
Ghai Essential Pediatrics8th
.pdf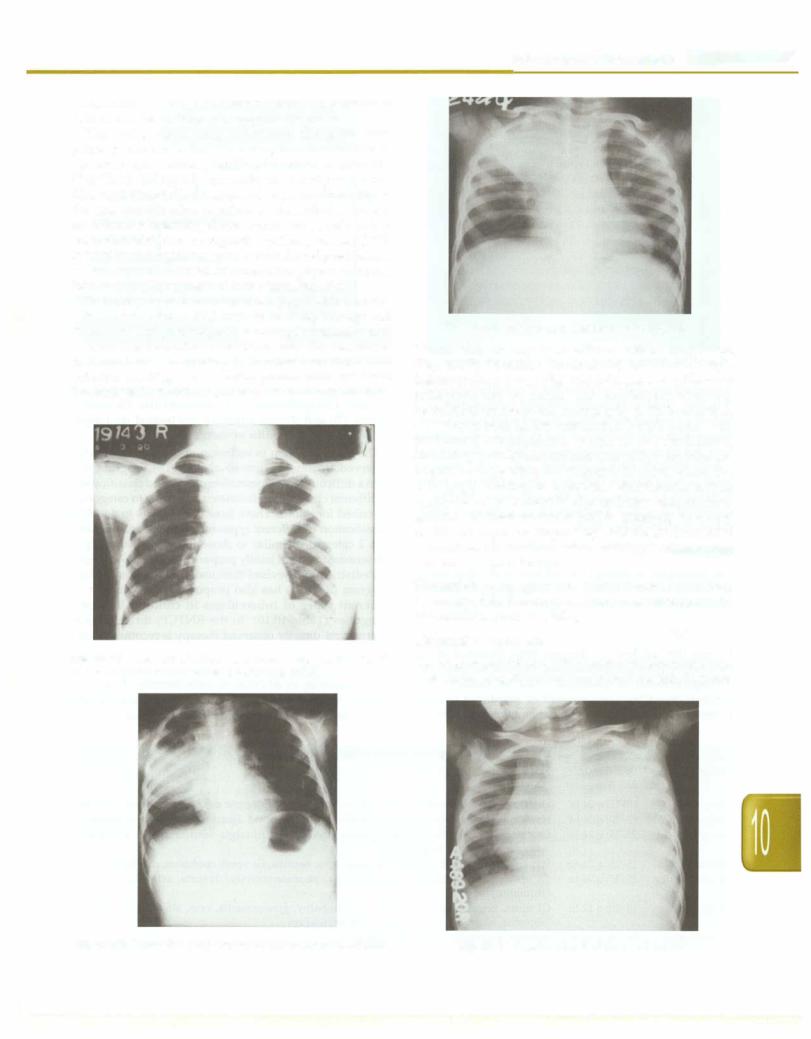
tuberculosis. In extrapulmonary tuberculosis, presence of lesions on chest radiograph supports diagnosis.
The typical chest X-ray appearance of a pulmonary primary complex is that of an airspace consolidation of variable size, usually unifocal and homogeneous (Fig. 10.16). Enlarged lymph nodes are usually seen in the hila, right paratracheal region. Adenopathy alone may be the sole manifestation of primary tuberculosis. There is no consensus regarding the most common site of involve ment. Consolidation in progressive primary disease (PPD) is usually heterogeneous, poorly marginated predilection of involvement of apical or posterior segments of upper lobe or superior segment of lower lobe (Fig. 10.17).
There may be features of collapse (Fig. 10.18). Bronchi ectasis may occur in PPD because of (i) destruction and fibrosis of lung parenchymal resulting in retraction and irreversible bronchial dilatation, and (ii) cicatricial bronchostenosis secondary to localized endobronchial infection resulting in obstructive pneumonitis and distal bronchiectasis. In children, cavitary disease is uncommon.
Fig. 10.16: X-ray film of primary pulmonary complex showing left hilar adenopathy and ill-defined parenchymal lesion
Infections and Infestations -
Fig. 10.18: Collapse consolidation of right upper lobe
Pleural effusion may occur with or without lung lesion (Fig. 10.19). In miliary tuberculosis, there are multiple lesions of size 2-5 mm (Fig. 10.20). Occasionally, the chest radiograph may be normal and lymphadenopathy may be detected on computed tomography (CT). In addition, CT features such as low attenuation lymph nodes with peripheral enhancement, lymph node calcification, branching centrilobular nodules and miliary nodules are helpful in suggesting the diagnosis in cases where the radiograph is normal or equivocal. Other features such as segmental or lobar consolidation and atelectasis are non specific. Contrast enhanced MRI is emerging as a very useful technique for diagnosing CNS tuberculosis, as it demonstrates the localized lesions, meningeal enhancement and the brainstem lesions.
Histopathology. Lymph nodes, liver and other tissues may be examined for histological evidence of tuberculosis by fine needle aspiration cytology.
