionally transmitted to humans by infected ticks. The virus is highly contagious and human-to-human trans mission in householdandhospital setting isnotuncommon. Outbreaks have been reported from various countries including Pakistan. It wasfirstreported from India in 2011 fromGujarat.Thepresentationisthatofa viralhemorrhagic fever with fever, body pain, headache, profuse bleeding, leukopenia, thrombocytopenia, altered liver functions, deranged coagulation parameters, rhabdomyolysis and renal failure. The disease mimics dengue with salient differences being early and more rapid platelet fall and rhabdomyolysis. Diagnosis is by specific PCR. Treatment is supportive; early administration of ribavarin is beneficial. Strict isolation of affected patients is crucial to prevent nosocomial transmission.
Hantaviruses cause two important clinical syndromes: hemorrhagic fever andrenal syndrome (HFRS) in Europe andAsiaincluding India andhantaviruscardiopulmonary syndrome in America. Rodents are natural hosts and humans acquire infection by inhalation of aerosols contaminated by rodent excreta or saliva. In India, HFRS and asymptomatichantavirusinfection has been reported from Tamil Nadu. The disease presents as a febrile illness with body pain, headache, thrombocytopenia, elevated liver enzymes, bleeding and renal failure. Leukocytosis with shift to left differentiates it from dengue. Diagnosis is byspecific IgM antibodies. Treatment includesribavarin and supportive care.
Nipah virus is an important cause of encephalitis increasingly reported from West Bengal, India. Its natural asymptomatic hosts are fruit bats who can transmit infection and disease to pigs and humans. Human-to human transmission has also been reported. Clinical features in humans are fever followed by features of encephalitis and sometimes pneumonia and respiratory distress. Mortality can be as high as 70% and there are residual sequelae in survivors. Treatment is only symptomatic. Prevention centers around limiting human exposure to raw date palm juice contaminated by fruit bat excreta and infected pigs.
Chandipura virus, a rhabdovirus, is implicated as a cause of epidemic viral encephalitis in children in several states in India but not abroad. It is transmitted by bite of infected sandflies and is associated with high mortality and neurologic sequelae.
Suggested Reading
Chandy S, Abraham S, Sridharan G. Hantaviruses: an emerging public health threat in India? A review. J Biosci 2008;33:495-504

....e.s.s.e.n.t.ia•I-P•e•dia-.t .ri•c•s ----------------- |
--- |
|
- |
-------------- |
|
|
|
|
Nipah virus. Wkly Epidemiol Rec 2011;86:451-5
Patel AK, et al. First Crimean-Congo hemorrhagic fever outbreak in India. J Assoc Physicians India 2011;59:585-9
Rao BL, et al. A arge outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet 2004;364:869-74
COMMON BACTERIAL INFECTIONS
Staphylococcal Infections
Staphylococcus a gram-positive coccus is a very common cause ofbothcommunityacquired andnosocornialdisease in children.
Etlopathogenesls
Staphylococci are functionally classified on basis of production of an enzyme and virulence factor coagulase. Coagulase positive staphylococcus is termed as S. aureus while S. saprophyticus and S. epidermidis are important coagulase negative staphylococci (CONS). CONS usually colonize the skinof allpeople and S. aureus thenares, axilla and perineum of around 20-25% of the population. Staphylococcal infection is acquired usually by direct contact with an infectedpatient or carrier and sometimes contaminated objects. Airborne spread is less common. Predisposing factors for staphylococcal infections include breach in the mucocutaneous barrier, previous viral infections such as measles, depressed immunity and prosthetic material such as shunts, central venous catheters and prosthetic joints.
pyopericardium an illness with high rates of constrictive pericarditis that often requires pericardiectomy.
S. aureus is the commonest cause of musculoskeletal infections such as osteomyelitis, septic arthritis and pyomyositis. CNS infections such as meningitis usually occur following trauma or neurosurgery. S. aureus is also a common etiologic agent of subdural empyema, brain abscess and shunt infections. Enterotoxin producing S. aureus is a common cause of food poisoning that is characterized by fever and profuse vomiting.
Toxic shock syndrome (TSS) results from a locally non invasive toxigenic strain of S. aureus which is characterized by fever, shock, erythematous skin rash, hepatic derangement, sensorial changes and high mortality. Disseminated staphylococcal disease is another illness usually seen in previously healthy children and most commonly reported in India. It is characterized by suppurative staphylococcal infections at multiple sites either together or serially and a prolonged course.
