
Ghai Essential Pediatrics8th
.pdf
severe antibody-enhanced infection than viruses in other geographic area. Serotype cross-reactive antibodies generated from previous primary infection with a particular dengue viral serotype are not highly specific for the other serotypes involved in secondary infections. Hence, they bind to the virions but do not neutralize them, and instead increase their uptake by cells, which express Fe. receptors on their surfaces, like tissue dendritic cells, monocytes and macrophages. Such antibody-coated virions are taken up more rapidly than uncoated virus particles and this leads to enhanced antigen presentation by the infected dendritic cells to the T cells, leading to the more rapidactivationand proliferationof memory T cells.
The cytokines produced by the activated T cells have several important effects that lead to the pathogenesis of the severe dengue infections (DHF/DSS). Cytokines are alsoimplicatedinthepathogenesis ofvascularcompromise and hemorrhage in dengue virus infection. Endothelial cell dysfunction in dengue virus infection manifests as diffuse increase in capillary permeability, which is responsible for the microvascular leakage, hemo concentration and circulatory insufficiency. The transient nature of plasma leakage suggests that it could be mediated by a soluble mediator.
Dengue viral infection is commonly associated with thrombocytopenia, the cause of which is molecular mimicry between dengue virus proteins and endogenous self proteins. There is generation of antibodies against dengue virus proteins (especially NSl), which cross-react with platelet surface proteins and thus cause thrombo cytopenia. There is activation of blood clotting and fibrinolytic pathways. Mild disseminated intravascular coagulation, liver injury and thrombocytopenia together contribute to hemorrhagic tendency. Central nervous systeminvolvement alsohasbeen identified and has been attributed to direct neurotropic effect of dengue virus.
Pathology
There are usually no gross or microscopiclesions that may account for death, except when massivegastrointestinal or intracranial bleeding causes death. Presence of viruses in tissues mainly leads to hemodynamic alterations with generalized vascular congestionand increased permeability, and mast cell recruitmentin lungs. These findingshave also been seen in animal models. Variable hepatic involvement hasbeenreported-diffusehepatitiswithmidzonalnecrosis and steatosis, focal areas of necrosis and normal histology in some children. Dengue virus antigen can be detected usingirnrnunohistochemistry in hepatocytes from necrotic areas. Absence of recruitment of polymorphonuclear cells and lymphocytes has been observed in the liver lesions of patients who died from DHF.
Clinical Manifestations
Dengue infection has varying clinical presentations and often with unpredictable clinical evolution and outcome.
Infections and Infestations -
Incubation period is 4-10 days. Most infections are subclinical. Infants and young children may present with anundifferentiatedfebrile illness. The classicpresentation of dengue fever is usually seen in older children, adoles cents and adults and can be described under three phases.
Febrile phase. It is characterized by sudden onset high grade fever that may last for 2-7 days. There may be facial flushing, skin erythema, generalized bodyache, myalgia, arthralgia, headache, anorexia, nausea and vomiting. Occasionally, child mayhavesorethroat, injected pharynx and conjunctiva! injection. A positive tourniquet test may be seen in some patients. Minor hemorrhagic mani festations: petechiae and mucosal bleeding (e.g. nose and gums) may be seen in some patients. Liver may be enlarged and tender from 2-5 days and indicates risk for development of severe illness. There is progressive decrease in total white cell count and platelet count.
Critical phase. This phase is between 3 and 7 days of onset of fever when defervescence sets in. Child may develop bleeding and shock with fall in platelet count and increase in packed cell volume. Some children may develop organ dysfunction such as severe hepatitis, encephalitis or myocarditis and/or severe bleeding (may also develop without obvious plasma leakage or shock).
Recovery phase. After 24-48 hr in critical phase, a gradual reabsorption of extravascular compartment fluid takes place in 48-72 hr. General wellbeing improves, appetite returns, gastrointestinal symptoms abate, hemodynamic status stabilizes and diuresis ensues. Some patients may have a rash of "isles of white in the sea of red". Some may experience generalized pruritus, bradycardia and electrocardiographic changes; respiratory distress may occur due to pulmonary edema. The packed cell volume stabilizes or may be lower due to the dilution; leukocyte count starts to rise soon after defervescence, while recovery of platelet count takes longer.
Differential Diagnosis
Differential diagnosis for dengue infection includes other hemorrhagic fevers, influenza, malaria, enteric fever, leptospirosis, less commonly meningococcemia and rickettsial infections. Malaria, leptospirosis, flu and enteric fever may also be coinfected with dengue. Wide spread chikungunya virus infections have occurred in various parts of India and South-East Asia. Its clinical mani festations are similar to dengue. However, fever is of shorterduration,thrombocytopenia andbleedingare less. Other clinical features that are more common in chikun gunya are skin eruptions, mucosal lesions, polyarthralgia and encephalopathy. Sincedengueas well as chikungunya infections are endemic in most parts of India, both infections may occur together.

___E_s_s_e_n_t.ia_r_P_e _d.ia.ritcs __________________________________
The following clinical and laboratory features suggest presence of severe dengue infection:
Clinical criteria. Acute onset high-grade fever,hemorrhagic manifestations (at least a positive tourniquet test), tender hepatomegaly, effusion in body cavities and or shock.
Laboratory criteria. Thrombocytopenia (1,00,000 cells per cubic mm or less or less than 1-2 platelets per oil immersion field), rising hematocrit.
Laboratory Investigations
During the course of illness, children with severe dengue infectionshowincreasingpacked cellvolume,lowplatelet count and decreasing leukocyte count with increasing lymphocytes. A lowleukocytecountin achildwithfebrile illness during the endemic season suggests possible dengue infection. However, malaria and typhoid/ paratyphoid may also present with low leukocyte count.