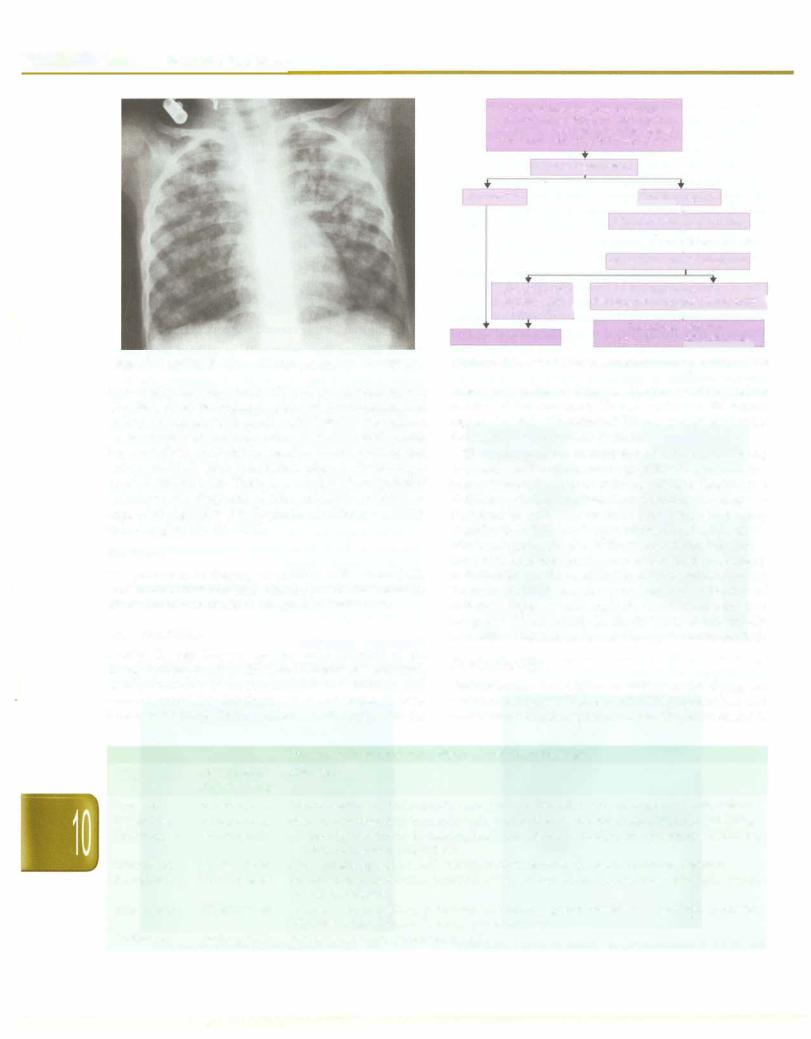
Diagnostic Algorithm
The diagnosis of tuberculosis disease in children continues to be challenging. Even in advanced nations, the diagnosis
Fig. 10.17: Progressive pulmonary disease showing consolidation |
Fig. 10.19: Massive pleural effusion on left side |
- Essential Pediatrics
Fever and/or cough for >2 weeks +/- loss of weight or no weight gain +/- history of contact with suspected or confirmed case of active tuberculosis
Sputum examination
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AFB present |
|
|
ISputum ..negative |
|||||||||
|
|
|
|
|
Oral |
antibiotics |
|
|
for 7-10 days |
||||
|
|
|
|
|
|
|
|
!Symptoms persist |
|||||
|
|
Mantoux |
|
|
Chest X-ray and Mantoux test |
||||||||
|
|
positive |
l |
Mantoux negative |
or |
||||||||
|
|
and abnormal |
Mantoux positive, chest X-ray normal] |
||||||||||
|
|
chest X-ray |
|
|
|
|
|||||||
|
|
|
Investigate |
•further for |
|
||||||||
|
|
|
|||||||||||
|
Treat for tuberculosis |
|
tuberculosis and other diagnosis |
||||||||||
|
|
|
|
||||||||||
Fig. 10.20: Miliary shadows with right paratracheal adenopathy |
Fig. 10.21: Proposed diagnostic algorithm for pulmonary tuberculosis |
||||||||||||
is most often made by combination of a positive tuberculin skin test, chest radiograph, physical examination and history of contact with adult patient with tuberculosis. Newer diagnostic methods such as PCR and serodiagnosis have not given encouraging results. Newer staining and culture methods have found their place in the manage ment of tuberculosis. There is a need to develop better techniques for diagnosis of tuberculosis in children. A suggested algorithm for diagnosis of pulmonary tuber culosis is given in Fig. 10.21.
Treatment
The principles of therapy in children with tuberculosis are similar to that of adults. The drugs used for treatment of tuberculosis in children are given in Table 10.9.
Drug Regimens
During the last few yr, changes have occurred in the therapeutic approach to childhood tuberculosis as a result of large number of treatment trials for children and concern about the development of resistance to anti tuberculosis drugs. Short-course chemotherapy, with the
treatment duration of 6 months, has become the standard practice. Extrapulmonary TB (osteoarticular TB, neuro logical TB) and disseminated TB are treated for longer durations of 9-12 months or more.
The major problem in inclusion of children in directly observed treatment short-course (DOTS) program has been a difficulty in demonstration of AFB and classification of different clinical manifestations according to categories described for adults. There have been efforts to develop classification of different types of childhood tuberculosis into 2 categories similar to those for adults. Recently a consensus statement jointly prepared by Indian Academy of Pediatrics and Revised National Tuberculosis Control Program (RNTCP) has also proposed a classification of different types of tuberculosis in children into two categories (Table 10.10). In the RNTCP, thrice weekly intermittent directly observed therapy is recommended.
Corticosteroids
Corticosteroids, in addition to antitubercular drugs, are useful in treatment of patients with CNS tuberculosis and occasionally pulmonary tuberculosis. These are useful in
|
|
Table 10.9: Doses and side effects of antitubercular drugs |
Drug |
Dose (mg/kg/ |
Side effects |
|
day; frequency) |
|
Isoniazid |
10-15; q 24 hr |
Hepatotoxicity, hypersensitivity rash, fever, peripheral or optic neuritis, psychosis, seizures |
Rifampicin |
10-20; q 24 hr |
Nausea, vomiting, hepatotoxicity, flu-like syndrome, blood dyscrasia, arthralgia, wheezing |
Streptomycin |
20-25; q 24 hr |
Ototoxicity (vestibular or hearing loss), rash, fever, arthralgia, neuromuscular blockade, |
|
|
peripheral neuritis, anaphylaxis |
Ethambutol |
15-25; q 24 hr |
Hypersensitivity (rash, fever), joint pain, optic neuritis, GI upset, confusion, dizziness |
Pyrazinamide |
25-35; q 24 hr |
GI upset, hepatotoxicity, hyperuricemia, photosensitivity, dysuria, arthralgia, fever, |
|
|
thrombocytopenia |
Ethionamide |
15-20; q 12 hr |
GI upset, hepatotoxicity, peripheral neuropathy, gynecomastia, rash, alopecia, headache, |
|
|
diplopia, blurred vision, tremors, hypothyroidism |
Cycloserine |
15-20; q 12 hr |
Seizures, psychosis, peripheral neuritis |

Infections and Infestations -
|
Table 10.10: Standardized clinical categories and clinical conditions |
|
|||
WHO categories |
Conditions in adults |
Suggested conditions |
Daily |
Intermittent (DOTS)* regimen |
|
|
|
in children |
regimen# |
Intensive phase |
Continuation phase |
Category I |
Sputum positive pulmonary TB |
Pulmonary primary complex |
2 HRZE + |
2 H3R3Z3E3 |
4 H3R3 |
|
Serious extrapulmonary disease |
Progressive primary disease |
4 HR |
|
|
|
Abdominal TB |
Tubercular lyrnphadenitis |
|
|
|
|
Osteoarticular TB |
Pleural effusion |
|
|
|
|
Genitourinary TB |
|
|
|
|
|
Neurological TB |
|
|
|
|
|
Pericardia! TB |
|
|
|
|
Category II |
Relapse |
Relapse |
2 SHRZE + 2 S3H3R3Z3E3 + |
5 H3R3E3 |
|
|
Treatment failure |
Treatment failure |
1 HRZE + |
I H3R3Z3E3 |
|
|
Return after default |
Interrupted treatment |
SHRE |
|
|
(interrupted treatment)
It is mandatory to screen all children in the household of an adult patient with sputum positive tuberculosis for evidence of tuberculosis. H isoniazid; E ethambutol; R rifampicin; S streptomycin; Z pyrazinarnide
il'fhe numerical before the letters denotes number of months for which the drug is to be given, e.g. 4 HR means 4 months of INH and rifampicin; the subscript after the letters refers to the number of doses per week. Thrice weekly regimens are used in Revised National Tuberculosis Control Program using directly observed treatment short-course (DOTS). The duration of therapy is longer in osteoarticular and neurological tuberculosis
settings where the host inflammatory reaction contributes significantly to tissue damage. Short-courses of corti costeroids are indicated in children with endobronchial tuberculosis that causes localized emphysema, segmental pulmonary lesions or respiratory distress. Some children with severe miliary tuberculosis may show dramatic improvementwithcorticosteroids if alveolocapillary block is present. While significant improvement in symptoms can occur in children with pericardia! effusion, steroids do not alter outcome of pleural effusion.The most commonly used medication is prednisolone, at doses of 1-2 mg/kg/day for 4-6 weeks.
