
Ghai Essential Pediatrics8th
.pdf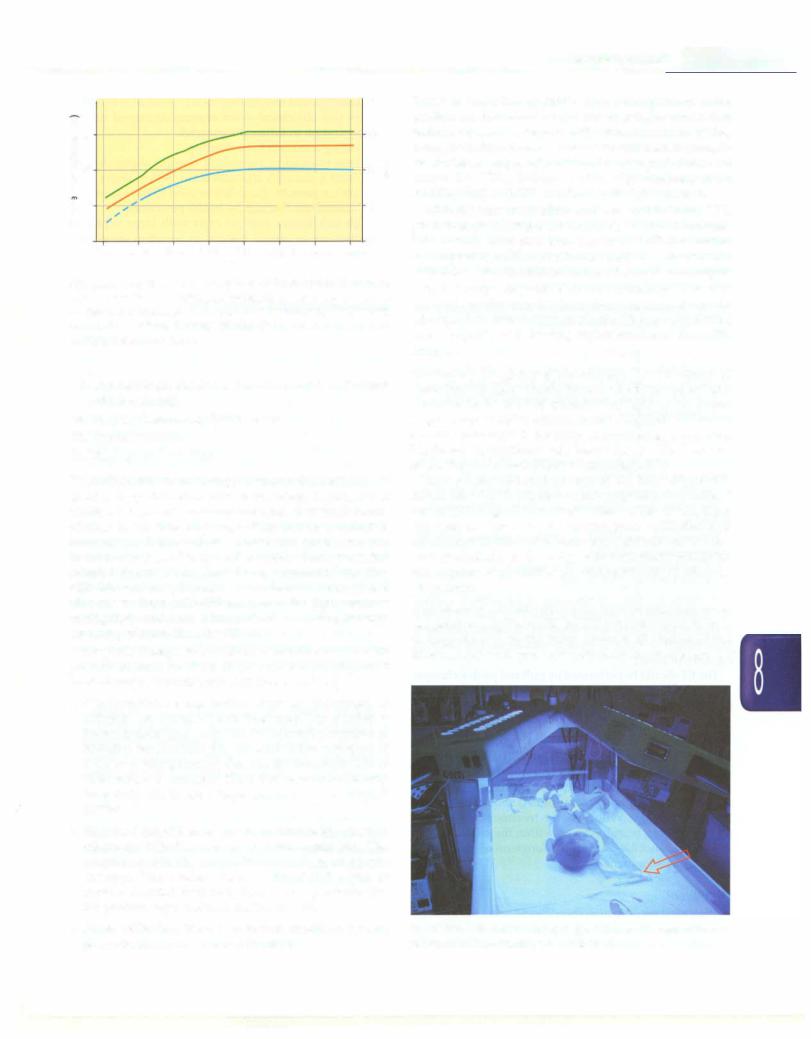
-----------------------------------N_e_w_b_o_rnrnf_a_nts
'6 |
30-,--------------------,-30 |
||||
f 25 |
|
25 |
|
||
C: |
|
|
|
|
|
:ZS |
|
0 |
|
0 |
|
.0 |
|
|
|
||
2 |
|
|
2 |
||
E |
|
|
r i |
|
E |
2 |
|
|
15 |
|
|
15 |
|
||||
.!ll |
|
|
I |
|
|
|
|
|
|
|
|
|
10 Birth 24 h 48 h |
10 |
|
||
|
72 h 96 h 5 days 6 days 7 days |
|
|||
|
|
|
Age |
|
|
Fig. 8.52: Guidelines for exchange transfusion in infants 35 or more weeks' gestation. - Infants at lower risk (> 38 week and well) - Infants at medium risk(> 38 week + risk factors or 35-37 6/7 week and well) - Infants at higher risk (35-37 6/7 week + risk factors) (Adapted from AAP 2004)
i.Cholestasis (stool color, urine color, direct andindirect bilirubin levels)
ii.Ongoing hemolysis, G6PD screen
iii.Hypothyroidism
iv.Urinary tract infection
Phototherapy Phototherapy remains the mainstay of treating hyperbilirubinemia in neonates. Photocopy is highlyeffective andcarriesan excellentsafetytrackrecord of over 50 yr. It acts by converting insoluble bilirubin (unconjugated) into soluble isomers that can be excreted in urine and feces. Many review articles have provided detailed discussion on phototherapy related issues. The bilirubin molecule isomerizes to harmless forms under blue-green light (460-490 nm); and the light sources havinghigh irradianceinthisparticular wavelength range are more effective than the others.
For phototherapy to be effective, bilirubin needs to be present in skin so there is no role for prophylactic phototherapy. Phototherapy acts by several ways:
• Configurational isomerization: Here the Z-isomers of bilirubin are converted into E-isomers. The reaction is instantaneous upon exposure to light but reversible as bilirubin reaches into the bile duct. After exposure of 8-12 hr of phototherapy, this constitutes about 25% of TSB, which is nontoxic. Since this is excreted slowly from body this is not a major mechanism for decrease in TSB.
• Structural isomerization: This is an irreversible reaction where the bilirubin is converted into lumirubin. The reaction is directly proportional to dose of photo therapy. This product forms 2-6% of TSB which is rapidly excreted from body thus is mainly responsible for phototherapy induced decline in TSB.
• Photo oxidation: This is a minor reaction, where photo-products are excreted in urine.
Types of phototherapy lights. The phototherapy units available inthemarket have a variety of light sources that include florescent lamps of different colors (cool white, blue, green, blue-green or turquoise) and shapes (straight or U-shaped commonly referred as compact florescent lamps, i.e. CFL), halogen bulbs, high intensity light emitting diodes (LED) and fibro-optic light sources.
With the easy availability and low cost in India, CFL phototherapy is beingmostcommonly used device. Often, CFL devices have four blue and two white (for exami nationpurpose)CFLs butthiscombinationcan be replaced with 6blue CFLs inorderto increasetheirradianceoutput.
In last couple of years, blue LED is making inroads in neonatal practice and has been found to at least equally effective. LED has advantage of long life (up to 50,000 hr) and is capable of delivering higher irradiance than CFL lamps.
Maximizing the efficacy of phototherapy. The irradiance of phototherapy lights should be periodically measured and a minimum level of 30 microW/cm2 /nm in the wave length range of460 to 490nm must be ensured. The lamps should be changed if the lamps are flickering or ends are blackened, if irradiance falls below the specified level or as per the recommendation of manufacturers.