Serum chemistry may show decrease in total protein and albumin, which is more marked in patients with shock. Levels of transaminases are raised. A higher increase in SGOT than SGPT suggests a possibility of dengue infection rather than other viral infections. In severe cases there may be hyponatremia, and acidosis along with increase in urea and creatinine.
X-ray film of the chest or ultrasound examination may show varying degrees of pleural effusion, commonly on the right side, occasionally bilateral. Ultrasonography of abdomen may show ascites and enlarged gallbladder due to wall edema.
Confirmation of diagnosis ofdengue maybe established by following:
i.Direct methods, including (a) virus isolation by culture; (b)genomedetection byPCR; (c) NSl antigen detection
ii.Indirect methods, including (a) IgM detection; and
(b) IgG detection
Virus isolation or PCR requires the sample to be obtained within the first 5 days of fever, is technically demanding, not universally available, expensive and hence of limited practicaluse. NSl antigen is ahighly conservedglycoprotein of dengue virus and secreted during the initial phase of illness. It disappears as the antibodies appear and hence declines as illness advances and in secondary dengue infections. The specificity is near 100% and sensitivity in thefirstfourdays of illness is 90% in primary dengue and 70% in secondary dengue infection.
Antibody determination needs careful interpretation. Following primary dengue infection, 80% of patients will have detectable IgM antibodies by day 5 and 99% by day 10. IgM antibodies peak by day 14 and are undetectable by two to three months. IgG antibodies rise later, peak to levels lower than IgM, decline slowly and remain detectable at low levels for life. Therefore, diagnosis of primary dengue infection is based on elevation of IgM.
Management
Patients with dengue infection can be classified as asymptomatic, or symptomatic as follows:
i.Undifferentiated fever
ii.Dengue without warning signs
iii.Dengue with warning signs
iv.Severe dengue infection.
Undifferentiated fever. Patients may have nonspecific symptoms. Treatments consist of paracetamol for fever and regular monitoring for development of any compli cations.
Dengue infectionwithout warning signs. Patients with fever, bodyaches, rashes or minor bleeding may be treated symptomatically. Fever and bodyaches are best treated with paracetamol. Salicylates and other nonsteroidal anti inflammatory drugs should be avoided as these may predisposetomucosalbleeds. Childshouldbeencouraged to drink plenty of fluids. There is no specific therapy. In epidemic situations the primary care physician/health care worker shouldmonitor the patient for warning signs (given below) along with hematocrit and platelet counts if possible.
Dengue with warning signs. Children with suspected dengue infection who have any of the following needs hospitalization: (i) abdominal pain or tenderness; (ii) persistent vomiting; (iii) clinical fluid accumulation; (iv) mucosal bleed; (v) lethargy, restlessness; (vi) liver enlargment >2 cm; and (vii) laboratory features like increase in packed cell volume (hematocrit) with concurrent with rapid decrease in platelet count.
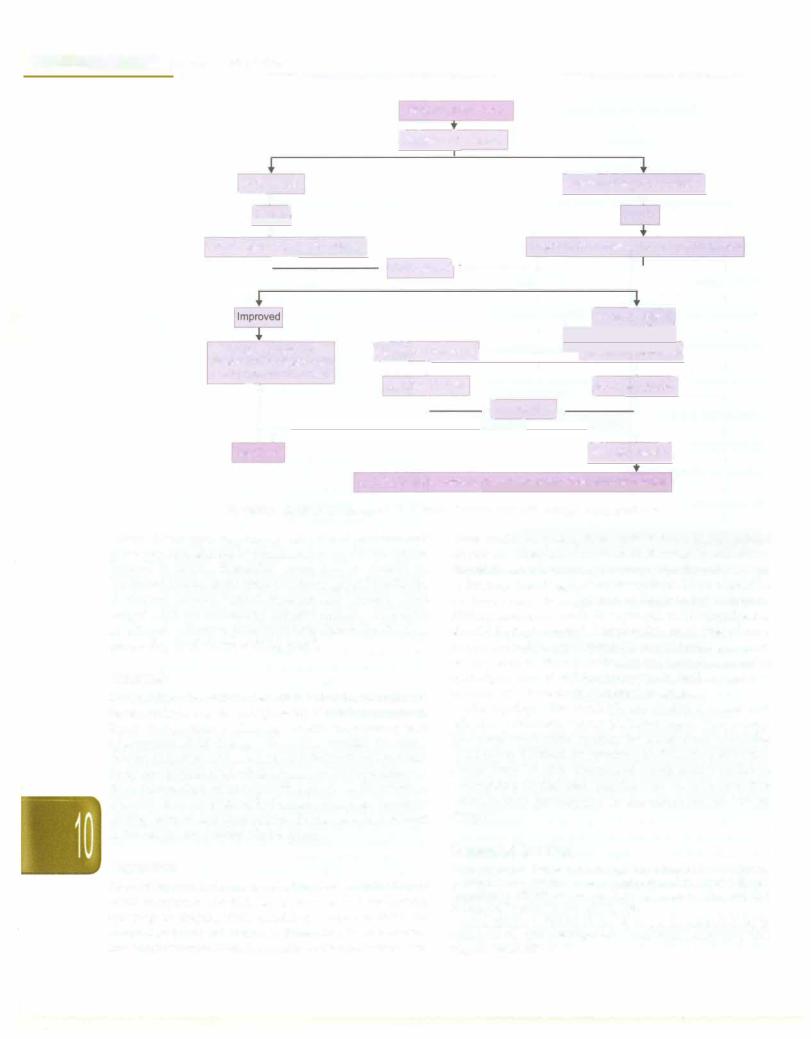
These patients require admission to the hospital and need intravenous fluids, preferably crystalloids. All children without hypotension should be given Ringer lactate or normalsaline infusion at a rate of 7 ml/kg over one hour. After one hour if the hematocrit has decreased and vital parameters are improving; fluid infusion rate should be decreased to 5 ml/kg over next hour and to 3 ml/kg/hr for 24-48 hr with frequent monitoring of hematocritandvitalparameters. When the patient is stable as indicated by normal blood pressure, good oral intake and urine output, the child can be discharged (Fig. 10.9).