CONS are usually pathogens of lower virulence than S. aureus. Since they colonize the skin, they are often cultured ascontaminants if blood culturesarenotcollected by aseptic techniques. They are commonly implicated in bacteremia in low birth weight babies or in those with central venous catheters, subacute infective endocarditis, CNSshunt infections, infections associated withperitoneal dialysis catheters and prosthetic joints, urinary tract infections and postoperative surgical site infections.
Clinical Features
S. aureus can cause a myriadofclinicalinfectionsinvolving almost all organs of the body. Infections are associated with suppuration and often require drainage and pro longed antibiotic therapy.
Commonest are infections of skin and soft tissues like furuncles, impetigo, carbuncles, abscesses and cellulitis. In some situations, the bacteria invade the fascia and muscle causing necrotizing fasciitis, an infection that is associated with very high morbidity and mortality. Staphylococcal scalded skin syndrome is another bullous infection commonly seen in infants produced by exfoliative toxin producing S. aureus that can lead to massive desquamation and denudation.
S. aureus is an important cause of respiratory infections such as sinusitis, otitis media, pneumonia, lung abscess and empyema. Staphylococcal pneumonia commonly occurs after antecedent viral infections, is rapidly progressiveandassociatedwithahighrateofcomplications such as pneumatoceles, abscess and empyema. S. aureus is the commonest cause of acute infective endocarditis in both patients with native and prosthetic valves and sometimes with no risk factors. It is rapidly progressive, locally destructive and is associated with significant complications. It is also the commonest cause of
Treatment
The most important treatment strategies are surgical drainage and antibiotics.
Antibiotic therapy of staphylococcal infections has become complicated due to evolving resistance in staphylococci. More than 90% of the current day organisms are resistant to penicillin due to production of a beta lactamase/penicillinase that destroys the beta lactam ring of penicillin. Most of them however are sensitive to penicillinase resistant penicillins such as cloxacillin/methicillin andcephalosporins and are termed as MSSA. Some staphylococci however have acquired resistance to methicillin by production of an altered penicillin binding protein (PBP) and are termed as MRSA. MRSA weretillsometimebackonly reported as causative agents of hospital acquired infections but are now also being reported in community acquired infections.
The drug ofchoicefor treating MSSA infections is cloxa cillin. Other alternatives are first generation cephalo sporins (cephalexin, cefadroxil or cefazolin), second generation cephalosporins (cefuroxime)and coamoxiclav, clindamycin. If MRSA infections are proven or suspected, drugs like vancomycin, linezolid and teicoplanin are required.
Most S. aureusinfectionsneedremovalof any prosthetic material to ensure cure and prolonged therapy ranging

from 2 weeks for bacteremia and up to 6 weeks for osteomyelitis, septic arthritis and endocarditis.
Suggested Reading
Baranwal AK, Singhl SC, Jayashree M. A 5-yr PICU experience of disseminated staphylococcal disease, Part 1: Clinical and microbial profile. J Trop Pediatr 2007;53:245-51
Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am 2009;23:35-52
Pneumococcal Infections
Pneumococcus is one of the most common bacterial causes of pediatric infections particularly pneumonia. It is currently estimated that 50% of deaths due to severe pneumonia are caused by pneumococcus. This means that of the 400,000 deaths in children below age 5 in India due to acute respiratory infections, 200,000 are perhaps due to pneumococcus.
Etiopathogenesis
Pneumococcus is a gram-positive diplococcus with a thick polysaccharide capsule. This capsule is the most important virulence factor and determines the various serotypes of the pneumococcus. Almost 90 serotypes of pneumococcus exist but only a handful cause disease. Serotypes 1, 4, 5, 6 A and 6B, 9V, 14, 18C, 19A, 19F and 23 are those that commonly cause human disease and are incorporated in the vaccines (Chapter 9). Pneumococci colonize the nasopharynx, colonization rates are highest in young children. Colonization can lead to infection in some individuals.Risk factors for pneumococcal disease include extremes of age (age less than two yr), splenic dysfunction, immunodeficiency especially HIV, any chronic disease and CSF leaks.