Expose maximal surface area of the baby (Fig. 8.53). Avoid blocking the lights by any equipment (e.g. radiant warmer), a large diaper or eye patch, a cap or hat, tape, dressing or electrode, etc. ensure good hydration and nutrition of the baby. Make sure that light falls on the baby perpendicularly if the baby is in incubator. Minimize interruption of phototherapy during feeding sessions or procedures.
Administering phototherapy. Make sure that ambient room temperatureis optimum25° to28°Ctopreventhypothermia or hyperthermia in the baby. Remove all clothes of the baby except the diaper. Cover the baby's eyes with an eye
Fig. 8.53: A jaundiced baby receiving phototherapy with two overhead units and biliblanket pad (arrow)

___E_s_s_e_n_ti_a _i _P_e _d_ia_t_ri_cs_ _______________________________
patch, ensuring that it does not block baby's nostrils. Place the naked baby under the lights in a cot or bassinet if weight is more than 2 kg or in an incubator or radiant warmer if the baby is small (<2 kg). Keep the distance betweenbabyandlight30 to 45 cm (or as per manufacturer recommendation).
Ensure optimum breastfeeding. Baby can be taken out for breastfeeding sessions and the eye patch can be removed for better mother-infant interaction. However, minimize interruption to enhance effectiveness of phototherapy. There is no need to supplement or replace breast milk with any other types of feedor fluid (e.g. breast milk substitute, water, sugar water, etc.).
Monitoringandstoppingphototherapy. Monitor temperature of the baby every 2 to 4 hr. Measure TSB level every 12 to 24 hr.
Discontinue phototherapy once two TSB values 12 hr apart fall below current age specific cut offs. The infant should be monitored clinically for rebound bilirubin rise within 24 hr after stopping phototherapy for babies with hemolytic disorders.
Exchange Transfusion
Double volume exchange transfusion (DVET) should be performed if the TSB levels reach to age specific cut-off for exchange transfusion (Fig. 8.52 and Table 8.25) or the infantshowssignsofbilirubin encephalopathyirrespective of TSB levels.
Indications for DVET at birth in infants with Rh isoimmunization include:
i. Cord bilirubin is 5 mg/dl or more ii. Cord Hb is 10 g/dl or less
At birth, if a baby shows signs of hydrops or cardiac decompensation in presence of low PCV (<35%), partial exchange transfusion with 50 ml/kg of packed red blood cells should be done to quickly restore oxygen carrying capacity of blood.
TheET should be performedby pulland pushtechnique using umbilical venous route. Umbilical catheter should be inserted just enough to get free flow of blood.
Followup
Babies with serum bilirubin ;?.20 mg/dl and those who require exchange transfusion should be kept under followup in the high-risk clinic for neurodevelopmental outcome. Hearing assessment (BERA) should be done at 3 months of age. With prompt treatment, even very elevated serum bilirubin levels within the range of 25 to 29 mg/dl arenot likely to result inlongtermadverseeffects on neurodevelopment.
Prevention
•Antenatal investigation should include maternal blood grouping. Rh positive baby born to a Rh negative mother is at higher risk for hyperbilirubinemia and
requires greater monitoring.AntiD (RhoGam)injection after first obstetrical event ensures decreased risk of sensitization in future pregnancies.
•Ensuring adequate breastfeeding
•Parent education regarding danger signs should include yellowish discoloration below knees and elbows or persistent jaundice beyond 15 days as reason for immediate checkup by health personnel.
•High risk babies such as ones with large cephalo hematoma or family history of jaundice should be followed up after 2-3 days of discharge.
CONGENITAL MALFORMATIONS
Tracheoesophageal Fistula (TEF)
Upper part of esophagus is developed from retro pharyngeal segment and the lower part from pregastric segmentof the first part of theprimitivegut.Atfour weeks of gestation, the laryngotracheal groove is formed. Later, two longitudinal furrows develop to separate the respiratory primordium from the esophagus. Deviation or altered cellular growth in this septum results in formation of tracheoesophageal fistulae. Incidence is 1 in 4000 live births. In the most common variety (over 80% of cases), the upper part of the esophagus ends blindly and the lower part is connected to the trachea by a fistula.
Clinical Features
The presence of maternal polyhydramnois and single umbilical artery should alert the health provider to look for atresia of the upper digestive tract. Association of congenital anomalies of vertebrae, anorectalregion,heart, kidneys or limbs should also arouse suspicion. The newbornbabyhas excessive drooling soonafterbirthwith frothing. There is choking and cyanosis on feeding. Overflow of milk and saliva from esophagus and regurgitationofsecretion through the fistulous tract (when present) into the lungs results in aspiration pneumonia.
Diagnosis
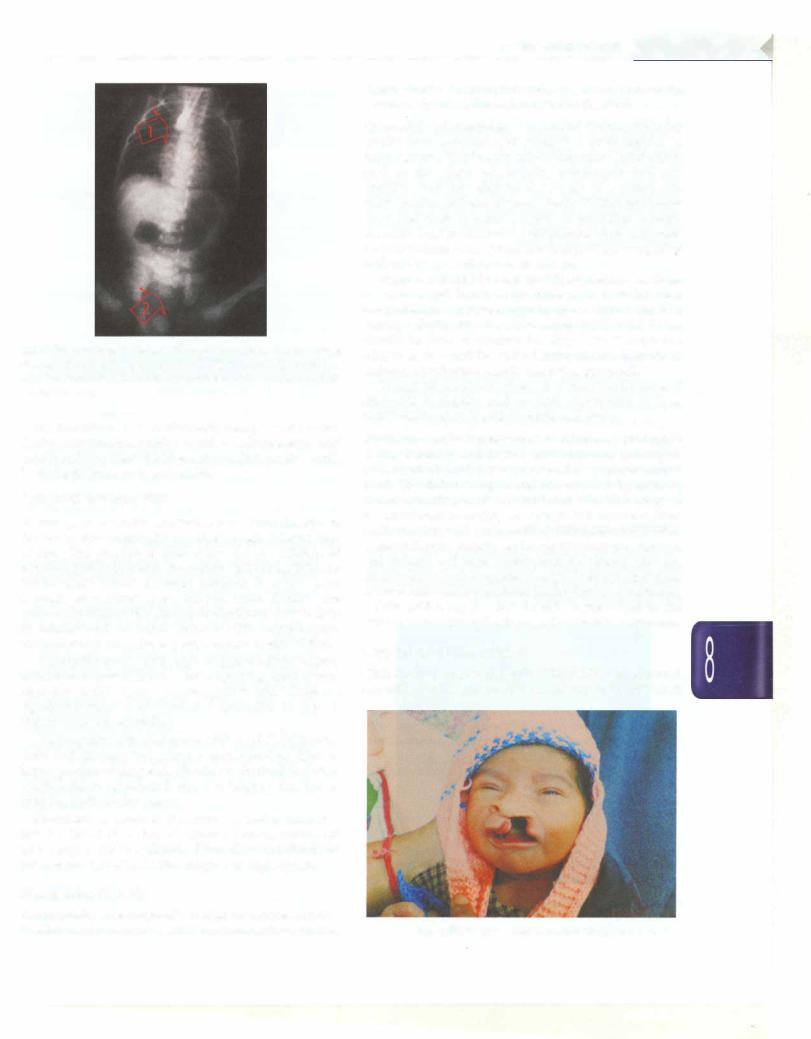
A stiff red rubber catheter cannot be passed into stomach as it gets arrested at a distance of 7-10 cm from the mouth (Fig. 8.54). A skiagram may be obtained after instilling 1-2 ml of air through the catheter. It is not advisable to use barium as a contrast material since it may be aspirated in lungs.