If at one hour, the hematocrit is rising and vital parameters donot show improvement, fluid infusion rate is increased to 10 ml/kg over next hour. In case of no further improvement, fluid infusion rate is further increased to 15 ml/kg over next hour (third hr). If no improvement is observed in vital parameters and hematocrit at the end of 3 hr; colloids or plasma infusion in doses of 10 ml/kg is administered. Once the hematocrit and vital parameters are stable the infusion rate is gradually reduced and discontinued over 24-48 hr.
Severedengue. Children presenting/developing any of the following are included:

Dengue fever •with risk factors
|
|
|
[ Hospitalize |
|
|
|
|
||||||||
|
|
|
Ringer lactate |
•(RL) 7 ml/kg/hr |
|
|
|
|
|||||||
|
Assessment at one hr;•vitals and hematocrit |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
No improvement |
|
|
|
|
Improvement |
|
|
|
|
|||||
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
i |
|
|
|
|
||
|
RL 10 ml/kg/hr |
|
|
|
|
|
|||||||||
|
|
|
|
|
|
• |
|
|
|
|
|||||
|
|
|
|
|
RL 5 ml/kg/hr |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
Further improvement |
|||||||
|
Assessment at 2 hr |
|
|
|
|
||||||||||
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|||
|
No improvement |
|
|
|
|
j |
|||||||||
|
|
|
|
|
RL 3 ml/kg/hr |
||||||||||
|
i=- |
|
|
|
|
+ |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
--l===---- |
|||||||
|
RL 15 ml/kg/hr |
|
|
|
|
ntinue IV fluids unlil1 |
|||||||||
|
|
|
|
fstable for 24 hr |
|
J |
|||||||||
|
Assessment at 3 hr |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
Discharge when stable for 24-48 |
hr |
|||||||||||
|
- |
|
|
|
|||||||||||
|
No improvement |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
f-- |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
1 Colloids 10 ml/k - -- |
|
|
|
|
|||||||||||
|
|
..- |
|
|
|
|
|
|
|
|
|
|
|
|
|
rNo improve |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||||
I Look for anemia, acidosis and myocardial dysfunction: |
|
|
|
|
|||||||||||
|
treat accordingly |
|
|
|
|
|
|
|
|
|
|
|
|
||
Fig. 10.9: Management algorithm for dengue fever with risk factors
•Severe plasma leakage leading to:
-Shock
-Fluid accumulation with respiratory distress
•Severe bleeding as evaluated by clinician
•Severe organ involvement
-Liver: AST or ALT 2'.1000 IU/1
-CNS: Impaired consciousness
-Heart and other organs
Children classified as severe dengue should be hospitalized (preferably in Pediatric Intensive Care Unit) andtreated with normal saline or lactated Ringer solution; 10-20 ml/kg is infused over one hr or given as a bolus if blood pressure is unrecordable (earlier known as dengue shock syndrome DSS IV). In critically sick children it is preferable to establish two IV lines, one for administration of normal saline and other for infusing 5% dextrose and potassium. If there is no improvement in vital parameters and the hematocrit is rising; colloid 10 ml/kg is infused rapidly. Alternatively, if hematocrit is falling without any improvement in vital parameters; blood transfusion should be given with the presumption that lack of improvement isdueto occult bloodloss (Fig. 10.10). Once improvement starts then fluid infusion rate is gradually
Infections and Infestations -
decreased. In addition to fluid management, oxygen should be administered to all patients with shock.
Management of bleeding
a.Petechial spots or mild mucosa/ bleed but hemodynamically stable. Such patients needs supportive care including bed rest, maintenance of hydration and monitoring. Avoid IM injections. There is no role of prophylactic platelet rich plasma (PRP) infusions even with severe thrombocytopenia. Avoid any procedures predispos ing to mucosal trauma.
b.Severe bleeding and hemodynamic instability, excessive mucosa/ bleed. These patients should be treated with blood transfusion and monitoring. There is little evidence to supportthe practice of transfusing platelet concentrates and/or fresh-frozen plasma for severe bleeding. When bleeding cannot be managed with fresh whole blood or fresh-packed cells and there is possibilityof DIC, fresh-frozen plasma andplateletrich plasma may be considered.
Management of fluid overload
Fluid overload with stable haemodynamic status and is out of the critical phase (more than 24-48 hr of defervescence). In such patients stop intravenous fluids but continue close moni toring. If necessary, give oral or intravenous furosemide 0.1-0.5 mg/kg/dose once or twice daily, or a continuous infusion of furosemide 0.1 mg/kg/hr. Monitor serum potassium and correct the ensuing hypokalemia.
Fluid overload with stable haemodynamic status but is still within the critical phase. Reduce the intravenous fluid accordingly. Avoid diuretics during the plasma leakage phase because they may lead to intravascular volume depletion. Patients with fluid overload and hypotension with low or normal hematocrit levels may have occult hemorrhage. Further infusion of large volumes of intravenous fluids will lead to a poor outcome. Careful fresh whole blood transfusion should be initiated as soon as possible. If the patient remains in shock and the hematocrit is elevated, repeated small boluses of a colloid solution may help.
