
Ghai Essential Pediatrics8th
.pdf
---------------------------------------N_u_tr-it-ion
3-4 mEq/kg/day for at least 2 weeks. Potassium can be givenassyrup potassium chloride;thecommonpreparation available has 20 mEq of potassium/15 ml.
Onday 1, 50% magnesium sulfate (equivalent to4 mEq/ ml) should be given at 0.3 ml/kg to a maximum of 2 ml intramuscularly. Thereafter, 0.8-1.2 mEq/kg magnesium should be given orally as a supplement mixed with feeds.
Step 5: Treat/Prevent Infection
Infection may not produce the classical signs of fever and tachycardia in severely malnourished children. Instead, severe infection may be associated with hypothermia. Localizing signs of infection are often absent. The most common sites for infection are the skin, the alimentary tract, the respiratory tract (including the ears, nose and throat) and the urinary tract. Majority of the infections and septicemia are caused by gram-negative organisms. Therefore, all severely malnourished children should be assumed to have a serious infection on their arrival in hospital. In addition, hypoglycemia and hypothermia are considered markers of severe infection in children.
The following investigations are done for identifying infections: (i) Hb, TLC, DLC, peripheral smear, (ii) urin alysis antituberculous and culture, (iii) blood culture, (iv) chest X-ray, (v) Mantoux test, (vi) gastric aspirate for AFB, (vii) peripheralsmearformalaria (in endemic areas), and (viii) CSF examination (if meningitis is suspected).
All children with suspected infection should be treated with broad spectrumparenteralantibiotics; ampicillinand gentamicin or amikacin (Table 6.12). Antimalarial and antituberculous treatmentshould only be given when the particular conditions are diagnosed.
Response to treatment will be indicated by resolution of initial symptoms and signs of infection, if any. The child's activity, interaction with parents and appetite should improve.Ifthereisnoimprovement ordeterioration of the symptoms/signs of infection, the child should be screened for infection with resistant bacterial pathogens, tuberculosis, HIV and unusual pathogens.
Prevention of Hospital Acquired Infection
The health care personnel should follow standard precautions.The effectiveness of hand hygiene should be
emphasized to all health care providers, attendants and patients. It is essential that adequate safety measures are taken to prevent the spread of hospital acquired infections, since these children are at higher risk of acquiring infections due to their compromised immune status.
Step 6: Correct Micronutrient Deficiencies
All severely malnourished children have vitamin and mineral deficiencies. Micronutrients should be used as an adjunct to treatment in safe and effective doses. Up to twice the recommended daily allowance of various vitamins and minerals should be used. Although anemia is common,ironshouldnotbe given initiallydue to danger of promoting free radical generation and bacterial proliferation. It should be added only after a week of therapy when the child has a good appetite and starts gaining weight.
Vitamin A deficiency is not an infrequent association and is an important cause of blindness caused by keratomalacia. Vitamin A should thereforebe given to all severely malnourished children on day 1 at 50,000 IU, 100,000 IU and 200,000 IU for infants 0-5 month, 6-12 months and children>1 yr of age unless there is definite evidence that a dose has been given in the last month. In presence of xerophthalmia, the same dose should be repeated on thenextdayand2weekslater. Children>1 yr but weighing <8 kg should receive half the age related dose. In presence of clinical evidence of xerophthalmia the administration of vitamin A should be considered an emergency as the changes may progress to keratomalacia within hours.
Vitamin K should be administered in a single dose of 2.5 mg intramuscularly at the time of admission. Daily multivitamin supplements containing thiamine 0.5 mg/ 1000 kcal, riboflavin 0.6 mg/1000 kcal and nicotinic acid (niacin equivalents) 6.6 mg/1000 kcal should be given. It is better to give a formulation that is truly multivitamin (e.g. one that has vitamin A, C, D, E and B12). Folic acid 1 mg/day (5 mg on day 1), zinc 2 mg/kg/day and copper 0.2-0.3 mg/kg/day should be given daily. Iron 3 mg/kg/ day shouldbe added oncechild startsgainingweight,after the stabilization phase.