Clinical Features
Pneumococcal infections are distributed like a pyramid, the base of the pyramid being noninvasive disease like otitis media, sinusitis and pneumonia and the apex comprising of invasive disease like bacteremic pneumonia, bacteremia and meningitis. It is estimated that for every 1000 cases of otitis media there is 1 case of meningitis. Other less common invasive diseases due to pneumococci are osteomyelitis, septic arthritis, cellulitis and peritonitis.
Pneumococcus is responsible for 30% of all acute bacterial meningitis and is associated with high rate of complications like subdural empyema, morbidity and mortality. With increasing vaccination against Haemophilus influenzae, the percentage contribution of pneumococcus towards meningitis will increase further. Pneumococcal bacteremia presents as fever without focus in infants and children below age 3 and needs aggressive therapy. It is estimated that pneumococcus causes 30-50% of radiologic/ severe pneumonia. Pneumococcal pneumonia has lobar distribution with necrosis and empyema being common complications.
I
Infections and Infestations
Diagnosis
The gold standard for diagnosis of pneumococcal disease is culture. However, culture yields are poor because of several reasons. Pneumococcus, unlike Salmonella, is difficult to culture especially if antibiotics have been administered. Special media containing sheep blood are required and delays in transportation and improper storage further reduce recovery. Isolation rates fromblood are low and the ideal sample of lung aspirate cannot be obtained in routine clinical practice.
Other tests useful in diagnosis are Gram stain (Fig. 10.12), latex agglutination tests and PCR in CSF and pleural fluids.
Treatment
Penicillin and its derivatives such as ampicillin and amoxicillin are the drugs of choice for treatment of pneumococcal infections. The cephalosporins particularly ceftriaxone are also satisfactory alternatives. Like many other bacteria, resistance in pneumococcus is being increasingly reported. Resistance to beta lactams is due to altered penicillin binding protein (PBP) and may be of intermediate or high level. Intermediate resistance can be overcome by using higher doses of penicillin/amoxicillin but high level resistance requires use of alternative drugs like fluoroquinolones or vancomycin.
Prevention of pneumococcal disease is discussed in Chapter 9.
Suggested Reading
WHO position paper. Weekly Epidemiologic record 2012;87:129-44
Diphtheria
Diphtheria is an acute bacterial infection caused by gram positive bacillus, Corynebacterium diphtheriae.
Etiopathogenesis
The secretions and discharges from infected person or carrier are the main source of infection. The infection is transmitted by contact or via droplets of secretion. The
portal of entry is commonly the respiratory tract. The
"••
Fig. 10.12: Gram stain of pus showing abundant gram-positive diplococci suggestive of pneumococci

__E_s_s_e_n_t.ia•l•P•de-ia.ritc s _________________________________
incubation period of the disease is 2-5 days. C. diphtheriae proliferate and liberate powerful exotoxin which is the principal cause of systemic and local lesions. The exotoxin causes necrosis of the epithelial cells and liberates serous and fibrinous material which forms a grayish white pseudomembrane which bleeds on being dislodged. The surrounding tissueis inflamedandedematous. The organs principally affected by the exotoxin include the heart, kidney and myocardium.
Clinical Features
The onset is generally acute with fever, malaise and headache. The child has a toxic look. The clinical features depend on the site of involvement. The commonest form is faucial/tonsillopharyngeal diphtheria in which there is redness and swelling over the fauces. The exudates coalesce to form agrayish white pseudomembrane, which extends to surrounding areas. The cervical lymph nodes are enlargedleading to a bull neck appearance. Sore throat, dysphagia and muffled voice are frequently present. In nasal diphtheria there is unilateral/bilateral sero sanguinous discharge from the nose and excoriation of upper lip. In laryngotracheal diphtheria, the membrane over the larynx leads to brassy cough, stridor and respiratory distress. Diphtheritic lesionsmayoccasionally also be found in skin and conjunctiva.
The commonest complication is respiratory failure due to occlusion of the airways by the membrane. Myocarditis generally occurs by second week of illness and can lead to symptoms of congestive cardiac failure, arrhythmias and sudden death.
Neurological complications include: (i) palatal palsy, which occurs in second week and is clinically manifested by nasal twang and nasal regurgitation; (ii) ocular palsy in third week; (iii) loss of accommodation, manifested by visual blurring and inability to read; and (iv) generalized polyneuritis occurs by 3rd to 6th weeks of illness. Renal complications include oliguria and proteinuria.