Other supportive care Other organ dysfunction (liver, kidney) shouldbemanaged.There is no therapeutic utility of corticosteroids, intravenous immunoglobulins or recombinant activated factor VIL Broad spectrum anti biotics are only indicated in case of superadded bacterial infection. Blood transfusion (20 ml/kg) is indicated when shock persists despite declining hematocrit values (which are indicative of adequate fluid replacement) due to overt or internal hemorrhage.
All children with hypotension should receive oxygen inhalation by nasal cannula, face mask or oxygen hood.
Monitoring
In view of the dramatic course of severe dengue, monitoring of the patient is crucial in thefirst fewhour of

Kabra SK, Lodha R, Singha! T. Dengue infections. In Medical Emergencies in ChjJdren, 5th ed, Singh M (Ed), New Delhi, Sagar Publications, 2012
Kabra SK,Jain Y, Singha! T, Ratageri VH. Dengue hemorrhagic fever: clirucal manifestations and management. Indian J Pediatr 1999;66: 93-101
Chikungunya
Chikungunya is an acute disease, which results in fever, arthritis and skin rash, caused by an enveloped virus capable of replicating in mosquitoes. Because of severe arthriticsymptoms, the disease is given theSwahili name of chikungunya (that which bends up). Since an outbreak of chikungunya in Tanzania in 1952, large epidemics were reported in South Africa, India (1971), South-East Asia and Philippines. Re-emergence of chikungunya disease occurred in India during 2005-06, causing 1.3 million cases in 13 states, chiefly Andhra Pradesh and Karnataka.
Epidemiology
The rural cycle of chikungunya transmission involves
Aedes africans, A. fancifer and wild primates, while the urbancycle involves A. aegypti and humans. In rural cycle (seen inAfrica) the disease is endemic with a small number of cases occurring in most year. In urban areas, the out breaks are sporadic and explosive with infection of a large population within weeks. In Asia, the virus may be main tained in urban cycle, with A. aegypti, or require reinocu lation before onset of epidemic. Outbreaks typically occur during the rainy season, associated with the population densityof themosquito vector, whichbreeds inhousehold containers and puddles with peak activity in mid-morning and late afternoon. After anepidemic, the diseasetypically vanishes for year, because a large proportion of the population is immune.
Clinical Features
The disease has a sudden onset, with an incubation period of 2-12 days. Infection is characterized by fever, headache, fatigue, nausea, vomiting, musclepain, rash and joint pain. Fever rises abruptly to 103-104°F and is accompanied by rigors. The acute phase lasts for 2-3 days. Joint pain appears suddenly and is often very severe in intensity; the arthralgia/ arthritis is polyarticular, migratory and predominantly affects the small joints of hands, wrist, ankle and feet, with less involvement of larger joints. Headache is present in 80% of cases. Photophobia and retro-orbital pain may also occur. An itchy, transient maculopapular rash appears 4-8 days later affecting the trunk and limbs. Inguinal lymph nodes may be enlarged. The joint pains may continue for many months after the illness. Fatalities are rare and associated with young age, thrombocytopenia and shock. Rarely encephalopathy may occur in infants and young children.
Infections and Infestations -
Diagnosis
Chikungunya should be suspected in patients who presents with the characteristic triad of fever, rash and arthritis. Viremia is present in most patients during the initial 2-4 days of disease and may be isolated in cell cultures. Polymerasechainreactioncan be used to confirm the infection. Virus specific IgM antibodies may be detected by capture ELISA and hemagglutination inhibition assays by 5-7 days of illness.
Treatment
Nospecifictreatment is available. Symptomatic treatment in the form of rest, fluids and ibuprofen, naproxen, acetaminophen, or paracetamol may relieve symptoms. Aspirin should be avoided during acute phase of illness.
Prevention and Control
Strategies for prevention and control include breaking the transmission cycle of A. aegypti and by holding the mosquito population at extremely low levels. A live attenuated vaccine that induces longterm production of neutralizing antibodies, is being examined.
Suggested Reading
Griffin DE. Alphaviruses. In: Krupe DM, Howley PM. Fields Virology, 5th edn. Philadelphla: Lippincott Williams & Wilkins 2007:1047-8
Guidelines on clirucalmanagementof chikungunyafever. WHO2008 Sebastian MR, LodhaR, KabraSK. Chikungunyainfection inchildren.
Indian J Pediatrics 2009;76:185-9
HUMAN IMMUNODEFICIENCY VIRUS (HIV)
HIV infection has become an important contributor to childhood morbidity and mortality, especially in many developing countries. The World Health Organization (WHO) estimated that 34 millionpersonsworldwide were living with HIVinfectionat the end of 2011; 2.5 million of these were childrenunder 15 yr of age. More than 90% of HIV-infected individuals live in developing nations.Sub SaharanAfrica accounts fornearly90% oftheworld'stotal population of HIV-infected children. India and Thailand dominate the epidemic in South-East Asia, with more recent expansion into Vietnam, China and Cambodia.
Worldwide, it is estimated that nearly 2 million require antiretroviral treatment. At present only 25% of such children have an access to the antiretroviral therapy. Without access to antiretroviral therapy, 20% of vertically infected children will progress to the acquired immuno deficiency syndrome (AIDS) or death in their first yr of life and more than half of HIV-infected children will die before their fifth birthday.
HIV-1 and HIV-2. HIV-1 and HIV-2 are members of the Retroviridae family and belong to the Lentivirus genus. The HIV-1 genome is single-stranded RNA that is 9.2 kb in size. The genome has three major sections: the gag region, which encodes the viral core proteins (p24, pl7,

- |
--E•s•s•e•n•t-ia•l•P-e.d.ia.t.ri•c•s----------------------------- |
---- |
|
- |
p9, p6; these are derived from the precursor p55), the pol region, which encodes the viral enzymes (reverse transcriptase [p51], protease [plO], and integrase [p32]); and the env region, which encodes the viral envelope proteins (gp120 and gp41).