Management of an Infant Born to Mother with Tuberculosis
Congenital tuberculosis is rare. The fetus may be infected either by hematogenously through umbilical vessels or through ingestion of infected amniotic fluid. In the former situation, there will be primary focus in liver and in latter it will be in lungs. It is difficult to findtheroute of transmis sion in a newborn with multiple focus of infection. It is difficult to differentiate between congenital and post natally acquired tuberculosis. Infants born to mothers with active tuberculosis should be screened for evidence of disease by physical examination, tuberculin test and X-ray film of chest. If physical examination and investi gations are negative for disease, the infant should receive isoniazid prophylaxis at doses of 10 mg/kg/day for 6 months. After three months, the patients should be examined for evidence of infection and a repeat tuberculin test is done. If tuberculin test is negative, the infant can be immunized with BCG and INH can be stopped. If tuberculin test is positive but the infant is asymptomatic, INH prophylaxis is continued for another 3 months. Infants with congenital tuberculosis should betreated with four drugs (isoniazid, rifampicin, pyrazinamide,
ethambutol) in the intensive phase followed bytwo drugs (isoniazid, rifampicin) during maintenance phase for next 4 months.
Management of a Child in Contact with an Adult with Tuberculosis
Nearly one-third of children (aged less than 5 yr) in contact with adults with active tuberculosis disease may have evidence of tuberculosis infection. The infection is more commonly associatedwith youngerage, severe malnutrition, absence of BCG vaccination, contact with an adult who is sputum positive and exposure to environmental tobacco smoke. Children below 5 yr of age in contact with adult patientswithpulmonarytuberculosisshouldbetreatedwith INH prophylaxis at a dose of 10 mg/kg/day for 6 months.
Monitoring of Therapy
Response to treatment can be judged using the following criteria: clinical, radiological, bacteriological, and labo ratory test.
Clinical Criteria
Clinicalimprovementassessestheresponseoftherapy.The child should be seen every 2-4 weeks initially, then every 4--8 weeks. On each visit, improvement in fever, cough, appetite and subjective wellbeing is assessed. The child is examined for weight gain and improvement in chest findings. Compliance is assessed by talking to parents and checking medications on each visit. Majority of children show improvement in symptoms within a few weeks.
In the presence of poor response or worsening of symp toms or signs, the initial basis of diagnosis is reviewed, especially, if there are no problems with compliance. Assessment for possibility of drug resistant tuberculosis should be made. After the treatment is over, followup every 3-6 months for next 2 yr is desirable.

__E_s_s_e _n_t.ia•l•P•e•d-ia.t .ri_cs __________________________________
Radiological Criteria
Clinical improvement precedes radiological clearance of lesion on chest X-ray films. The first chest X-ray during therapy should be done after 8 weeks, i.e. at the end of intensive phase. In patients who show increase or little change in radiological features coupled with delayed clinical response, prolongation of intensive phase by one month is suggested. Further films are taken after 4 weeks andchild,ifbetter, shouldbe shiftedto continuation phase; else the child is investigated for failure of treatment and drug resistance. The degree of radiological clearance can be graded as (i) complete clearance, (ii) moderate to signi ficantclearance ( Yi-% clearance), and (iii) mildclearance (decrease in size)or (iv)no clearance orappearanceofnew lesion. One should not attempt to treat till complete radiological clearance, improvement in the X-ray may continue to occur even afterstoppage of therapy.
Microbiological Criteria
Most childhoodpulmonary tuberculosis is paucibacillary. In children, where isolation ofM. tuberculosis was possible at the time of diagnosis, every effort should be made to document disappearance of bacilli during therapy.
Drug Resistant Tuberculosis
Children in the following categories are at risk of drug resistant tuberculosis: Contact with adult patients who have proven drug resistant tuberculosis; unsatisfactory response to antituberculosis drugs and children who initially respond to antituberculosis drugs and then show deterioration. Appearance of new lymph nodes on treatment, persistence of shadow or isolated non clearance of X-ray film of the chest should not be considered an indicator of drug resistant tuberculosis.
It is important that clinicians should neither miss nor make over diagnosis of drug resistant tuberculosis. Prob lems with over diagnosis and its treatment are many fold as second line drugs are less effective, have more side effects and are expensive. A physician can suspect drug resistant tuberculosis on basis of criteria given above, but before making a diagnosis all attempts should be made to demonstrate AFB from appropriate samples and get its culture and sensitivity.
Suggested Reading
Chauhan LS, Arora VK. Management of pediatric tuberculosis under the Revised National Tuberculosis Control Program (RNTCP). Indian Pediatrics 2004;41:901-5
Kumar A, Gupta D, Nagaraja SB, et al. Updated National guidelines for pediatric tuberculosis in India, 2012. Indian Pediatr 2013;50:301-06.
Seth V, Kabra SK (Eds). Essentials of Tuberculosis in Children, 3rd edn. New Delhi; Jaypee Publishers, 2010
Mycobacteria Other than Tuberculosis
Atypical mycobacteria or nontuberculous mycobacteria or mycobacteria other than tuberculosis (MOTT) are
environmental pathogens that are being increasingly recognized as cause of human disease. More than 125 NTM species have been recognized till date and new species arebeing identified constantly.NTMareclassified depending on the rapidity of growth in media as rapid growers which grow within 7 days and slow growers as those which take longer to grow. Acquisition of NTM is through contact with the environment and human to human or animal to human transmission almost never occurs. Though asymptomatic infection can occur, there is no recognized latent infection or reactivation disease. NTM infections may occur in previously healthy or those with underlying immunodeficiency like HIV. The main clinical syndromes recognized with NTM infection are pulmonary, disseminated, lymph node disease and skin and soft tissue infections.