Table 6.12: Recommended antibiotics for infections in severely malnourished children
Type of infection
No obvious infections or complications
Infected child or complications
For septic shock or no improvement or worsening
Meningitis in initial 48 hr Dysentery
Recommended antibiotics
Oral cotrimoxazole (5 mg/kg 12 hourly of trimethoprim) or oral amoxicillin 10 mg/kg 8 hourly for 5 days
IV ampicillin 50 mg/kg/dose 6 hourly and IV gentamicin 5-7 mg/kg/day in 1-2 doses; add IV cloxacillin 100 mg/kg/day 6 hourly if staphylococcal infection is suspected; revise therapy based on the culture sensitivity report
Add third generation cephalosporin, i.e. IV cefotaxime 100 mg/kg/day 8 hourly
IV cefotaxime 200 mg/kg/day IV 6 hourly with IV amikacin 15 mg/kg/day 1-2 doses Ciprofloxacin 20 mg/kg/day in 2 divided doses; IV ceftriaxone 50 mg/kg/day 12 hourly if child is sick or has already received nalidixic acid

Essen tiaiPed ia tr ics |
_____________ |
_____________ |
|
__ |
_ _ _ _ _ _ _ _ _ _ _ _ _ - _ _______ |
|
|
|
|
|
|
Emergency treatment of severe anemia If a severely malnourished child has severe anemia with a hemoglobin less than 4 g/dl or between 4 and 6 g/dl but with respiratory distress, a blood transfusion should be given with whole blood 10 ml/kg bodyweight slowly over 3 hr. Furosemideshould be given at the start of the transfusion. If the severely anemic child has signs of cardiac failure, packedcellsrather than whole blood shouldbe transfused.
The hemoglobin concentration may fall during the first week of treatment. This is normal and no transfusion should be given. In mild to moderate anemia, iron should be given for two months to replete iron stores but this should not be started until after the initial stabilization phase has been completed.
One should begin with 80 kcal/kg/day and gradually increase to 100 kcal/kg/day. To fulfill this, one should start with 2 hourly feeds of 11 ml/kg/feed. Night feeds are essential. The volume of feeds are increased gradually while decreasing the frequency of administration. The calories are increased only after the child can accept the increased volume of feeds.
Step 8: Achieve Catchup Growth
Once appetite returns, higher intakes should be encouraged. Starter F-75 feeds should be gradually replaced with feeds which have a higher calorie density (100 kcal/100 ml) and have at least 2.5-3.0 g protein/100 ml. These feeds are called F-100 diets (Table 6.14). It is recommended that each successive feed is increased by 10 ml until some is left
Step 7: Initiate Re-feeding
Feeding should be started as soon as possible with a diet which has osmolarity less than 350 mOsm/1; lactose not more than 2-3 g/kg/day; appropriate renal solute load (urinary osmolarity <600 mOsm/1); initial percentage of calories from protein of 5%; adequate bioavailability of micronutrients and low viscosity. The preparation should be easy to prepare and socially acceptable and there should be facilities for adequate storage, cooking and refrigeration.
Feedingshouldbe started as soon as possible as frequent small feeds. If child is unable to take orally with a cup and spoon or takes <80% of the target intake, nasogastric feeds should be initiated. Breastfeeding should be continued ad libitum. The suggested starter formulae are usually milk based, such as starter F-75 (with 75 kcal/100 ml and 0.9 g of protein/100 ml). Older children could be started on cereal based diets (Table 6.13).