The major external viral protein of HIV-1 is a heavily glycosylated gp120 protein which contains the binding site for the CD4 molecule, the most common T lymphocyte surface receptor for HIV. Most HIV strains have a specific tropism for one of the chemokines: the fusion-inducing molecule, CXCR-4, which has been shown to act as a co receptor for HIV attachment to lymphocytes, and CCR-5, a chemokine receptor that facilitates HIV entry into macrophages.
Following viral attachment, gp120 and the CD4 molecule undergo conformational changes, allowing gp41 to interact with the fusion receptor on the cell surface. Viral fusion with the cell membrane allows entry of viral RNA into the cell cytoplasm. Viral DNA copies are then trans cribed from the virion RNA through viral reverse transcriptase enzyme activityandduplicationof the DNA copies produces double-stranded circular DNA. Because the HIV-1 reverse transcriptase is error-prone, many mutations arise, creating wide genetic variation in HIV-1 isolates even within an individual patient. The circular DNA is transported into the cell nucleus where it is integrated into chromosomal DNA; this is called as the provirus. The provirus can remain dormant for extended periods.
HIV-1transcriptionis followed bytranslation. A capsid polyproteinis cleaved to produce, among other products, the virus-specific protease (plO). This enzyme is critical forHIV-1 assembly. The RNAgenomeisthen incorporated into the newly formed viral capsid. As the new virus is formed, itbudsthrough thecellmembrane andisreleased.
HIV-2 is a rare cause of infection in children. It is most prevalent in Western and Southern Africa. If HIV-2 is suspected, a specific test that detects antibody to HIV-2 peptides should be used.
Transmission. Transmission of HIV-1 occurs via sexual contact,parenteralexposuretoblood,orverticaltransmission from mother to child. The primary route of infection in the pediatric population is vertical transmission. Most large studies in the United States and Europe have documented mother-to-child transmission rates in untreated women between 12 and 30%. In contrast, transmission rates in Africa and Asia are higher, up to 50%.
Vertical transmission of HIV can occur during the intrauterine or intrapartum periods, or through breast feeding. Up to 30% of infected newborns are infected in utero. The highest percentages of HIV-infected children acquire the virus intrapartum. Breastfeeding is an important route of transmission, especially in the developing countries. The risk factors for vertical transmission include preterm delivery (<34 week
gestation), a low maternal antenatal CD4 count, use of illicit drugsduringpregnancy, >4 hr durationofruptured membranes and birthweight <2500 g.
Transfusions of infected blood or blood products have accounted for a variable proportion of all pediatric AIDS cases. Heat treatment of factor VIII concentrate and HIV antibody screening of donors hasvirtually eliminatedHIV transmission to children with hemophilia. Blood donor screening has dramatically reduced, but not eliminated, the risk of transfusion-associated HIV infection. Sexual contact is a major route of transmission in the adolescent population.
Natural History
Before highly active antiretroviral therapy (HAART) was available, three distinct patterns of disease were described in children. Approximately 10-20% of HIV-infected newborns in developed countries presented with a rapid disease course, with onset of AIDS and symptoms during the first few months of life and, if untreated, death from AIDS-related complications by 4 yr of age. In resource poor countries, >85% of the HIV-infected newborns may have such a rapidly progressing disease.
It has been suggested that if intrauterine infection coincides withthe period of rapid expansion of CD4 cells in the fetus, it could effectively infect the majority of the body's immunocompetent cells. Most children in this group have a positive HIV-1 culture and/or detectable virus in the plasma in the first 48 hr of life. This early evidence of viral presence suggests that the newborn was infected in utero. In contrast to the viralload in adults, the viral load in infants stays high for at least the first 2 yr of life.
The majority ofperinatallyinfectednewborns (60-80%) present with a second pattern-that of a much slower progression of disease with a median survivaltimeof 6 yr. Many patients in this group have a negative viral culture or PCR in the 1st week of life and are therefore considered to be infected intrapartum. In a typical patient, the viral load rapidly increases by 2-3 months of age (median 100,000copies/ml) andthenslowlydeclines over a period of 24 months. This observation can be explained partially by theimmaturity of theimmune system in newborns and infants. The third pattern ofdisease (i.e. longterm survivors) occurs in a small percentage (<5%) of perinatally infected children who have minimal or no progression of disease with relatively normal CD4 counts and very low viral loads for longer than 8 yr.
HIV-infected children have changes in the immune system that are similar to those in HIV-infected adults. CD4 cell depletion may be less dramatic because infants normally have a relative lymphocytosis. Therefore, for example, a value of 1,500 CD4 cells/mm3 in children <1 yr of age is indicative of severe CD4 depletion and is comparableto<200CD4 cells/mm3 inadults. Lymphopenia is relatively rare in perinatally infected children and is

usually only seen in older children or those with endstage disease.
B cell activation occurs in most children early in the infection as evidenced by hypergammaglobulinemia associated with high levels of anti-HIV-1 antibody. This response may reflect both dysregulation of T cell suppression of B-cell antibody synthesis and active CD4 enhancement of B-lymphocyte humoral responses.
CD4depletion and inadequate antibody responses lead to increased susceptibility to various infections and the clinical manifestations vary with the severity of immuno deficiency.
Clinical Manifestations
The clinical manifestations of HIV infection vary widely among infants, children and adolescents. In most infants, physical examination at birth is normal. Initial signs and symptoms may be subtle and nonspecific, such as lymphadenopathy,hepatosplenomegaly, failure to thrive, chronic or recurrent diarrhea, interstitial pneumonia, or oral thrush and may be distinguishable from other causes only bytheirpersistence.Whereassystemic andpulmonary findings are common in the United States and Europe, chronic diarrhea, wasting and severe malnutrition predominate in Africa. Symptoms found more commonly in children than adults with HIV infection include recurrent bacterial infections, chronic parotid swelling, lymphocytic interstitial pneumonitis and early onset of progressive neurologic deterioration.