Lymphaticdiseaseis the most common manifestation of NTM disease in children. It usually presents as painless cervical adenitis in children aged 1-5 yr with no systemic symptoms. The main differential is tuberculous lyrnpha denitis. The definitive diagnosis is by culture; the usual causative organisms are MAC (Mycobacterium avium intracellulare), M. scrofulaceum and M. haemophilum.
Treatment is complete excision of the lymph nodes. Pulmonary disease due to NTM usually occurs in adults with underlying pulmonary problems and is usually due to MAC, M. kansasii and M. abscessus. Disseminated NTM disease is seenmainly in adults and less commonly children with advanced HIV infection is usually due to MAC and presentsasfever,weightloss,nightsweats,abdominalpain, diarrhea and anemia. Blood cultures are positive. Skin and soft tissue infections are usually a consequence of trauma or health care procedures. These are usually due to M. abscessus, M. chelonae, M. fortuitum, M. ulcerans and M. marinum.Theyhavebeenimplicatedininfections following injections, central lines, peritoneal dialysis catheters, laparoscopy, liposuction, cosmetic procedures, implants and prosthesis, LASIK and surgery. These mycobacteria species are usually hardy, resist the commonly used disinfectants andhence occur when surgical equipment is rinsed withtapwater andinadequately disinfected. Usually these infections present as indolent abscesses that do not respond to the usual antibiotics.
Microbiologic diagnosis of NTM infections is possible in specialized laboratories where identification and speciation is done by advanced biochemical/molecular methods. Treatment of NTM disease depends on the causative organism and its sensitivity. MAC is usually treatedwith combination therapy consisting of a macrolide (clarithromycin/azithromycin), rifampicin,ethambutoland an aminoglycoside (only in initial stages) for around 12-18 months. Treatment ofrapidlygrowingmycobacteria group of organisms depends on the antimicrobial sensitivity and generally includes a combination of clarithromycin, quinolones, aminoglycosides (tobramycin/amikacin), cefoxitin, imipenem, minocycline and clofazirnine.

Suggested Reading
An Official ATS/IDSA Statement: Diagnosis, Treatment and
PreventionofNontuberculousMycobacterial Diseases. AmJ RespirCrit
Care Med 2007;175:367-416
Brucellosis
Brucellosis though a relatively uncommon chronic granulomatous infection is discussed here as it is often missed or misdiagnosed as tuberculosis. It occurs world wide especially in the Middle Eastern countries and has been reported from India.
Etiopathogenesis
Brucellaspeciesareintracellulargram-negativecoccobacilli. The classification of brucella species is based on its preferred hosts namely B. melitensis (sheep and goats), B. abortus (cattle), B. suis (pigs), B. canis (dogs). Brucellosis is a zoonosisandtransmissionto humans can occurthrough the consumption of infected unpasteurized milk and animal products, through direct contact with infected animal parts such as placenta, by inoculation of skin and mucous membranes, and by inhalation of infected aerosolizedparticles. The vast majorityofcases worldwide are attributed to B. melitensis.
Clinical Features
Human brucellosis can be an acute illness (<2 months), subacute illness (2-12 months) or chronic illness (>1 yr). The incubation period is usually between 7 days and 3 months. A history of exposure to animals especially drinking unpasteurized milk without proper boiling is present. Fever can be continuous or intermittent and persist for several months due to partial response to antibiotics. Fever is usually accompanied by profuse sweating, joint pains (particularly knee) and hepato splenomegaly and less common lymphadenopathy. In untreated cases complications such as spondylitis, osteoarthritis, meningoencephalitis, brain abscess, pneumonia and endocarditis can occur.
Diagnosis
Blood count usually reveals anemia, leukopenia and thrombocytopenia. The liver enzymes are mildly elevated (SAP and GGT more than transaminases).
The gold standard for diagnosis is blood culture, which hasgood sensitivity if done prior to antibiotic therapy and specimen incubated for 4 weeks. The sensitivity of bone marrow cultures is higher since organisms are present in large amounts in the reticuloendothelial system. Serologic diagnosis can be established by the serum agglutination test where titers above 1:160 or 1: 320 may be considered diagnostic or by IgG and IgM antibodies by ELISA.
The common differentials for brucellosis include tuber culosis, enteric fever, chronic malaria, HIV, sarcoidosis and lymphoproliferative disorders.
Infections and Infestations -
Treatment
Treatment of human brucellosis (adult or pediatric) is always with a combination of antibiotics for 6 weeks as all monotherapies and short treatments are characterized by unacceptably high relapse rates. Brucella is sensitive to many antibiotics but the regimens designed for the treatment of brucella infection should have one or more drugs that can penetrate macrophages and actin the acidic intracellular environment. The standard regime is a combinationof doxycycline with streptomycin for3 weeks followed by doxycycline and rifampicin to complete 3 more weeks. For children less than 8 yr of age combi nations containing rifampicin and TMP-SMZ with or without aminoglycosides are recommended. Regimes containing macrolides and quinolones are also being evaluated. Treatment is prolonged for endocarditis and neurobrucellosis.
Prevention
Preventive strategies includes control of disease in livestock and preventing human exposure particularly boiling milk before use.
Suggested Reading
Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis: Review. N Engl J Med 2005;352:2325-36
FUNGAL INFECTIONS
Fungi have becomeincreasinglycommoncause of disease in humans. Superficial fungal infections are detailed in the chapter on skin.
Invasive Candidiasis
Candida is a yeast like fungus with major species being
C. albicans, C. tropicalis, C. parapsilosis, C. krusei, C. glabrata.
It commonly causes superficial infections such as thrush, vaginitis, paronychia, etc. Colonization with candida at mucosal sites in patients on antibiotic therapy is also common. More seriousareinvasive infectionsthathappen in hospitalized individuals with impaired defenses. Common risk factors for invasive candidiasis include prolonged intensive care stay, broad spectrum antibiotic therapy, renalfailure, very low birthweight, corticosteroid and other immunosuppressive therapy, use of total parenteral nutrition especially intralipids, neutropenia and central venous catheters. The commonest form of invasive candidiasis is bloodstream infection and less commonly meningitis, endocarditis, osteomyelitis and septic arthritis. Clinical features are similar as bacterial sepsis and outcomes are poor if therapy is not initiated early.