On X-ray, an air bubble is seen in the stomach if there is communication between the lower part of the esopagus and trachea, which occurs in the commonest variety of tracheoesophageal fistula.In other variety, wherein there is no communication of esophagus and trachea, there will be no gas in stomach.
Management
The baby should be nursed supine or in an upright position and esophageal pouch should be gently sucked
-------------------------------N_e_w_b_o_r_n_i_n_fan ......ts -
Fig. 8.54: Esophageal atresia with tracheoesophageal fistula. Note the red rubber catheter stopping at T4 level (arrow 1 ). There is a double gas bubble sign indicating presence of concomitant duodenal atresia (arrow 2)
every five minutes, or continuously using a slow suction device. Intravenous fluids should be administered and infection, if any should be treated. Surgical repair should be undertaken as early as possible.
Anorectal Malformation
A variety of anorectal anomalies have been described. Thesemay be anatomically classifiedashigh, intermediate or low. The position is determined by the relation of terminal part of bowel to the puborectalis sling. High or intermediate lesions are more common in males. Anal stenosis or covered anus, anocutaneous fistulae are common in both sexes. Anovestibularfistula (low lesion) in females and anorectal agenesis with rectoprostatic urethral fistula (high lesion) are common in male infants.
Among the males with high or intermediate lesions, 80% are rectovesical fistulae, but among the females with high defect, 80% have a rectovaginal fistula. There are significant chances of associated anomalies in case of higher anorectal anomalies.
AnX-ray film of the abdomen is obtained 12-24 hr after birth, with the baby being kept in an inverted position. A lateral picture of the pelvis should be obtained to define whether the rectal pouch is above or below a line drawn from the pubis to the coccyx.
Treatment is surgical. Prognosis is better with low defects. About 80 to 90% of patients become continents after surgery for low defects. More than two-thirds of patients are incontinent after surgery of high defects.
Neural Tube Defects
Encephalocele. In encephalocele, the brain and/or its coverings herniate through a defect in the skull.
Congenital hydrocephalus. Congenital hydropcephalus
results from impaired CSP circulation or absorption in basal cisterns. This usually follows intrauterine infections such as toxoplasmosis, rubella, cytomegalovirus and syphilis, but may also be the result of a congenital malformation of the aqueduct, Dandy-Walker syndrome (posterior fossa cyst and a defect of cerebellar verrnis), Arnold-Chiari malformation (displacement of brainstem and cerebellum in the spinal canal) or multiple congenital malformations of the nervous system.
Diagnosis should be suspected if the head is too large or sutures and fontanels are wide open or if the head circumference increases rapidly (more than 1 cm in a fortnight during the first three months). CT or MRI scan should be done to confirm the diagnosis. The type of dilatation of ventricles indicates the site of obstruction. Isolated aqueductal stenosis has better prognosis.
Treatment should be directed at the specific cause if amenable to therapy and surgical intervention such as ventriculocaval or ventriculoperioneal shunt.
Myelomeningocele. It presents as membranous protrusion at the lumbosacral region and contains meninges, cerebrospinal fluid, nerve roots and a dysplastic spinal cord. The defect is open and not covered by skin. In contrast, meningocele is covered with skin. There may be no associated neurological deficit, but Arnold-Chiari malformation and congenital hydrocephalus are often associated. Severemotorand sensory deficit are common and urinary and fecal incontinence are usually present. Meningomyelocele is operated only if there is no paralysis oflowerlimbsandifthereisnobladder/bowelinvolvement.
Folic acid 4 mg per day should be prescribed to the women in periconceptional period to prevent recurrence.
Cleft Lip and Cleft Palate
Cleft lip is recognized readily (Fig. 8.55), but a careful inspection of the oral cavity is necessary to identify cleft
Anencephaly. Anencephaly is due to a defect in the |
|
developmentofneural axisandisnot compatiblewithlife. |
Fig. 8.55: Unilateral cleft lip and cleft palate |
___E_s_s_e_n_t_ia_P_e_d_iat_rics _____________________________
palate. A cleft of the soft palate can be easily missed unless the baby is examined carefully. Ventricular septal defect is a common associated anomaly with cleft palate.
In Pierre-Robinsyndrome, cleftpalate isassociatedwith retracted jaw (micrognathia) and large tongue, with a tendency for glossoptosis. Feeding is difficult in cases of cleft palate. For the first few days, gavage feeding or spoon-feeding may be done. Bottle feeding may be tried with a soft nipple with rubber flange, which close the cleft and help the baby in sucking. If this is not successful, palatal prosthesis may be used.
Management. Management of cleft palate requires a team effort involving a pediatrician, a plastic surgeon, orthodontist, ENT specialist and speech therapist. Cleft lip is repaired in the neonatal period. Operation for cleft palate is generally deferred until the second year.