The pediatric HIV disease is staged by using two parameters: clinical status (Table 10.4) and degree of immunologic impairment (Table 10.5).
Opportunistic Infections
Children with HIV infection and advanced or severe immunosuppression are susceptible to develop various opportunisticinfections.The importantpathogens include Pneumocystis jiroveci, Cryptosporidium, Cryptococcus, Isospora and CMV.
Respiratory Diseases
Pneumocystis pneumonia Pneumocystis jiroveci (pre viously P. carinii) pneumonia (PCP) is the opportunistic infection that led to the initial description of AIDS. PCP is one of the commonest AIDS defining illnesses in children in the US and Europe. However, data regarding the incidence of PCP in children in other parts of the world are scarce. The majority of the cases occur between 3rd and 6th months of life.
Even if a child develops PCP while on prophylaxis, therapy may bestartedwith cotrimoxazole. This is because the prophylaxis may have failed because of poor compliance, orunusualpharmacokinetics. Untreated, PCP is universally fatal. With the use of appropriate therapy, the mortality decreases to less than 10%. The risk factors
Infections and Infestations -
for mortality are the severity of the episode and the severity of the immunosuppression.
Recurrent bacterial infections In various studies from developing countries, up to 90% of HIV-infected children had history of recurrent pneumonias. Initial episodes of pneumonia often occur before the development of significantimmunosupression. Asthe immunosupression increases the frequency increases.
The common pathogens for community-acquired pneumonia in these children are Streptococcus pneumoniae, Haemophilusinfluenzaeand Staphylococcusaureus. However, in children with severe immunosuppression and in hospital-acquired infections, gram-negative organisms, such as, Pseudomonas aeruginosa gain importance.
The clinical features of pneumonia in HIV-infected children are similar to those in uninfected children. However, in severely immunocompromised children, the signsmay be subtle. Often, the response to therapy is slow and the relapse rates are high. Bacteremia may be more common, seen in up to 50%.
Choices of appropriate antibiotics are often made based on local patterns of etiologies and susceptibilities. In many settings, an appropriate choice would be a combination of a broadspectrumcephalosporin andan arninoglycoside. In areas where a large proportion of Staphylococcus aureus isolates areresistantto antistaphylococcalantibiotics,then the empiric inclusion of vancomycin, clindamycin, linezolid should be considered. Children with nonsevere pneumonia can be managed as outpatients using a second or a third generation cephalosporin or a combination like amoxicillin-clauvulinic acid. Since Pneumocystis jiroveci pneumonia cannot be excluded at the outset in most HIV infected children with severe respiratory infections, co trimoxazole should beaddedunlessanotherdiagnosishas been definitively made.
The principles of supportive care of HIV-infected children admitted with severe pneumonia are similar to those in non-HIV-infected children.
Tuberculosis With the spread of the HIV infection, there has beenresurgence intuberculosis.CoexistentTB and HIV infections accelerate the progression of both the diseases. HIV infected children are more likely to have extra pulmonaryanddisseminatedtuberculosis;thecourse isalso likely to be more rapid. An HIV infected child with tubercular infection is more likely to develop the disease than a childwithout HIVinfection.Theoverallrisk ofactive TB in children infected with HIV is at least 5- to 10-fold higher than that in children not infected with HIV.
All HIV-infected children with active TB should receive longer duration of antitubercular therapy. A 9-12 month therapy is preferred. A close followup is essential to diagnose nonresponse/drug resistance early.
Viral infections Infections caused byrespiratory syncytial virus, influenza and parainfluenza viruses result in

E_s_s_.en.t.ia•l•P•e•d ai.t.ri•cs -------------- |
---------- |
--- |
||
__ |
- |
------- |
|
|
Table 10.4: WHO clinical staging of HIV/AIDS in children with confirmed infection
Clinical stage 1
Asymptomatic
Persistent generalized lymphadenopathy
Clinical stage 2
Unexplained• persistent hepatosplenomegaly
Papular pruritic eruptions
Fungal nail infection
Angular cheilitis
Lineal gingival erythema
Extensive wart virus infection
Extensive molluscum contagiosum
Recurrent oral ulceration
Unexplained• persistent parotid enlargement
Herpes zoster
Recurrent or chronic upper respiratory tract infections (otitis media, otorrhea, sinusitis, tonsillitis)
Clinical stage 3
Unexplained• moderate malnutrition or wasting not adequately responding to standard therapy
Unexplained• persistent diarrhea (14 days or more)
Unexplained• persistent fever (above 37.5°C intermittent or constant, for longer than one month)
Persistent oral candidiasis (after the first 6-8 weeks of life)
Oral hairy leukoplakia
Acute necrotizing ulcerative gingivitis/periodontitis
Lymph node tuberculosis
Pulmonary tuberculosis
Severe recurrent bacterial pneumonia
Symptomatic lymphoid interstitial pneumonitis
Chronic HIV-associated lung disease including bronchiectasis
Unexplained anemia (<8 g/dl), neutropenia (<0.5 x 109/1) or chronic thrombocytopenia (<50 x 109/1).