Diagnosis is byfungalculturesandoccasionallybyPCR. It is important to differentiate colonization from true infection to avoid overtreatment. At the same time it is importanttohave ahighindexof suspicion sothat therapy
- Essential Pediatrics
is started early in patients with likelihood of infection. Fluconazole is the drug of choice especially because of ease of administration and availability of oral switchover. Fluconazole resistant candida are treated by amphotericin B , echinocandins and newer azoles like voriconazole.
Aspergillosis
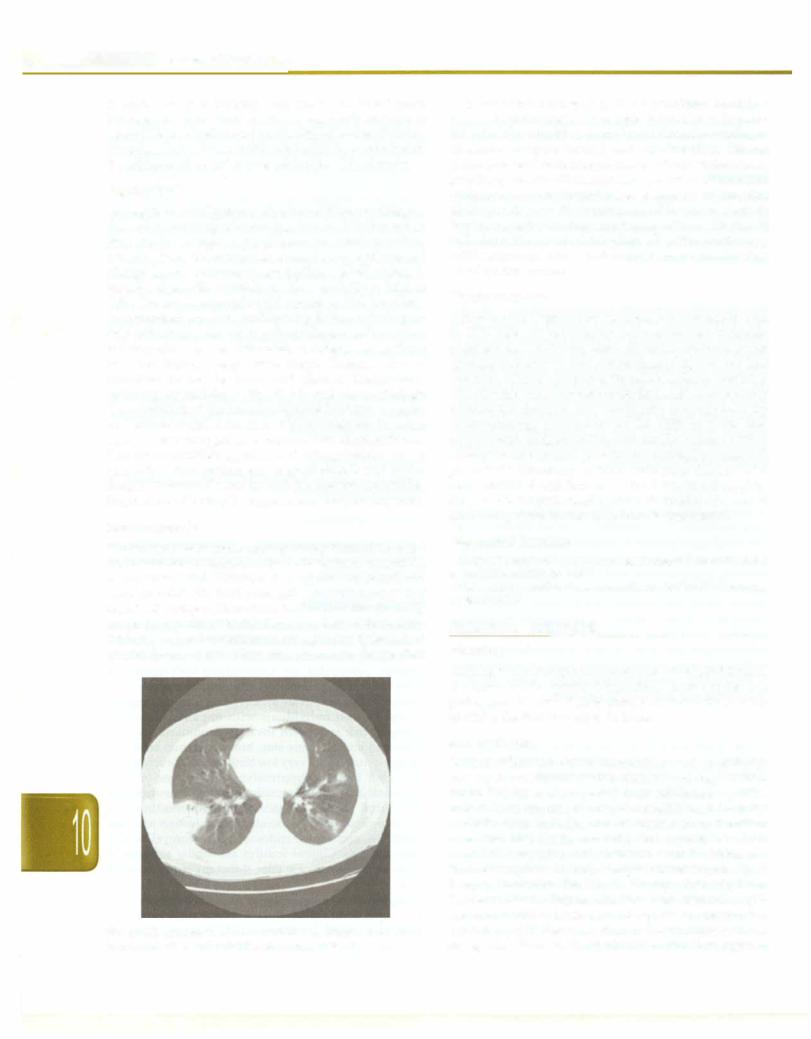
Aspergillusisaubiquitously distributedfilamentedfungus; the two common species causing human infection are A. fumigatusandA. niger. Aspergillus causes certainnon-inva sive infections like otomycosis, sinusitis. aspergilloma and allergic bronchopulmonary aspergillosis. More sinister is invasive aspergillosis which can have mortality as high as 50%. Invasive aspergillosis occurs in the immuno compromised; common predisposing factors include pati ents with cancer undergoing chemotherapy and resultant neutropenia, stem cell transplant recipients and patients on other immunosuppressive drugs. Common sites of involvement are the lungs and sinuses. Diagnosis is primarily by radiology (Fig. 10.22) and histopathologic demonstration of the invasive hyphae in biopsy samples and culture. Serial estimation of galactomannan in serum samples has emerged as a noninvasive diagnostic test. Treatment should be aggressive. Thedrugof choice is vori conazole. Other options are amphotericin B and caspo fungin. Fluconazole has no activity against aspergillus. Surgical resection may berequired in nonrespondingcases.
Mucormycosis
Mucormycosis or more appropriately termed as zygo mycosis refers to infectionwiththe filamentedfungiofthe genus Mucor and Rhizopus. The hyphae are broad and aseptate unlike those of Aspergillus that are narrow and septate.Zygomycosisisaninvasiveinfectionthatprimarily occurs in patients with risk factors such as diabetic keto acidosis, cancer chemotherapy, transplant recipients, iron overload and receipt of irnmunosuppressive drugs. Sites
Fig. 10.22: Multiple air space opacities with irregular borders and internal cavitation suggesting invasive aspergillosis
of involvement are mainly the nasal sinuses and less commonlypulmonary,gastrointestinalandskin/softtissue. Infection can sometimes occur due to direct inoculation in traumatic/surgical wounds and injection sites. Clinical featuresdependonthesiteinvolved;inthenasalformpain, swelling, bloody discharge and presence of blackish eschars on nasal examination are common. Confirmation of diagnosis is by demonstration of the characteristic hyphae on histopathologyandfungal cultures. Treatment includesradicalsurgicaldebridement,antifungal therapy with amphotericin B and correction of underlying predisposing factors.
Cryptococcosis
Infection with Cryptococcus neoformans is commonly seen in HIV infected individuals with advanced immuno suppression. The disease often affects the central nervous system; pulmonary and disseminated forms are less common. Clinical symptoms include headache, vomiting, alteredsensorium, signs of meningism and less commonly neurologic deficits. The diagnosis is confirmed by demonstrating cryptococci in the CSF by India ink, cryptococcal antigen testing and finally culture. CSF is usually under increased pressureandhashighprotein with pleocytosis. Treatment includes antifungal therapy with amphotericin B and flucytosine for 2 weeks followed by fluconazole for prolonged periods. Reduction of elevated pressure by serial lumbar punctures is also crucial.
Suggested Reading
Smith PB, Steinbach WJ, Benjamin DK. Neonatal candidiasis. Infect Dis Clin N Arn 2005;19:603-15
Steinbach WJ. Antifungal agents in children. Pediatr Clin North Arn 2005;52:895-915
PROTOZOAL INFECTIONS
----------------
Malaria
Malaria, the most important protozoa! disease in humans, is caused by the genus Plasmodium. Four species are pathogenic, P. vivax, P.Jalciparum, P. malariae and P. ovale, of which the first two occur in India.