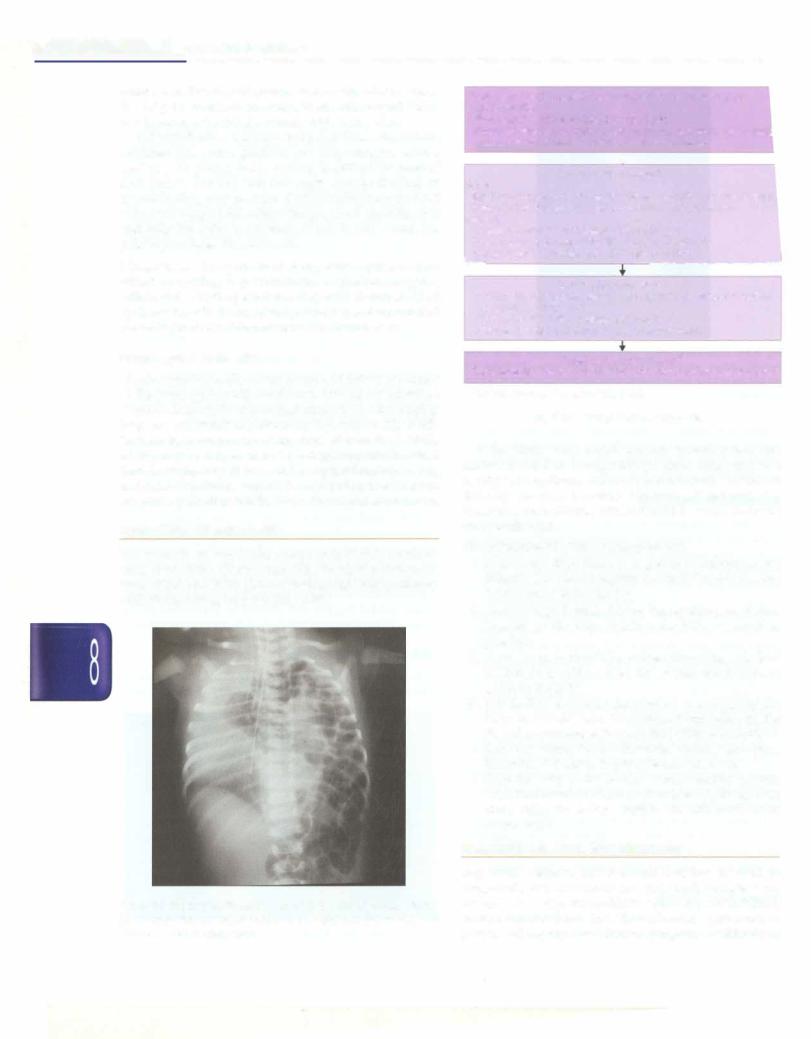
Diaphragmatic Hernia
Diaphragmatic herniaoccurs because offailure of closure of the pleuroperitoneal membrane. This allows intestinal loops toascend to the thorax thatcompressthe developing lung and can result in pulmonary hypoplasia (Fig. 8.56). These babies can present at any time after birth. At birth, a baby may be suspected to have diaphragmatic hernia if there is respiratory distress and a scaphoid abdomen. Bag and mask ventilation should be avoided in these babies. Surgicalrepairafterstabilization is thetreatmentof choice.
TRANSPORT OF NEONATES
Transport is an important component of sick newborn care. It requires careful attention to vital parameters, temperatureandbloodglucoselevelsaswellascoordination with the receiving hospital (Fig. 8.57).
Fig. 8.56: Diaphragmatic hernia: Note multiple air filled cysts in left hemithorax, shift of mediastinum to the right and the absence of outline of the left diaphragm
Determine the Indication* to transport the baby to higher health facility
Birth weight <1200 g or gestation <30 week
Sickness: severe respiratory distress,• shock, severe jaundice, maj malformations requiring surgery, refractory seizures
Prepare for transport
Baby
Stabilize (temperature, airway, breathing, circulation and blood sugar Secure IV line and give necessary treatment before transfer
Logistics
Counsel the parents and family before transport
|
Communicate with referral facility. Provide a brief note |
|
Arrange supplies, equipment and transport vehicle |
t |
............ - ------ |
|
Care during transport |
|
Monitor frequently (temperature, airway and breathing, circulation, |
|
IV canula and infusions |
|
Ensure that the baby receives feeds or fluid |
|
Stop the vehicle, if necessary, to manage problems |
|
Feedback after transport |
|
Communicate with referral team for condition at arrival and outcome |
|
* Indications may vary as per the facility |
|
Fig 8.57: Transport of sick neonates |
If the birth of an at-risk neonate is anticipated, the mother should be transported (in utero transport) to a facility with optimum maternal and neonatal care before delivery (in utero transfer). However, if referral of a neonate is unavoidable, efforts should be made to do the best possible job.
The principles of efficient transport are:
i.Make sure that there is a genuine indication for referral. Oneshouldexplaintheconditionandreasons for transport to the family.
ii.Correct hypothermia before transporting, as it may worsen on the way. Stabilize the baby as much as possible.
iii.A precise note should be written providing details of the baby's condition, need for referral and treatment given to the baby.
iv.The mother should be encouraged to accompany the baby. In case she cannot accompany immediately, she shouldbeencouraged toreachthefacilityattheearliest.
v.A doctor/nurse/dai/health workershould accompany the baby, if feasible, to provide care en route.
vi.Take the baby to the nearest referral facility (inform theminadvance onphoneorotherwise),bytheshortest route, using the fastest possible and affordable mode of transport.
FOLLOWUP OF HIGH RISK NEONATES
Improved perinatal and neonatal care has resulted in improved survival of many sick and small neonates who are atrisk forlongterm morbidities such as growth failure, developmental delay and visual/hearing problems. A proper and appropriate followup program would help in

prevention, early detection and appropriate management of these problems, thereby ensuring disability and morbidity free survival.
Who Needs Followup Care?
Table 8.26 lists the cohort of high risk infants who require followup services.
Table 8.26: Common newborn conditions requiring high risk followup care
Birth weight <1500 g and/or gestation <32 weeks
Perinatal asphyxia: Apgar score :,;3 at 5 min and/or hypoxic ischemic encephalopathy
Mechanical ventilation for >24 hr
Metabolic problems: Symptomatichypoglycemiaandhypocalcemia Infections: Meningitis and/or culture positive sepsis Hyperbilirubinemia >20 mg/dl or requirement of exchange transfusion
When to Followup?
The following should be the followup schedule:
•2 weeks after discharge
•At 6, 10, 14 weeks of postnatal age
•At 3, 6, 9, 12 and 18 months of corrected age and then 6 monthly until at least 5 yr.