Clinical stage 4b
Unexplained• severe wasting, stunting or severe malnutrition not responding to standard therapy
Pneumocystis pneumonia
Recurrent severe bacterial infections (e.g. empyema, pyomyositis, bone or joint infection, meningitis, but excluding pneumonia) Chronic herpes simplex infection (orolabial or cutaneous of more than one month's duration or visceral at any site) Esophageal candidiasis (or candidiasis of trachea, bronchi or lungs)
Extrapulmonary/disseminated TB Kaposi sarcoma
Cytomegalovirus infection: retinitis or CMV infection affecting another organ, with onset at age >1 mo Central nervous system toxoplasmosis (after one month of life)
Extrapulmonary cryptococcosis (including meningitis) HIV encephalopathy
Disseminated endemic mycosis (extrapulmonary histoplasmosis, coccidioidomycosis) Disseminated nontuberculous mycobacterial infection
Chronic cryptosporidiosis (with diarrhea) Chronic isosporiasis
Cerebral or B cell non-Hodgkin lymphoma Progressive multifocal leukoencephalopathy
Symptomatic HIV-associated nephropathy or HIV-associated cardiomyopathy
•unexplained refers to where the condition is not explained by other causes
bSome additional specific conditions can also be included in regional classifications, e.g. reactivation of American trypanosomiasis (meningoencephalihs and/or myocardihs) in Americas region, penicilliosis in Asia and HIV-associated rectovaginal fistula in Africa
symptomatic disease more often in HIV infected children in comparison to noninfected children. Infections with other viruses such as adenovirus and measles virus are more likely to lead to serious sequelae than with the
previously mentioned viruses. As RSV infection most oftenoccurs inchildreninthefirst 2 yr of life, duringwhich many of these may notbe severely immunocompromised, the severity of illness may not be different from the non-

Infections and Infestations -
|
Table 10.5: Severity of immune suppression based on CD4 levels children |
|
|
||
HIV-associated |
|
Age-related CD4+ cell values |
|
|
|
immunodeficiency |
'.'11 mo |
12-35 mo |
36-59 mo |
5 yr |
|
Not significant (normal) |
>35% |
>30% |
>25% |
>500 cells/mm3 |
|
Mild |
30-35% |
25-30% |
20-25% |
350--499 cells/mm3 |
|
Advanced |
25 -30% |
20-25% |
15-20% |
200-349 cells/mm3 |
|
Severe |
<25% or |
<20% or |
<15% or |
<200 cells/mm3 |
or |
|
<1500 cells/mm3 |
<750 cells/mm3 |
<350 cells/mm3 |
<15% |
|
HIV infected children. In children with AIDS, dissemi nated CMV is a known opportunistic infection, but pneumonia caused by this virus is rare. The principles of diagnosisandtreatment of these infections in HIV-infected children are similar to those in non-HIV infected children. Fungal infections Pulmonary fungal infections usually present as a part of disseminated disease in immuno compromised children. Primary pulmonary fungal infec tions are uncommon.
Pulmonary candidiasis should be suspected in any sick HIV-infected child with lower respiratory tract infection that does not respond to the common therapeutic modali ties. A positive blood culture supports the diagnosis of invasive candidiasis.
Lymphoid interstitial pneumonitis (LIP) LIP has been recognized as a distinctive marker for pediatric HIV infection and is included as a Class B conditio11 in the revised CDC criteria for AIDS in children. In absence of antiretroviral therapy, nearly 20%ofHIV-infectedchildren developed LIP.
The etiology and pathogenesis of LIP are not well understood. Suggested etiologies include: anexaggerated immunologic response to inhaled or circulating antigens, and/or primary infection of the lung with HIV, Epstein Barr virus (EBV), or both.
LIP is characterized by nodule formation and diffuse infiltration of the alveolar septae by lymphocytes, plasmacytoidlymphocytes,plasma cellsandimmunoblasts. There isno involvement of the blood vessels ordestruction ofthe lung tissue. Children withLIP havea relatively good prognosis compared to other children who meet the surveillance definition of AIDS.
LIP is usually diagnosed in children with perinatally acquired HIV infection when they are older than 1 yr of age,unlikePCP.MostchildrenwithLIP are asymptomatic. Tachypnea, cough, wheezingand hypoxemia may be seen when children present with more severe manifestations; crepitations are uncommon. Clubbing is often present in advanced disease. These patients can progress to chronic respiratory failure. Long standing LIP may be associated with chronic bronchiectasis. The presence of a reticulo nodular pattern, with or without hilar lymphadenopathy, that persists on chest radiograph for 2 months or greater and that is unresponsive to antimicrobial therapy is considered presumptive evidence of LIP. Care should be
taken to exclude other possible etiologies. A definitive diagnosis of LIP can only be made by histopathology.
Early disease is managed conservatively. The effect of antiretrovirals on LIP is probably limited. Steroids are indicated if children with LIP have symptoms and signs ofchronicpulmonarydisease,clubbingand/ or hypoxemia. Treatment usually includes an initial 4 to 12 week course of prednisolone (2 mg/kg/day) followed by a tapering dose, using oxygensaturationandclinical status as aguide to improvement. This is then followed by chroniclow dose prednisolone.
Gastrointestinal Diseases
The pathologic changes in the gastrointestinal tract of children with AIDS are variable and can be clinically significant.
A variety of microbescan causegastrointestinaldisease, including bacteria (salmonella, campylobacter, Myca bacterium avium intracellularecomplex),protozoa (giardia, cryptosporidium, isospora, microsporidia),viruses (CMV, HSV, rotavirus) and fungi (Candida). The protozoa! infections are most severe and can be protracted in children with severe immunosuppression. Children with cryptosporidium infestation can have severe diarrhea leading to hypovolemic shock. AIDS enteropathy, a syn drome of malabsorption with partial villous atrophy not associated with aspecificpathogen, is probably the result of direct HIV infection of the gut.