Epidemiology
Malaria afflicts 200-300 million patients each yr globally, causing about 650,000 deaths, chiefly in young children. While 70% cases are reported from sub-Saharan Africa, malaria is an important cause of morbidity and mortality in South Asia. In India, malaria causes about 2 million cases and 1000 deaths annually, the majority of which occur in association with infection with P. Jalciparum. Endemic regions include Orissa, Chhattisgarh, West Bengal, Karnataka, Jharkhand, Madhya Pradesh, Uttar Pradesh, Assam, Gujarat and Rajasthan. Malaria is also common in urban areas, particularly due to construction activities, population migration and inappropriate water storage anddisposal. The sixth Millennium Development

Goal aims for reduction in malaria mortality by 75% by 2015.
Transmission
The infectious stage of the parasite, the sporozoite, is transmitted to thehost by the bite of the female mosquito. Six species of anopheline mosquitoes are important in the transmission of the disease, namely Anopheles culicifacies
(rural), A. fluvitalis, A. stephensi (urban), A. minimus, A. philippinesis andA. sundaicus. The parasites develop in the vector's body and make it infectious if the mosquito is susceptible, feeds on human blood and lives for at least 10-12 days after an infective blood meal. Mosquitoes usually breed in edges of streams, water tanks, pits, cisterns and overhead tanks. A. stephensi breed in wells, cisterns, fountains and overhead tanks, A. fluviatilis in movingwaterandA. sundaicusinbrackishwater. Breeding sites such as burrowed pits, pools, ponds, marshy areas and unregulated irrigation channels are conducive to mosquito breeding and spread of malaria. Mosquitoes thrive best in temperature between 20 and 30°C, relative humidity 60% and in areas with good rainfall. The peak transmission season of malaria is between July and November.
Life Cycle of the Parasite
Hepatic or tissue phase in human host (Exoerythrocytic schizogony). The trophozoites injected by an infectious mosquito invade hepatocytes and reticuloendothelial tissues. In thehepatocyte,each parasite replicatesto form 2000 to 15000 merozoites in case of P. vivax infection and 40000 merozoites for P. Jalciparum. Merozoites released from hepatocytes invade red cells. This first hepatic phase is asymptomatic and constitutes the incubation period, lasting about 10 days.
En;throcytic schizogony. In erythrocytes, parasites develop into ring forms, mature trophozoites and then multi nucleated schizonts, which rupture and release more merozoites. Repeated cycles of erythrocyte invasion and ruptureleadto chills, fever,headache,fatigue,nonspecific symptoms and with severe malaria, signs of organ dysfunction.
Manifestations of severe malaria, including cerebral malaria, noncardiogenic pulmonary edema and renal failure are caused by high concentrations of P. Jalciparum infected erythrocytes in the microvasculature. Since mature P.Jalciparum organisms inthe erythrocytes adhere to endothelial cells, only ring forms circulate (except in severe infections) and levels of peripheral parasitemiamay be low despite substantial infection.
Gametocyticphase.After several stages ofschizogony,some merozoites are converted to gametocytes which are taken up by mosquitoes. These do not cause symptoms but are responsible for transmission of malaria.
Infections and Infestations -
Exoerythrocytic phase. Merozoites of P. vivax, but not those of P.Jalciparum, may go into a dormant stage (hypnozoite) in the liver and cause relapses by invading the blood stream weeks or even yr later. This intermittent release of schizonts in case of P. vivax and P. ovale may last for 2-3 yr and for P. malariae for 10-20 yr.
Mosquito. The gametocytes ingested by the mosquito multiply in the stomach (sporogonic cycle). Fertilization of female gametes generates motile and elongatedzygotes (ookinetes)that invade the midgut to develop into oocysts (resting stage), which later grow and rupture to release sporozoites. These reach the mosquitosalivary glands and may be inoculated in a new human host.
Immunity Against Malaria
Epidemiologic observations suggest that patients with sickle cell trait, thalassemia and glucose-6-phosphate dehydrogenase deficiency are relatively immune to mala ria. Homozygotes of sickle cell disease are not protected from malaria but heterozygotes are immune. Variations in HLA frequency may also determine the prevalence of malaria.
Clinical Patterns of Malaria
The clinical manifestations andseverityof malariadepend on the species of the parasite and endemicity of disease. Most cases of severe or complicated malaria are due to P. Jalciparum; recently there is an increasing number of reports due to severe disease byP. vivax. In highly endemic areas with "stable malaria" such as sub-Saharan Africa, children below 5 yr are most affected with severe anemia and cerebral malaria being prominent manifestations. In areas of lower endemicity all ages including children and young adults are affected.
The incubation period of malaria varies between 9 and 30 days, the least for P. Jalciparum and longest for P. malariae infections. The onset of the disease is not as characteristic as is believed, especially in infections with P.falciparum.The onsetof the disease is sudden with fever, headache, loss of appetite, lassitude and pain in the limbs. The fever may becontinuousor remittent for several days before itbecomesclassically intermittent. Theillness,then, is characterized by a cold stage (chills and rigors with headache, nausea, malaise and anorexia); hot stage (dry flushed skin, rapid respiration and marked thirst); and sweating stage (temperature falls by crisis). The classic intermittent fever is not usually seen in children.
On basis of severity malaria is classified as'complicated or severe' and uncomplicated malariawhich has treatment and prognostic implications. Severe malaria can affect virtually every organ system and has a mortality rate of approximately 20%. The criteria for severe malaria are listed below. Any of the criteria if present with asexual parasitemia with P.Jalciparum or P. vivax classifies malaria as severe and should be treated as such.

- Essential Pediatrics
Features of Severe Malaria
•Impaired consciousness or unrousable coma
•Prostration, i.e. generalized weakness so that the patient is unable walk or sit up without assistance
•Failure to feed
•Multiple convulsions -more than two episodes in 24 hr
•Deep breathing,respiratory distress (acidotic breathing)
•Circulatory collapse or shock, systolic blood pressure <70 mm Hg in adults and <50 mm Hg in children
•Clinical jaundice plus evidence of other vital organ dysfunction
•Hemoglobinuria
•Abnormal spontaneous bleeding
•Pulmonary edema (radiological)
Laboratory findings of severe malaria
•Hypoglycemia (blood glucose <2.2 mmol/1 or <40 mg/dl)
•Metabolic acidosis (plasma bicarbonate <15 mEq/1)
•Severe normocytic anemia (Hb <5 g/dl, packed cell volume <15%)
•Hemoglobinuria
•Hyperparasitemia (>2% or 100 000/µl in low intensity transmission areas or >5% or 250 000/µl in areas of high stable malaria transmission intensity)
•Hyperlactatemia (lactate >5 mmol/1)
•Renal impairment (serum creatinine >265 µmol/1).