What should be Done at Followup?
i.Assessment offeeding and dietary counseling: Parents should be asked about the infants' diet and offered dietary counseling at each visit. Breastfeeding frequency and adequacy should be assessed. The amount, dilution and mode of feeding should be noted if supplemental feeding is given. It is also important to record the duration of exclusive breastfeeding. If a baby is not gaining adequate weight on exclusive breastfeeding, take care of any illness or maternal problems which may interfere with feeding and milk output. If poor weight gain persists despite all measures to improve breast milk output supple mentation can be considered. Complementary feeding should be started at 6 months corrected age. Initially, semisolids should be advised in accordance with the local cultural practices.
ii.Growth monitoring: Growth (including weight, head circumference, mid-arm circumference and length) should be monitored and plotted on an appropriate growth chart at each visit.
iii.Developmentalassessment:Assessment of developmental milestones should be done according to the corrected age. The milestones should be assessed in four domains - gross motor, fine motor, language and personal-social. Infants who lag behind in any domain should undergo a formal developmental evaluation by a clinical psychologist using tests such as Developmental Assessment of Indian Infant II (DASII
Newborn Infants -
II). Age appropriate stimulation should be provided to these babies.
iv.Immunization: Immunization should be ensured according to chronological age. Parents should be offered the option of using additional vaccines such as Hemophilus influenzae B, typhoid, MMR.
v.Ongoing problems: Ongoing morbidities such as diarrhea, pneumonia occur more frequently in these babies and should require appropriate treatment.
vi.Neurological assessment: Muscle tone should be assessed, any asymmetry between the extremities should also be recorded. Any history of seizures or involuntary movements should also be recorded.
vii.Eye evaluation: An ophthalmologist should evaluate the baby for vision, squint, cataract and optic atrophy. Subjective visual assessment can be made from clinical clues as inability to fixate eyes, roving eye movements and nystagmus. Objective visual assessment should
be done with the Teller Acuity Card.
viii.Hearing evaluation: High risk infants have higher incidence of moderate to profound hearing loss (2.5-5% versus l%). Since clinical screening is often unreliable, brainstem auditory evoked responses (BAER/BERA) should be performed between 40 weeks PMA and 3 months postnatal age.
METABOLIC DISORDERS
Hypoglycemia
Hypoglycemia is defined as a blood glucose value of less than 40 mg/dl (plasma glucose less than 45 mg/dl).
Screening for hypoglycemia is recommended in high risk situations (Table 8.27). These babies should be screened for hypoglycemia at 2, 6, 12, 24, 48 and 72 hr after birth with reagentstrips (dextrostix).Babies showing blood sugar value of less than 40 mg/dl on reagent strip should be treated for hypoglycemia but should have confirmation of hypoglycemia by a lab test as reagent
Table 8.27: Common causes of hypoglycemia
In adequate substrate: Small for gestational age (weight for gestation <3rd percentile), gestation <35 week, birth weight <2000 g
Relative hyperinsulinemia: Infants of diabetic mother, large for date baby (weight for gestation >97th percentile), Rh isoimmunization.
Sickness: hypothermia, sepsis, asphyxia
strips have high false positive rates. Appropriate for gestational age babies who are breastfeeding adequately do not require any screening for hypoglycemia.
Clinical Features
Clinically the hypoglycemia may be asymptomatic or may manifest with a range of clinical features like stupor, tremors, apathy, cyanosis, convulsions, apneic spells,

___Essen ti al_Ped i a trics_________________________________
tachypnea, weak and high pitched cry, lethargy, difficulty in feeding, eye rolling, episodes of sweating, sudden pallor, hypothermia and rarely, cardiac arrest.
Management of Hypoglycemia
Prevention of hypoglycemia. All high risk babies should receive proper breastfeeding counseling and support. Adequacy of breastfeeding should be assessed and small babies not able to suck effectively on the breast, should receive expressed breast milk by alternate methods.
Asymptomatic babies. If thebloodsugaris morethan 20 mg/ dl in an asymptomatic baby, a trial of oral feeds is given and blood sugar be tested after 30-45 minutes. If repeat blood sugars values are above40mg/dl, frequent feeding is ensured with 6 hourly monitoring of blood sugar for 48 hr. However if blood sugar values persists below 40 mg/dl, baby should receive IV glucose infusion.
If the initial blood sugar value is less than 20 mg/dl, then intravenous glucose infusion is started.
Symptomatic babies: A bolus of 2 ml/kg of 10% dextrose should be given, followed immediately by glucose infusion at an initial rate of 6 mg/kg/min. Blood sugar is checked after 30-45 minutes and then 6 hourly. Repeat hypoglycemic episodes may be treated by increasing the glucose infusion rate by 2 mg/kg/min until a maximum of 12 mg/kg/min. If two or more consecutive values are >50 mg/dl after 24 hr of parenteral therapy, the infusion can be tapered off at the rate of 2 mg/kg/min every 6 hr, with glucosemonitoring. Taperinghas to be accompanied by concomitant increase in oral feeds.
routine screening. The symptoms when present may be of neuromuscular irritability: myoclonic jerks, jitteriness, exaggerated startle and seizures. They may represent the cardiac involvement like tachycardia, heart failure, prolonged QT interval, decreased contractibility. Apnea, cyanosis, tachypnea, vomiting and laryngospasm areother rare symptoms.
Treatment
Prevention. They should receive 40 mg/kg/day of ele mental calcium (4 ml/kg/day of 10% calcium gluconate). Infantstoleratingoral feeds mayreceivethis calciumorally q 6 hourly. Therapy should be continued for 3 days.
Asymptomatic hypocalcemia. They should receive 80 mg/kg/day elemental calcium for 48 hr. This may be tapered to 50% dose for another 24 hr and then discontinued.
Symptomatic hypocalcemia. They should receive a bolus dose of 2 ml/kg/dose. This should be followed by a continuous IV infusion of 80 mg/kg/day elemental calcium for 48 hr. Calcium infusion should be reduced to 50% of the original dose for the next 24 hr and then discontinued. The infusion may be replaced with oral calcium therapy on the last day.
Bradycardia and arrhythmia are known side effects of bolus IV calcium administration and bolus doses of calcium should be diluted1:1with 5% dextrose andgiven under cardiac monitoring. Skin and subcutaneous tissue necrosis may occur due to extravasation.