Diagnosis
There shouldbe a high index ofsuspicion. Rapiddiagnosis is enabled by Albert stain of a swab from the oropharynx and larynx. Culture, however, takes eight hr to become available. Faucialdiphtheriashould bedifferentiatedfrom acute streptococcal membranous tonsillitis (patients have high feverbut are less toxic and the membrane is confined to the tonsils), viral (adenovirus) membranous tonsillitis (highfever, sorethroat,membranoustonsillitis with normal leukocyte count, self limited course of 4-8 days), herpetic tonsillitis or aphthous stomatitis, thrush, infectious mononucleosis, agranulocytosis and leukemia.
Management
Themost important component oftherapyis neutralization of bacterial toxin by administration of antitoxin. Diphtheria antitoxin (IV/IM) shouldbeadministered soon
asinfectionwithdiphtheria bacilliis suspectedeven earlier than bacteriological confirmation before the bacteria have fixed to the tissues. The degree of protection offered by the diphtheria antitoxin is inversely proportional to the duration of clinical illness. Repeat doses of antitoxin may be given if clinical improvement is suboptimal.
Antibiotics such as penicillin or erythromycin should be used to terminate toxin production, limit proliferation of bacteria, to prevent spread of organism to contacts and to prevent the development of carriers. This should be followed by active immunization as clinical disease does not confer active immunity.
Bed rest is advocated for two to three weeks. Children should bemonitoredfor airwayobstruction andmanaged; tracheostomy may be required in some cases. Sudden exertion should be avoided and changes in rate and rhythm of heart should be looked for. Children with palatal palsy should be fed by nasogastric feeding. Generalized weakness due to polyneuritis is treated as for poliomyelitis or Guillain-Barre syndrome.
Prevention and Control
The patient should be isolated until two successive cultures of throat and nose are negative for diphtheria bacillus. All contaminatedarticlesfrom dischargesshould be disinfected. All household and other contacts should be observed carefully for development of active lesions, cultured for C. diphtheria and given chemoprophylaxis with oral erythromycin for 7 days or single dose benzathinepenicillin. Previously immunizedasymptomatic patients should receive a boosterdose of diphtheria toxoid. Those not fully immunized should receive immunization for their age (see Chapter 9).
Suggested Reading
Panchereon C. Clinical features of diphtheria in Thai children: a historic perspective. South-east Asian J Trop Med Public Health 2002; 33:352-4
Zakikhany K, Efstratiou A. Diphtheria in Europe: Current problems and new challenges. Future Microbiol 2012;7:(5)595-607
Pertussis (Whooping Cough)
Pertussis is an acute highly contagious respiratory tract infection, caused by Bordetella pertussis. It may affect any susceptible host but is more common and serious in infancy and early childhood. The disease is characterized by intense spasmodic cough. Similar illness is also caused by B. parapertussis, B. bronchoseptica and adenoviral infections 1, 2, 3 and 5. The worldwide prevalence of the illness has declined following widespread vaccination.
Epidemiology
Pertussis is extremely contagious with attack rates as high as 100% in susceptible individuals exposed to aerosol droplets. B. pertussis does not survive for prolonged periods in the environment. Chronic carriage in humans is not known. After intense exposure as in households,

the rate of subclinical infection is as high as 80% in fully immunized andnaturally immuneindividuals. Protection against typical disease wanes3-5 yr after vaccination and is unmeasurable after 12 yr. Coughing adolescents and adults are the major reservoir of B. pertussis and are the usual sources for index cases in infants and children.
Features
The incubation period of the disease is 7-14 days. The clinical presentation can be divided into three stages.
The catarrhal phase lasts for 1-2 weeks and is the most infectious period. The initial manifestations are indistin guishable from upper respiratory tract infections. The child has cough, coryza with little nasopharyngeal secretions. Unlike the upper respiratory infections, the cough does not improve in a few days but becomes more severe and frequent with the passage of time. Though the cough may not be typically paroxysmal in early stages, it tends to be annoying and frequent at night. The paroxysmal nature ofthe cough is suspected towards the latter part of this phase.