Diagnosis
Peripheral smear The gold standard for diagnosis of malariais careful examination ofaproperlyprepared thick film. Thick smears have as sensitivity of detecting 5-10 parasites/µ!. Thin smears have a lower sensitivity of 200 parasites/µ! but enable species identification.Microscopy alsoprovides information about theparasiteload (number of infected RBC/total RBC), prognosis (mature schizonts and pigmented neutrophils indicating a poor prognosis) and tracks response to therapy (Fig. 10.23). The main drawback is need for expertise and that they are time con suming (a careful examination of 100 fields needs 20 min). Sometimes peripheral smears may be negative due to partial antimalarial treatment or sequestration of parasitized cells in deep vascular beds. Repeating smears every 6-8 hourly at least three times is recommended if the clinical suspicion for malaria is high and the initial smear is negative.
Quantitative buffy coat (QBC) test is a new method for identifying the malarial parasite in the peripheral blood. It involvesstaining of the centrifuged and compressedred cell layer with acridineorange and its examination under UV light source. It is fast, easy and claimed to be more sensitive than the traditional thick smear examination. Disadvantages include need for special equipment, cost,
Fig. 10.23: Gametocyte of Plasmodium fa/ciparum
false positives due to staining artefacts and inability to speciate the parasite. QBC has been largely supplanted by the rapid diagnostic tests detailed below.
Rapid diagnostic tests detect malaria antigens (PfHRP2/ PMA/pLDH) from asexual and/or sexual forms of the parasite as color changes on antibody coated lines on the strips. In general, these are quick and simple to use, distinguish between the major forms of human malaria, and are useful when reliable microscopy is not available. Disadvantages include cost, lower sensitivity than microscopy (detect 100-200 parasites/µ!), variation in quality from batch to batch and need for rigorous storage conditions. In addition they do not give any information about the parasite load and cannot be used to monitor response to therapy. The OptiMALtest based on detection of parasite LDH (Fig. 10.24) and tests based on plasmo dium aldolase are superior to the Parasight F test which is based on detection of HRP 2 antigen of P. Jalciparum as the latter is positive even in past infection and cannot be used to diagnose P. vivax malaria.
Polymerase chain reaction (PCR) has been found to be highly sensitive and specific for detecting all species of malaria, particularly in cases of low level parasitemia but is not availablecommerciallyandhenceoflimitedpracticalutility.
|
I |
Negative |
|
|
|
I |
I |
P vivax |
|
|
|
I |
|
P falciparum |
Fig. 10.24: OptiMAL test for rapid diagnosis of malaria. Expected reaction patterns on the OptiMAL test strip for a negative patient, a patient with P. vivax malaria, and a patient with P. falciparum malaria

Tests for disease management and assessing severity
These include blood counts and culture, PT, PTT, blood glucose,electrolytes,pH,bicarbonate,chloride andlactate; chest X-ray for respiratory distress syndrome; serum bilirubin, transaminases and creatinine; and urine hemoglobin. The WHO consensus statement recommends that a lumbar puncture should be done in a patient presenting with acute febrile encephalopathy. However, in the Indian scenario, CSF examination is not required if the smear diagnosis of faciparum malaria is unequivocal.
Differential Diagnosis
Common clinical differentials for malaria include other tropical and monsoon infections like typhoid fever, leptospirosis, dengue, viral hepatitis. Cerebral malaria should be differentiated from other causes of acute febrile encephalopathy like meningitis and encephalitis. Patients with algid malaria (those in shock) mimic meningo coccemia and gram-negative shock.
Treatment of Uncomplicated Malaria
In a setting of suspected uncomplicated malaria, establishing a lab diagnosis is a must before giving empirical therapy. This is to prevent irrational therapy and consequent drug resistance and also to avoid missing other causes of febrile illness.
Vivax malaria The drug of choice for managing vivax malaria is still chloroquine. Resistance to chloroquine has been rarely reported in India. The total dose is 25 mg/kg; first dose given as 10 mg/kg and then 5 mg/kg at 0, 6, 24 and 48 hr respectively. Fever should be brought down before giving chloroquine to reduce the risk of vomiting. Radical therapy with primaquine is indicated for vivax malariatoeliminatetheexoerythrocyticstages in liver and reduce risk of relapses. Primaquine also has schizonticidal effect and due to this effect functions as combination therapy for vivax malaria along with chloroquine. The G6PD level should be checked prior to administering primaquine. The dose of primaquine is 0.25-0.3 mg/kg/ day for 14 days. The risk of relapse is reduced by two thirds with primaquine. For patients who relapse despite primaquine, higher doses of 0.5-0.75 mg/kg may be used. Primaquine is contraindicated in infants, pregnant and breastfeeding women. For patients who cannot be given primaquine, relapses may be prevented by administering chloroquine as suppressive therapy in a dose of 10 mg/ kg weekly.
Quinine and pyrimethamine sulfadoxine do not have adequate activity against vivax malaria and should not be used for treatment.
Uncomplicatedfalciparum malaria Falciparummalaria should always be treated with combination therapy. Both drugs should have independent mode of action and should be effective in the area where they are used. Hence
Infections and Infestations -
pyrimethamine-sulfadoxine is notcombinationtherapy as both partner drugs have similar mechanism of action. Similarly if an area has resistance to pyrimethamine sulfadoxine and mefloquine then these should not be used as part of combination therapy.
Artemisinin based combination therapy (ACT) is the treatment of choice for falciparum malaria (Table 10.13). Artemisinin (qinghaosu) is the antimalarialextractisolated from Artemisia annua. Artemisinin and its derivatives (artemether, artesunate, arteether) are the most rapidly acting of all antimalarials; they also have a broad time window of antimalarial effect from ring forms to mature trophozoites (like chloroquine andunlikequininethat acts only on mature forms). Artemether lumefantrine is the most commonly used oral ACT at this time. Other drugs such as mefloquine andpyrimethamine-sulfadoxine may be used in combination with artesunate in those areas where resistance to these drugs is uncommon. Artesunate amodiaquine is not available in India at present and dihydroartemisinin piperaquine is a new combination drug that may be introduced soon.