Followup and Outcomes
Hypoglycemia has been linked to longterm adverse outcomes. These babies are followed up and assessed at one month corrected age for vision/eye evaluation and at 3, 6, 9, 12 and 18 months corrected age for growth, neurodevelopment and vision and hearing loss.
Hypocalcemia
Hypocalcemia is definedas total serum calciumlevel of <7 mg/dl orionized calciumlevelof <4mg/dl.Hypocalcemia may be of early onset (<72 hr) or rarely late onset (>72 hr).
Early onset neonatal hypocalcemia: Commonly seen in preterms less than 32 weeks, infants of diabetic mothers, perinatal asphyxia and maternal hyperparathyroidism. Such babies are at increased risk of hypocalcemia.
Late onset hypocalcemia: Neonates born to mothers with vitamin D deficiency, babies on anticonvulsant therapy orwith malabsorption, those on cow milkfeeding, or with hypoparathyroidism are at riskof late onset hypocalcemia.
Clinical Presentation
Early onset hypocalcemia is usually asymptomatic unlike the late onset hypocalcemia variety and is diagnosed on
Drug Therapy and Breastfeed ing
Though most drugs given to mother get transferred into human milk, the amount is not significant and does not pose any risk to the baby. The clinician should evaluate each medication carefully, examine published data on the drug and advise the mother carefully about the use of medications while breastfeeding. Table 8.28 enlists the maternal medications which may influence the baby.
Maternal Medications and Fetal Hazards
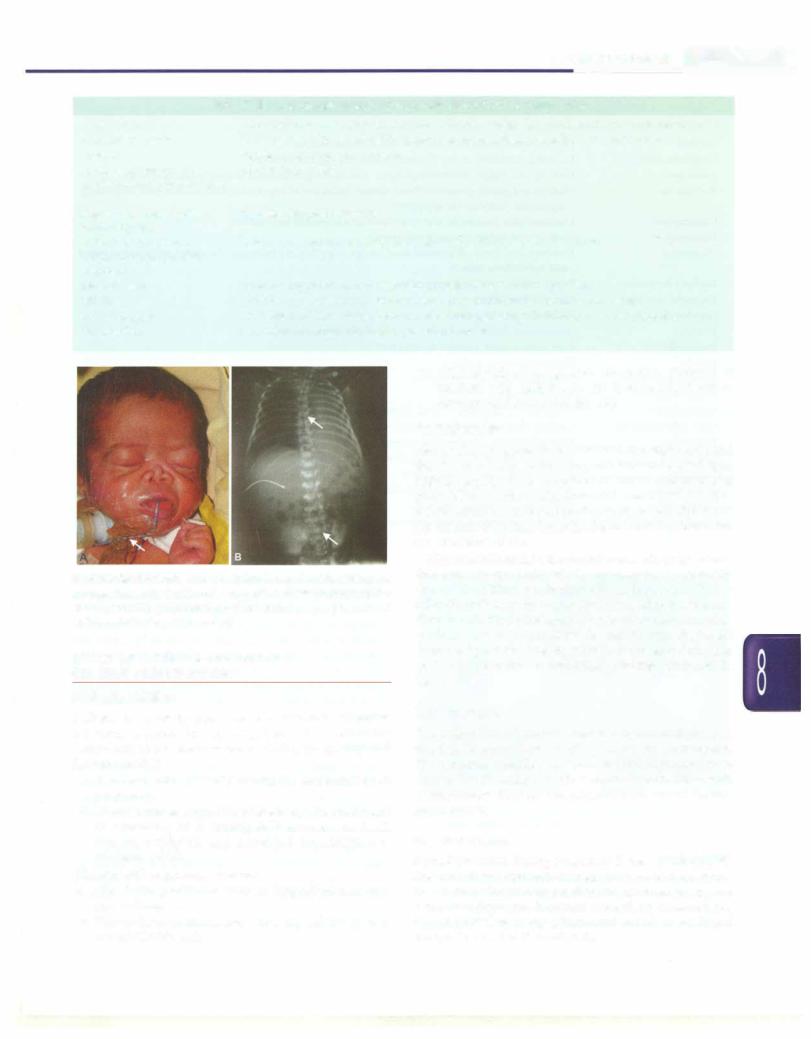
The risk by exogenous agents to the fetus is most pronounced during the periodofembryogenesisandmay result in abortion or congenital malformation (Fig. 8.58). In the latepartofpregnancy, these agents onlycause organ dysfunction or disturbances of enzyme systems. As a general principle, the use of drugs during pregnancy should be minimized. The benefits of medication to the mothermustalways be carefully weighedagainstthe risk to the fetus.
Drugs listed in Table 8.29 are known to be or suspected to be teratogenic when given during the first trimester of pregnancy.
|
|
Newborn Infants - |
|
Table 8.28: Maternal medications that confer high risk to breastfed infants |
|
Amiodarone |
Accumulation in breast milk may cause thyroid suppression and cardiovascular toxicity |
|
Anticancer agents |
Some agents with short hall-lives may permit breastfeeding with brief interruption |
|
Doxepin |
Sedation and respiratory arrest |
|
Drugs of abuse (cocaine, |
Should be avoided |
|
amphetamines, phencyclidine, |
||
heroin) |
|
|
Ergotamine, cabergoline, |
Ergotism has been reported |
|
bromocriptine |
|
|
Sodium or potassium |
Iodine concentration in milk may cause thyroid suppression in infants |
|
iodide; povidone-iodide |
|
|
solutions |
|
|
Methotrexate |
Immune suppression; concentration in gastrointestinal tract of infant |
|
Lithium |
Lithium concentrations in infant plasma may reach 33-40% of maternal levels |
|
Radioisotopes |
Brief interruptions advised; consult Nuclear Regulatory Commission recommendations |
|
Tetracycline |
Short-term use (up to 3-4 week) are not harmful |
|
vi. Higher risk of congenital anomalies. (Infants of mothers with diabetes are 20 times more at risk to develop cardiovascular defects)
Pathogenesis
Fig 8.58: Warfarin embryopathy. Maternal warfarin intake during first trimester has resulted inCA) severe hypoplasia of nasal bonesrequiring tracheostomy for (B) maintenance of upper airways (solid arrow) and epiphyseal stippling (open arrow)
EFFECT OF MATERNAL CONDITIONS
ON FETUS AND NEONATES
Diabetic Mellitus
Diabetes is one of the most common endocrine disorders affecting women during pregnancy. The following complications are likely to occur during pregnancy of a diabetic mother.
i.Fetus may die suddenly during the last trimester of pregnancy
ii.Macrosomia or large size of the body (Fig. 8.59) and its attending risks during delivery such as birth trauma, asphyxia and increased possibilities of cesarean section
iii.Neonatal respiratory distress
iv.Metabolic problems such as hypoglycemia and hypocalcemia
v.Polycythemia, increased viscosity of blood and hyperbilirubinemia
Maternal hyperglycemia leads to fetal hyperglycemia and that in turn leads to fetal hyperinsulinemia (Pederson hypothesis).Insulinis ananabolichormoneand promotes growth. Excessmaternalglucose and aminoacidsprovide the substrate for increasedsynthesisof protein, lipids and glycogen in the fetus. Large fetal size is mostly due to the accumulation of fat.