Chronicliverinflammation is relativelycommonin HIV infected children. In some children, hepatitis caused by CMV, hepatitis B or C viruses, or mycobacteria may lead to liver failure and portal hypertension. It is important to recognize that several of the antiretroviral drugs such as didanosine and protease inhibitors may also cause reversible elevation of transaminases.
Pancreatitis is uncommon in HIVinfected children. This may be the result of drug therapy, e.g. didanosine, lami vudine, nevirapine,or pentarnidine. Rarely, opportunistic infections such asmycobacteria or CMV may be responsi ble for acute pancreatitis.
The principles of management of these conditions are similar to those in non-HIV-infected children.
Neurologic Diseases
The incidence of central nervous system involvement in perinatally infected children may be more than 50% in

- |
|
s |
--------- |
•sE•e•s.n.itla•P-ed•ia•tri•c• ------------ |
- |
||
|
- |
---------- |
|
developing countries but lower in developed countries, with a median onset at about one and a half yr of age. The mostcommonpresentationisprogressiveencephalopathy with loss or plateau of developmental milestones, cognitive deterioration, impaired brain growth resulting in acquired microcephaly and symmetric motor dys function. Meningitisduetobacterial pathogens, fungisuch as Cryptococcus and a number of viruses may be responsible. Toxoplasmosis of the nervous system is exceedingly rare in young infants, but may occur in HIV infected adolescents; the overwhelming majority of these cases have serum IgG antitoxoplasma antibodies as a marker of infection. The management of these conditions is similar to that in non-HIV-infected children; the response rates and outcomes may be poorer.
Cardiovascular Involvement
Cardiac abnormalities in HIV-infected children are common, persistent and often progressive; however, the majority of these are subclinical. Left ventricular structure and function progressively may deteriorate in the first 3 yr of life, resulting in increased ventricular mass in HIV infected children. Children with encephalopathy or other AIDS-defining conditions have the highest rate of adverse cardiac outcomes. Resting sinus tachycardia has been reported in up to nearly two-thirds and marked sinus arrhythmia in one-fifth of HIV-infected children. Gallop rhythm with tachypnea and hepatosplenomegaly appear to be thebestclinicalindicatorsof congestiveheartfailure. Electrocardiography and echocardiography are helpful in assessing cardiac function before the onset of clinical symptoms.
Renal Involvement
Nephropathy is an unusual presenting symptom of HIV infection, more commonly occurringinoldersymptomatic children. Nephrotic syndrome is the most common manifestation ofpediatric renal disease, with azotemia and normal blood pressure. Polyuria, oliguria and hematuria have also been observed in some patients.
Diagnosis
All infants born to HIV-infected mothers test antibody positive at birth because of passive transfer of maternal HIVantibody across the placenta. Most uninfectedinfants lose maternal antibody between 6 and 12 months of age. As a small proportion of uninfected infants continue to have maternal HIV antibody in the blood up to 18 months of age, positive IgG antibody tests cannot be used to make a definitive diagnosis of HIV infection in infants younger than this age. In a child older than 18 months of age, demonstration of IgG antibody to HIV by a repeatedly reactive enzyme immunoassay (EIA) and confirmatory test (e.g. Western blot or immunofluorescence assay) can establish the diagnosis of HIV infection. Although serologic diagnostic tests were the most commonly used
in the past, tests that allow for earlier definitive diagnosis in children have replaced antibody assays as the tests of choice for the diagnosis of HIV infection in infants.
Specific viral diagnostic assays, such as HIV DNA or RNA PCR, HIV culture, or HIV p24 antigen immune dissociated p24 (ICD-p24), are essential for diagnosis of young infants born to HIV infected mothers. By 6 months of age, the HIV culture and/or PCR identifies all infected infants, who are not having any continued exposure due to breast feeding.HIVDNA PCR is the preferredvirologic assay in developed countries. Plasma HIV RNA assays may be more sensitive thanDNA PCR for early diagnosis, but data are limited. HIV culture has similar sensitivity to HIVDNA PCR; however,itis moretechnically complex and expensive and results are often not available for 2--4 week compared to 2-3 days with PCR. The p24 antigen assay is less sensitive than the other virologic tests. Figure 10.11 shows the suggested algorithm for diagnosis of HIV infection in infants. The national program (Early Infant Diagnosis) now uses HIV DNA PCR test on dried blood spot samples; the positive tests need confirmation using a HIV DNA PCR on a whole blood sample.
Management
ThemanagementofHIVinfected child includes antiretro viraltherapy,prophylaxis and treatment of opportunistic infections and common infections, adequate nutrition and immunization.
Antiretroviral Therapy
Decisions about antiretroviral therapy for pediatric HIV infected patients are based on the magnitude of viral replication (i.e. viral load), CD4 lymphocyte count or percentage and clinical condition. A child who has WHO stage 3 or 4clinicaldiseaseshouldreceiveARTirrespective of the immunologic stage. Children who are asymptomatic or have stage 1 or 2 disease may be started on ART if they have evidence of advanced or severe immunosupression. However,currentevidenceshows that young children less than 2 yr of age have a higher risk of mortality without ART. The World Health Organization now recommends initiation of ART for all HIV infected children less than 2 yr ageirrespectiveof clinicalsymptomsandtheimmunologic stage.
Availability of antiretroviral therapy has transformed HIVinfection from a uniformly fatalconditionto a chronic infection, where children can lead a near normal life. The currently available therapy does not eradicate the virus and cure the child; it rather suppresses the virus replication for extended periods of time. The 3 main groups of drugs are nucleoside reverse transcriptase inhibitors (NRTI), non nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI). Highly active antiretroviral therapy (HAART) is a combination of 2 NRTis with a PI or a NNRTI.
The national program for management of HIV infected children recommends a combination of zidovudine, lamivudine and nevi.rapine as the first line therapy. The