|
Table 6.14: Catchup diets |
|
Diet contents |
|
F-100 |
(per 100 ml) |
|
Catchup |
Cow milk/toned dairy milk (ml) |
95 |
|
|
||
(Approximate measure of one katori) (3/4)
Sugar (g) |
5 |
(Approximate measure of one |
(1) |
level teaspoon) |
|
Cereal: Puffed rice (g) |
|
(Approximate measure of one |
|
level teaspoon) |
|
Vegetable oil (g) |
2 |
(Approximate measure of one |
(1/2) |
level teaspoon) |
|
Water to make (ml) |
100 |
Energy (kcal) |
101 |
Protein (g) |
2.9 |
Lactose (g) |
3.8 |
F-100 Catchup (cereal based)
75
(1/2) 2.5 (1/2)
7
(2)
2 (1/2)
100
100
2.9
3
|
Table 6.13: Starter diets |
|
Diet contents (per 100 ml) |
F-75 Starter |
F-75 Starter (cereal based) |
|
|
Example: 1 |
Cow milk or equivalent (ml) |
30 |
30 |
(Approximate measure of one katori) |
(1/3) |
(1/3) |
Sugar (g) |
9 |
6 |
(Approximate measure of one level teaspoon) |
(l1h) |
(1) |
Cereal: Powdered puffed rice• (g) |
|
2.5 |
(Approximate measure of one level teaspoon) |
|
(3/4) |
Vegetable oil (g) |
2 |
2.5 |
(Approximate measure of one level teaspoon) |
(1/2) |
(1/2) |
Water: make up to (ml) |
100 |
100 |
Energy (kcal) |
75 |
75 |
Protein (g) |
0.9 |
1.1 |
Lactose (g) |
1.2 |
1.2 |
F-75 Starter (cereal based) Example: 2
25 (1/4) 3 (1/2) 6
(2)
3 (3/4) 100 75 1.2 1.0
• Powdered puffed rice may be replaced by commercial precooked rice preparations (in same amounts)
Whereverfeasible, actual weighing of the constituents shouldbe carriedout. Household measure shouldbe used only as an alternative, as they may not be standardized
The above charts give the composition for 100 ml diet. Wherever, there is a facility for refrigeration, 1 liter diet could be prepared by multiplying the requirement of each constituent by 10

--------------------------------------N_u_t_ri-ti_o_n__
uneaten. The frequency of feeds gradually decreased to 6 feeds/day and the volume increased till the child is being offered 200 ml/kg/day and 4-6 g/kg/day of protein. Breastfeeding should be continued ad libitum.
Ready to use therapeutic food (RUTF) In the 1990s, the
RUTF was developed, which has allowed much of the management of severe malnutrition to move out of hospitals,byshorteningthedurationofinpatient treatment from an average of 6 weeks to only 5-10 days.This energy dense, mineral and vitamin enriched ready to use therapeutic foodwithasimilarnutrient profile but greater energy and nutrient density than F-100 has greatly improvedcosteffectivenessoftreatingseveremalnutrition. F-100, although extremely effective during rehabilitation phase in inpatient centers, is very vulnerable to bacterial contamination and must be used within a couple of hours of being made. This restricts its use to inpatient facilities. RUTF is an oil based paste and as such can be stored at home unrefrigerated with little risk of microbial contamination for several months. The daily amount of RUTF tobe consumed varies according to body weight as follows: 3-4.9 kg: 105-130 g; 5-6.9 kg: 200-260 g; 7-9.9 kg: 260-400 g and 10-14.9 kg: 400-460 g. This amount is to be given along with plenty of water in 2-3 hourly feeds. The child should continue to receive other foods and brestfeedingduringmedical nutritiontherapywithRUTF.
Complementary foods should be added as soon as possible to prepare thechild for home foods at discharge. They should have comparable energy and protein con centrations once the catchup diets are well tolerated. Khichri, dalia, banana, curd-rice and other culturally acceptable and locally available diets can also be offered liberally (see Table 6.2).
Special diets for diarrhea For children with persistent diarrhea, who do not tolerate low lactose diets, lactose free diet can be started. In these diets, carbohydrates (rice, sugar and glucose) can be given in varying proportions according to the patients' individual tolerance to achieve optimal balance between osmolarity and digestibility.
Monitoring progress during treatment Ifthere is a good weight gain of>10 g/kg/day, the same treatment should becontinuedtillrecovery.Ifthereisamoderateweightgain of 5-10 g/kg/day; food intake should be checked and the children should be screened for systemic infection. In case of poor weight gain of <5 g/kg/day possible causes like inadequate feeding, untreated infection, psychological problems and coexisting infections like tuberculosis and HIV should be looked for and managed appropriately.
Step 9: Provide Sensory Stimulation and
Emotional Support
Delayed mental and behavioraldevelopment often occurs in severe malnutrition. In addition to the above manage ment, one should encourage a cheerful, stimulating
environment; structured play therapy for at least 1530 min/day; physical activity as soon as the child is well enough and tender loving care.