The paroxysmal stagelasts for 2-6 weeks in which cough progresses to episodic paroxysms of increasing intensity ending with high-pitched inspiratory whoop. The whoop is produced by the air rushing in during inspiration through the half-open glottis. Thewhoopmay not always be present in infants who present with apneic or cyanotic spells. The child coughs up thick tenacious mucus. The paroxysms of cough may occur frequently and terminate by vomiting. Repeated thrusting of tongue over the teeth causes ulceration of the frenulum of the tongue. Paroxysms ofcough are precipitated by food, cold air and cold liquids. In infants <3 months, this stage may be considerably prolonged.
In the convalescent phase the intensity and paroxysms of cough decrease gradually over 1-4 weeks. The vomiting becomes less frequent. Appetite, general condition and health gradually improve.
Complications
Respiratory complicationsincludeotitismedia,pneumonia, atelectasis, emphysema, bronchiectasis, pneumothorax and pneumomediastinum
•Neurological complications include seizures and encephalopathy (2-7%).
•Bleeding episodes, e.g. epistaxis, retinal or sub conjunctival bleeds, intracranial hemorrhage.
•Inguinal hernia, rectal prolapse.
•Malnutrition due to persistent vomiting and disincli nation to eat because of fear of paroxysms of cough with attempts at feeding.
•Flare up of tuberculosis.
Diagnosis
The diagnosis of whooping cough is based on clinical features. There may be a lymphocytic leukocytosis and
Infections and Infestations -
low ESR. Specific diagnosis depends on isolation of the organism from nasopharyngeal swab or cough plate cultured on Bordet-Gengou medium, which is often positive in the catarrhal and paroxysmal stage. Other conditions that present with prolonged episodes of spasmodic cough include adenoviral infection, endo bronchial tuberculosis, inhaledforeign body and reactive airway disease.
Management
General measures include providing adequate nutrition and hydration and avoiding factors aggravating cough. The antibiotic of choice is erythromycin (40-50 mg/kg/ day in 3 divided doses) given for 14 days. It terminates the respiratory tract carriage of B. pertussis thus reducing the period of communicability but does not shorten the course ofillness. Nebulization withsalbutamolis effective in reducingbronchospasmandcontrolling boutsof cough. If nebulization is not possible, salbutamol may be given orally. Cough suppressants and antihistaminic agents should be avoided.
Prevention
Chemoprophylaxiswitherythromycinisrecommendedfor closefamilycontactsespeciallychildren<2-yr-old.Children under 7 yr of age should be vaccinated (Chapter 9).
suggested Reading
Wood N, McIntyre P. Pertussis: Review of epidemiology, diagnosis, management and prevention. Paediatric Respiratory Reviews 2008;9:201-12
Zouari A, Srnaoui H, Kechrid A. The diagnosis of pertussis: which method to choose? Crit Rev Microbiol 2012;38(2):112-21
Enteric Fever
The term enteric fever includes typhoid fever caused by Salmonella enterica vartyphi andparatyphoidfevercaused by S. enterica var paratyphi A, BorC.Paratyphoidinfections constitute about 20% of all cases of enteric fever worldwide. As enteric fever is a disease transmitted by the feco-oral route, its greatest burden is in resource limited countries where water supply and sanitary conditionsarepoor. Ina community-basedstudyin urban slums of Delhi the incidence was estimated to be 980/ 100,000 population. Enteric fever is the most common cause of fever lasting for more than 7 days in clinical practice in India.
Etiopathogenesis
S. enterica serotypetyphi/paratyphi is agram-negative,non lactose fermenting, flagellate bacterium. The somatic or 0 antigen is shared among various salmonellae; the flagellar or H antigen is specific to the serovar. S. enterica var typhi also possesses a Vi polysaccharide capsule.
The infective dose of typhoid/paratyphoid bacillus varies from 103 to 106 organisms. The organism must

__E_s_s_e_n_t .ia•l•P•e•d·i-at.r.ics_________________________________
survive the gastric barrier to reach the small intestine; hence, conditions which reduce gastric acidity, such as use of antacids, H2 receptor blockers and proton pump inhibitors, reduce the infective dose. On reaching the small intestine, the organism penetrates the mucosa and infects the lymphoid follicles and subsequently the draining mesenteric lymph nodes and the liver and spleen. It multiplies in the reticuloendothelial system and after incubation period varying from 7 to 14 days spills into the bloodstream and is widely disseminated, especially to liver, spleen, bone marrow, gallbladder and the Peyers patches of the terminal ileum. This spill marks the onset of clinical manifestations of enteric fever. Infection leads to both local and systemic immune responses, which are, however, inadequate to prevent relapse or reinfection.