Hyperinsulinemia in the neonate causes hypoglycemia. The cause of hypocalcemia is not clear but is probably due to diminished production of parathormone. Hyper bilirubinemia may be due to the increased red cell mass. Since insulin blocksinductionof enzyme system, thismay explain lower production of surfactant. Reduced surfactant pool and the higher risk of preterm deliveries explains higher risk of respiratory distress syndrome in these babies.
Management
The infant should be screened for malformations and injuries. Frequent breastfeeding should be encouraged. The neonate should be monitoredfor blood glucose levels during first three days of life. The other morbidities such as respiratory distress, hyerbilirubinemia should treated appropriately.
Hypothyroidism
Hypothyroidism during pregnancy if treated adequately does not affectpregnancyoutcomes; however, inadequate treatment of the mother predisposes the fetus to adverse neurodevelopment. Neonate should be screened for hypothyroidism using either cord blood or on blood sample taken after 72 hr of birth.

___E s s e n tial P e d iatrics_________________________________
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _
|
Table 8.29: Common teratogenic drugs |
Drugs or chemical |
Teratogenic effect |
Alcohol |
Growth retardation; cardiac, limb and facial anomalies |
Amphetamines |
Learning disability, motor incoordination, hepatic calcification |
Androgens |
Cleft lip and palate, tracheoesophageal fistula, congenital heart disease, masculinization |
Barbiturates |
Cleft lip andpalate, congenital heart disease, induction of hepatic microsomal enzymes, respiratory |
|
depression, withdrawal symptoms |
Chloroquine |
Deafness after prolonged use, hemolysis in susceptible individuals |
Diazepam |
Cleft lip and palate, apnea, hypothermia |
Dicumarol |
Bleeding, fetal death, depressed nasal bridge, stippling of phalanges, choanal atresia, cardiac, renal |
|
and ophthalmic defects |
Diphenylhydantoin |
Facial, cardiac and limb anomalies |
Excessive smoking |
Growth retardation |
Gentamicin |
Eighth nerve damage |
Heroin |
Intrauterine death, low birth weight, sudden infant death |
Indomethacin |
Low birth weight, platelet dysfunction |
Iodides |
Hypothyroidism, goiter |
Lithium carbonate |
Congenital heart disease, goiter |
Propylthiouracil |
Hypothyroidism |
Methotrexate |
Congenital malformation, fetal death |
Oral contraceptives |
Cardiac, limb and visceral anomalies |
Progestins |
Masculinization, advanced bone age |
Quinine |
Deafness, neurologic anomalies, thrombocytopenia |
Radiation |
Microcephaly, mental retardation |
Tetracycline |
Staining of teeth, enamel hypoplasia, inhibition of bone growth, congenital cataracts |
Tolbutamide |
Fetal death, thrombocytopenia |
Vitamin D (heavy dose) |
Mental retardation, supravalvular aortic stenosis, ventricular opacities, elfin facies |
should not be separated from the mother. Exclusive breastfeeding is encouraged. The infant should be given isoniazidprophylaxis (5mg/kg/day)and is evaluatedat 6 weeks of age. If there is any evidence of tubercular infection in the baby (clinically or radiologically), the infant should be started on antitubercular therapy. If the infant does not have any evidence of tuberculosis at 6 weeks, the isoniazid therapy continued for 6 months and the infant given BCG vaccine after 2 weeks ofcessation of therapy.
Syphilis
Fig. 8.59: Infant of diabetic mother. Note the large size of the baby with broad shoulders and torso and a relatively smaller head
Tuberculosis
If the mother has active pulmonary tuberculosis that has been treated for less than 2 months before birth or the diagnosis of tuberculosis was made afterbirth, the baby is at risk to acquire infection from the mother. Such babies
Syphilis infection can be transmitted to the infant who have significant disease and its sequelae. If the mother was diagnosedtohave syphilis andshe received adequate treatment at least one month before delivery, the infant does not require any treatment.
If the mother did not receive any treatment or inadequate treatmentorher treatment status isnotknown and the neonate does not have any signs of congenital syphilis, the infant should be treated with procaine or benzathine penicillin. Along with the baby, the mother and her partner should also be treated.

I
----------------------------------N_e_w_b_o_r_ni_nfa_ n_ts....-,.
If the infant shows signs of congenital syphilis, he should be treated with crystalline penicillin for 10 days.
The infant should be followed up in four weeks to examine the baby for growth and signs of congenital syphilis.
Hepatitis B Infection
The women who have hepatitis B infection (active or carrier stage) can transmit the infection to their babies. Such babies should receive hepatitis B vaccine within 12 hr of birth, whichcanpreventperinataltransmission of hepatitis B virus significantly. Hepatitis B immuno globulins (HBIG; 200IU, IM) can be given to enhance the protection but it is costly and there are availability issues.
HIV infection
Most children living with HIV acquire the infection through mother-to-child transmission (MTCT). HIV infec tion can be transmitted from an infected mother to her fetus during pregnancy, delivery, or by breastfeeding.
Prevention
In the absence of any intervention, the risk of perinatal transmission is 15-30% in non-breastfeeding populations. Breastfeeding by an infected mother increases the risk by 5-20% to a total of 20-45%.
The risk of MTCT can be reduced to under 2% by interventions that include antiretroviral (ARV) prophyl axis given to women during pregnancy and labor and to theinfantin the first6 week oflife, obstetrical interventions including elective cesarean delivery (prior to the onset of labor and rupture of membranes) and complete avoidance of breastfeeding.