Step 10: Prepare for Followup after Recovery
The child is said to have recovered when his weight for height is 90% of the NCHS median and he has no edema. Thechildis still likely to have a low weight for agebecause of stunting.
Criteria for discharge and failure of response Ideally 6-8 weeks of hospitalization is required for complete recovery. The child is said to have recovered when his weight for height is 90% of the NCHS median or has 15% weight gain, and he has no edema. The child may be discharged earlier if it is certain that the final stages of recovery will not be jeopardized by early discharge.
Severelymalnourishedchildrenare ready for discharge when the child:
•Is alert and active, eating at least 120-130 kcal/kg/day with a consistent weight gain (of at least 5 g/kg/day for 3 consecutive days) on exclusive oral feeding
•Is receiving adequate micronutrients
•Is free from infection
•Has completed immunization appropriate for age
•The caretaker has been sensitized to home care.
The caregiver should be advised to bring child back for regular followup checks, ensure booster immunizations, make sure that vitamin A is given every six months, feed frequently with energy and nutrient dense foods and give structured play therapy.
Criteria for discharge before recovery is complete
For some children, earlier discharge may be considered if effective alternative supervision is available. Domiciliary care should only be considered if the child:
•Is aged>12 months
•Has a good appetite with satisfactory weight gain
•Has completed antibiotic treatment
•Hastaken2weeksof potassium/magnesium/mineral/
vitamin supplement (or continuing supplementation at home is possible).
It is important to be sure that the mother/caretaker has the financial resource to feed the child, is specifically trained to give appropriate feeding (types, amount, frequency), lives within easy reach of the hospital, is trained to give structured play therapy and is motivated to follow advice given.
Care at home For children being rehabilitated at home, it is essential to give frequent meals with a high energy and protein content. One should aim at achieving at least 150 kcal/kg/day and adequate protein (at least 4 g/kg/ day). This would require feeding the child at least 5 times perday withfoods that containapproximately100 kcaland 2-3g protein per 100g offood. A practicalapproachshould be taken using simple modifications of usual staple home

___E_s_s_e_n_t _ia_l_P_e_d_ i _at_r_ics_________________________________
;,
I
foods.Vitamin, iron and electrolyte/mineral supplements can be continued at home. High energy snacks should be given between meals (e.g. milk, banana, bread, biscuits). The child should be assisted and encouraged to complete each meal.
|
|
Community-based Therapeutic Care |
|
|
A growing number of countries and international relief |
|
|
agencies have adopted a new concept called the |
|
|
Community-based Therapeutic Care (CTC) for the |
|
|
management of severe acute malnutrition. The CTC |
|
|
concept combines facility or inpatient management of |
|
|
severe acute malnutrition with complications, and |
|
|
community-based management of severe acute mal |
|
|
nutrition without complications or mild or moderate |
|
|
malnutrition. These facility-based and community-based |
|
|
components of management should be closely linked so |
|
|
that children who are too ill to be treated at the community |
|
|
level or who are not responding to treatment can be |
|
|
referred to the facility level and those receiving facility |
|
|
based treatment who have regained their appetites can |
|
|
be transferred for continued care in the community. For |
|
|
this community-based therapeutic care approach a |
|
|
suggested classification and treatment system for acute |
|
|
malnutrition is given in Table 6.15. |
: |
···· ol |
Phenomena Encountered during |
Nutritional Rehabilitation |
||
Pseudotumor cerebri Overenthusiastic nutritional |
||
; |
|
correction in malnourished infants may be accompanied |
|
|
|
|
|
by transient rise of intracranial tension. The phenomenon |
|
|
is benign and self limiting. |
|
|
Nutritional recovery syndrome It refers to a sequence of |
|
|
events seen in children who are being treated with very |
|
|
highquantityofproteins duringthecourseofrehabilitation. |
|
|
It presents as (i) abdominal distention, (ii) increasing |
|
|
hepatomegaly, (iii) ascites, (iv) prominent thoracoabdo |
|
|
minal venous network, (v) hypertrichosis, (vi) parotid |
|
|
swelling, (vii) gynecomastia, (viii) eosinophilia, and |
|
|
(ix) splenomegaly. Its development may be related to |
endocrinal disturbances, possibly by an increase in the estrogen level and by a variety of trophic hormones produced by the recovering pituitary gland.