Clinical Features
Thereisnoappreciabledifferencebetweenthemanifestations of typhoid and paratyphoid fever. The hallmark of enteric fever is fever which starts as a low grade fever and then shows stepwise increase peaking to as high as 103-104°C by the end of the first week. This pattern differentiates it from viral fever where the peak is usually at the onset of fever. With fever, there is associated malaise, dull headache, anorexia, nausea, poorly localized abdominal discomfort, mild cough and malaise. There may be diarrhea; constipationinchildren is rare. Physical findings are unremarkable with the exception of a coated tongue, tumid abdomen and sometimeshepatosplenomegaly.The rash described in Western textbooks is seldom or never seen in Indian subjects. Infants and young children with enteric fever may have diarrhea as a predominant manifestation or a short-lasting undifferentiated febrile illness. In the absence of treatment fever may continue for 3-4 weeks followed by natural remission or by development of complications.
Complications
The commonest intestinal complications are bleeding or perforation seen in the 2nd or 3rd week of illness in 10-15% adult patients, but less frequently in children. Bleeding is due to erosion of a necrotic Peyers patch through the wall of a vessel and is usually mild but can, sometimes, be life-threatening. Perforation is a dreaded complication manifesting as acute abdomen, with high mortality unless appropriately treated.
The term severe or complicated enteric fever is used for patients presenting with neurological complications such asdelirium,coma, obtundation, stupor and/orshock and isassociatedwithmortalityrates as highas50%. Other complications of enteric fever include splenic abscesses, hepatitis, cholecystitis, pneumonia, disseminated intra vascular coagulation and other manifestations such as psychosis, ataxia or meningitis. The case fatality rate is less than 1% in appropriately treated cases but may be 1020% in inadequately treated or complicated cases.
Relapse Relapse may occur in 5-15% of treated cases, usually due to the organism with the same susceptibility as the originalattack and is relatively a milder illness. Rate of relapse is dependent on choice of drug therapy. It is higher with beta lactams such as cefixime or ceftriaxone as compared to quinolones and azithromycin.
Carrier state Although 5-10% adult patients may shed salmonella in stool following an acute attack for up to 3 months, only 1-4% excrete bacilli for more than 1 yr. These individuals are potential sources of infection for family members and contacts and for the community if they are in occupations that involve food-processing. There is no data on carrier prevalence in children and routine culture of stool following recovery from enteric fever is not recommended.
Diagnosis
Leukocyte counts may be normal to low with absolute eosinopeniaandneutrophilicpredominance.Anemia and thrombocytopenia may occur in advanced illness. There may be mild elevation of transaminases to 2-3 times normal (SGOT higher than SGPT). A high C reactive protein (CRP) helps to differentiate enteric from viral fevers especially dengue.
The gold standard for diagnosis is blood culture. The sensitivity is greatest in the first week at around 90% but drops to 40% in the 4th week. Salmonella is an easy organism to culture and use of bile broth media and automated culture systems such as BACTEC improve recovery. Sufficient blood should be collected (10 ml in adults and 5 ml in children) and a blood: media ratio of 1:5 should be maintained. Bone marrow cultures have higher yield as compared to peripheral blood cultures as Salmonella is a pathogen of the reticuloendothelial system and should be done when patients present in later stages of the illness. Stool and urine cultures are not recom mended. Antimicrobial susceptibilitytestingof the isolate is important.
The Widal test detects presence of IgG and IgM anti bodies to H(flagellar antigen) of S. enterica var typhi and paratyphi A and B, and O (somatic antigen) common to typhi and paratyphi A and B. Anti O titers are both IgG and IgM that rise and decline early, while anti Hare primarily IgG that rise and decline late in course of the disease. The conventional method of interpretation of the Widal test has been to demonstrate four-fold rise in antibody titers in two samples. A single titer of at least 1:160 for both O and His also considered positive. The sensitivity of the test is low in the first week of illness and in patients treated with prior antibiotics. Specificity is low owing to anamnestic reactions, prior vaccination, cross reactivity with other Enterobacteriaceae and subclinical infections in endemic areas. Other tests such as Tubex and Typhidot that detect IgM antibodies against typhoid have not proven superior to the Widal test.