Approach to a womenwith HIV infection and her infant is summarized in Fig. 8.60.
Breastfeeding
Mothers known to be HIV-infected should only give commercial infant formula milk as replacement feeding when specific conditions are met (referred to earlier as AFASS-Affordable, Feasible, Acceptable, Sustainable and Safe in the 2006 WHO recommendations on HIV and Infant Feeding)
If replacement feeding is not feasible, mothers known to be HIV-infected (and whose infants are HIV uninfected or of unknown HIV status) should exclusively breastfeed their infants for the first 6 months of life, introducing appropriate complementary foodsthereafter and continue breastfeeding for the first 12 months of life. Breastfeeding should then stop once a nutritionally adequate and safe diet without breast milk can be provided.
Immunization
HIV exposed or infected but asymptomatic children should receive all standard vaccines as per national schedule. HIV infected children with immune suppression or symptoms should receive all standard vaccines except BCG, OPV and varicella vaccines. Consider HiB and pneumococcal vaccines in all HIV exposed children (irrespective of symptoms or CD4 count).
Suggested Reading
Rapid Advice: Use of antire troviral drugs for treating pregnantwomenandpreventing HNinfection ininfants.World Health Organization,WHO Press, Geneva, 2009; available at www .who .in t/i r i s/bi t s t r ea m/ 1 0665/442 49/1/ 9789241598934_eng.pdf
|
HIV infected mother (diagnosed by ELISA test) |
|
||||||||
|
|
|
|
|
|
|
|
|
||
|
|
Clinical assessment and CD4 count of mother |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ART to prevent MTCT |
|
|||||
ART indicated for mother |
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Two options |
|
||||||
|
|
|
|
|||||||
|
|
|
1. Triple ART start at 14 week of gestation until |
|
||||||
|
|
|
one week after exposure to breast milk has stopped |
|||||||
|
|
|
||||||||
|
|
|
2. Antepartum AZT starting at 14 week+ of gestation |
|||||||
|
|
|
|
|
|
l |
__, |
|||
|
|
|
+ intrapartum single dose NVP + AZT + 3TC |
|
||||||
|
|
|
+ postpartum AZT + 3TC for 7 days* |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
Infant
Mother received only AZT for ART
Breastfed: Daily NVP from birth until one wk after all exposure to breast milk has ended
Not breastfed: NVP or AZT for 6 week
Mother received triple drug ART during pregnancy and entire breastfeeding: NVP or AZT for 6 week
Fig. 8.60: Approach to an infant born to HIV-infected mother. • Efavirenz (EFV) based regimens should not be newly-initiated during the first trimester
of pregnancy' single dose nevirapine (Sd-NVP) and zidovudine (AZT) with lamivudine (3TC) intraand postpartum can be omitted if mother receives more than 4 weeks of AZT during pregnancy. ART antiretroviral therapy; AZT (4 mg/kg PO per dose twice a day for infant); Sd NVP (10 mg/day for infants <2.5 kg, 15 mg/ day for infants 2.5 kg or more)

Immunization and
Immunodeficiency
Aditi Sinha, Surjit Singh
IMMUNITY
The immunesystemrecognizes microorganisms and other foreign material, discriminates it from self and mounts an appropriate response to eliminate it. There are two major components of immunity: the innate immune sys tem and the adaptive immune response. Innate immunity is primitive, nonspecific, has no memory and provides the first line of defense against infections, while the adaptive immune system is highly evolved, specific and has memory, characterized bya rapid rise inimmune response when exposed again to the microorganism.
Innate Immune System
The skin and mucous membranes provide an important mechanical barrier to infection. Gastric acidity is an effective physiologic barrier as very few microorganisms can survive the low acidic pH in stomach.
The complement system consists of multiple serum proteinscirculating asinactiveprecursors.Oncetriggered, these proteins activate each other sequentially to generate activecomponents. There are threepathways of activation of the complement cascade. The classical complement pathway is triggered by activation of Clq by antibody antigen complexes or polyanions (heparin, protamine, nucleicacidsfromapoptoticcells).Thealternativepathway is continuously active at low levels due to spontaneous C3 lysis, and is amplifiedby binding of complement compo nents to pathogen (e.g. bacterial lipopolysaccharides or endotoxin,yeastcellwall). Thelectinpathwayis activated by binding ofmannosebinding lectintomannoseresidues on pathogen cell surface. Activation of the classical pathway results in low levels ofC4, C2andC3; activation of alternative pathway is characterized by reduced levels of C3 and normal levels of C4 and C2. Activation of C3 by eitherpathwayresultsinformationofthemembraneattack complex, which binds to the surface of bacteria, fungi and viruses leadingto theirlysis. C3b componentcan opsonize
immune complexes or foreign cell surface anaphylatoxin, includingC3a,C4aandCSa, bindtoreceptorsonmastcells andbasophils,resultingintheir degranulation andrelease of histamine and intracellular enzymes. C3a and CSa inducetheadherenceofmonocytes,macrophageandneu trophilsto vascular endothelialcellscausing extravasation and chemotaxis at the site of inflammation.
Cellular components of innate immunity comprises polymorphonuclear leukocytes, macrophages and natural killer (NK) cells. These ingest extracellular material by phagocytosis. The activation of myeloperoxidase in phagolysosomes results in production of superoxide that oxidizes and inactivates microbial proteins.
Adaptive Immune System
Adaptiveimmuneresponsesdevelopthroughcooperation between lymphocytes and antigen presenting cells following specific antigenic challenge, show tremendous diversityandexhibitimmunologicalmemory. Thecompo nents of adaptive immune system are lymphocytes, macrophages and antigen presenting cells. These cells developandmature in theprimary lymphoidorgans (bone marrow, thymus) and interact with foreign antigens in secondary lymphoid organs (spleen, lymph nodes, mucosa associatedlymphoidtissues, e.g. tonsilsandPeyer patches).
Lymphocytes constitute 20-40% of white cells in the peripheral blood and are classified as B cells, T cells and NK cells. T cells are identified by the presence of T cell antigen receptor (TCR), which is associated with CD3 complex to form the TCR-CD3 complex that remains unchanged during cell division. The TCR recognizes antigen only when it is bound to MHC molecules on the surface of antigen presenting cells. Mature T lymphocytes are distinguished into CD4+ cells and CD8+ cells based on the presence of membraneglycoprotein molecules. The normal ratio of the number of CD4+ and CD8+ cells in
184