Oral quinine with clindamycin or doxycycline (in children aged more than 8 yr) is alternative treatment for but is associated with disadvantages such as poor tolerability of oral quinine and prolonged therapy.
Chloroquine should not be used for treatment for falciparummalariaunless thereisdemonstrable sensitivity to the drug in a particular area; similarly mefloquine and pyrimethamine sulfadoxine monotherapy is not recom mended. At the end of therapy a single gametocidal dose of primaquine 0.75 mg/kg is recommended to reduce community transmission of malaria.
Uncomplicated mixed malaria It should be treated as
P. Jalciparum malaria with ACT. The preferred ACT for mixed malaria is dihydroartemisinin piperaquine, which is not available. Use of artemether lumefantrine may be associated with higher rates of recrudescence of vivax malaria. Primaquine should also be used as mentioned earlier.
Treatment of Complicated or Severe Malaria
The treatment of severe malaria is a medical emergency as it is associated with high mortality rates of up to 20%. If the suspicion of malaria is strong then treatmentshould be initiated without waiting for confirmation of diagnosis. Severe malaria whether due to falciparum or vivax should be treated similarly. Supportive care and treatment of complications are as important as antimalarial therapy.
Antimalarial therapy Treatment should be parenteral as most patients are not able to take orally and the bioavailability of oral drugs is unpredictable. The choice is between use of artemisinin based combination therapy or quinine. Results of meta analysis indicate that at present, in children in the Indian subcontinent the two drugs have equal efficacy. ACT is much easier to

E_s_s_e_n_t.ai•l•P•e•d-iat.ri.·c·s ----------- |
---------- |
- |
|
__ |
------------ |
|
|
administer with fewer side effects than quinine; it is however more expensive.
Artemisinin based therapy Parenteral formulations of artesunate, artemether and arteether are commercially available; artesunate is theagentfor which most safety and efficacy data is available. Artesunate is available as a dry powder which is reconstituted with sodium bicarbonate and given as a bolusinjection.Artemether and arteether are given by the intramuscular route. The dose of artesunate is 2.4 mg/kg and that of artemether 3.2 mg/kg given stat and then repeated after 12 hr and 24 hr and then daily. Parasite counts start declining 5-6 hr after institution of therapy with artemisinin derivatives, unlike quinine. Asexual parasitemia generally disappears after a mean time of 72 hr. Once the patient is better, treatment can be shifted to oral and a full course of oral ACT given earlier.
Artemisinin derivatives have a good safety profile. Local reactions after intramuscular administration are rare and much less frequent as compared to IM quinine. Cardiotoxic effects in the form of prolongation of the QT interval in patients being administered high dose artemether have been reported. No clinical significance of this has been noted except that artemisinins should not be combined with other cardiotoxic drugs such as quinine/halofantrine. In two human trials, use of artemether was associated with more frequent convulsions and longer recovery time from coma as compared to quinine, longterm sequelae being comparable in both the groups.
Quinine based therapy Quinine acts principally on the mature trophozoite stage of parasite development; it does not prevent sequestration or further development of formed schizonts and does not kill the pre-erythrocytic or sexual stage of Plasmodiumfalciparum.
Parenteral quinine is available asdihydrochloride salt in concentrations of 300 mg/ml. It is readily photodegraded and should be stored in brown glass ampoules in the dark. Photodegradation is insignificant over short periods (<24 hr) when quinine is dissolved in 0.9% saline or dextrose. Quinine must always be given by rate controlled intravenous infusion and never by bolus orpush injection. It is recommended to administer a loading dose of quinine i.e. 20 mg salt/kg diluted in 10 ml/kg of normal saline or dextrose over a period of 4 hr at treatment onset. The objective of loading dose is to provide therapeuticlevels as early as possible in the course of treatment without overshoot to toxic levels. The loading dose should be avoided if there is reliable evidence that the patient has received quinine/halofantrine/mefloquine in the past 24 hr (both halofantrine and mefloquine produce additive cardiac toxicity). After the loading dose, quinine should be continued at a dose of 10 mg salt/kg as infusion over 2 hr every 8 hr. Intramuscular quinine is anotheralternative for initial therapy if facilities for controlled IV quinine administration are not available. Quinine should not be given subcutaneously as this may cause skin necrosis.
The parasite counts start declining only after 24 hr, slower than artemisininderivatives and may even increase in the first 24 hr. The patient should be switched to oral quinine as soon as possible. If parenteral quinine has to be continued beyond 48 hr or if renal failure supervenes, the maintenance dose should be reduced to 5-7 mg salt/ kg to avoid quinine toxicity. Total duration of therapy is 7 days. A second drug such as doxycyclineor clindamycin should be added. A single dose of primaquine 0.75 mg/ kg is recommended on completion of quinine therapy to eradicate gametocytes and prevent transmission.
The only contraindication to use of quinine is reliable evidence of severe quinine allergy and G6PD deficiency. Thrombocytopenia, jaundice, renal failure, hypotension are not contraindications for quinine administration. Minor side effects including tinnitus, deafness, headache, nausea and visual disturbances termed as "cinchonism" are common with quinine therapy in conscious patients but do not warrant dose reduction. They however reduce compliance with the 7-day treatment regimen. Serious side effects with parenteral quinine are rare if it is administered properly. Quinine produces prolongation of the QTc interval in the ECG. Routine ECG monitoring during quinine infusion is not required if there is no evidence of preexisting heart disease. Quinine is known to produce hypoglycemia through stimulatory action on the pan creatic beta cells. Quinine can cause marked intravascular hemolysis or black water fever and in this setting change of therapy to artemisinin derivatives may be required. Quinine can rarely cause immune mediated thrombo cytopenia and this must be suspected if platelet counts fail to recover with clinical improvement.
Supportive Therapy in Severe Malaria
•Admit to intensive care if available
•Rapid clinical assessment with respect to level of consciousness, blood pressure, pallor, rate and depth of respiration, hydration status. Fundus should be examined. The patient should be weighed
•Relevant investigations are sent
•Parenteral antimalarial therapy should be started empirically awaiting confirmation of diagnosis. Doses should be calculated as mg/kg body weight. Doses of salt and base should not be confused (quinine is prescribed as salt)
•If blood glucose cannot be determined or if hypoglycemia documented, glucose should be given
•Good nursing care especially if patient is unconscious
•Oxygen therapy and other respiratory support if required
•Appropriate fluid therapy to avoid under/overhydra tion. One of the most important points is not to ascribe deep/rapid breathingin achild with severemalaria and anemia to congestive heart failure. In most of these children it has been demonstrated that rapid breathing