Encephalitis like syndromes Up to one-fifth of patients with kwashiorkor may become drowsy within 3-4 days afterinitiationofdietarytherapy.Most oftenthecondition is self limiting. Occasionally, it may be accompanied by progressive unconsciousness with fatal outcome. Even more rarely a transient phenomena marked by coarse tremors, parkinsonian rigidity, bradykinesia and myo clonus may appear several days after starting the dietary rehabilitation. These encephalitis states are considered to be the result of toomuch proteins in the diet.
Prevention of Malnutrition
Improvement of nutrition status of children is an essential component of health care.
Prevention at National Level
Nutrition supplementation. This can be done by improve ment of food and feeding; by fortification of staple food; iodination of common salt and food supplementation.
Nutritional surveillance. Surveillance defines the character and magnitude of nutritional problems and selects appropriate strategies to counter these problems.
Nutritional planning. Nutritional planning involves a political commitment by the government, formulation of a nutrition policy and planning to improve production and supplies of food and ensure its distribution.
Prevention at Community Level
a.Health and nutritional education. Lack of awareness of the nutritional quality of common foods, irrational beliefs about certain foods and cultural taboos about feedingcontributeto the development of malnutrition. People should be informed of the nutritional quality ofvariouslocallyavailableandculturallyaccepted low cost foods.
Table 6.15: Suggested classification and treatment system for malnutrition
Suggested classification |
Suggested treatment |
Severe acute malnutrition with complications like anorexia, lower respiratory tract infection, high fever, severe dehydration, severe anemia or lethargy
Severe acute malnutrition without complications where the child is clinically well, alert and has a good appetite
Moderate acute malnutrition without complications where weight for height is between 70 and 80%; without edema, or MUAC is 11-12.5 cm
Stabilization center (SC): inpatient care, also known as 'phase 1 treatment', useful for acutely malnourished children with medical complications and no appetites managed using standard WHO guidelines
Outpatient therapeutic program (OPT): home based treatment and rehabilitation with specially formulated ready to use therapeutic feed (RUTF) provided on a weekly or two weekly basis; medical treatment using simplified medical protocols and regular followup for children with severe acute malnutrition without complications Supplementary feeding program (SFP): take home ration for children with moderate acute malnutrition without complications

---------------------------------------N_u_t_ri-t _o_....n -
b.Promotion of education and literacy in the community, especially nonformal education and functional literacy among village women.
c.Growth monitoring. The growth should be monitored periodically on growth cards. Velocity of growth is more meaningful than the actual weight of a child.
d.Integrated health package. Primary health care package should be made available to all sectors of population including preventive immunization, oral hydration, periodicdeworming and early diagnosis and treatment of common illnesses.
e.Vigorous promotion of family planning programs to limit family size.
Prevention at Family Level
a.Exclusive breastfeeding of infants for first 6 months of life should be vigorously promoted and encouraged.
b.Complementaryfoods should be introduced in the diet of infants at the age of 6 months.
c.Vaccination.
d.Iatrogenic restriction of feeding in fevers and diarrhea should be discouraged.
e.Adequate time should be allowed between two pregnancies so as to ensure proper infant feeding and attention to the child before the next conception.
Integrated Child Development SeNices (/CDS)
Programme
The ICDS programme is an intersectoral program which seeks to directly reach out to children, below six years, especially from vulnerable groups and remote areas. The Scheme provides an integrated approach for converging basic services through community-based workers and helpers. The services are provided at a center called the 'Anganwadi'. A package of six services is provided under the ICDS Scheme:
a.Supplementarynutrition. ThenormsaregiveninTable 6.16
b.Immunization. Immunization of pregnant women and infants is done against the six vaccine preventable diseases.
c.Nonformal preschool education.
d.Health check-up. This includes health care of children less than six years of age, antenatal care of expectant mothers and postnatal care of nursing mothers. These services are provided by the ANM and Medical
Table 6.16: Honns for supplementary nutrition in ICDS
Beneficiaries |
Calories, kcal |
Protein, g |
Children <3 yr |
300 |
8-10 |
Children 3-6 yr |
300 |
8-10 |
Severely malnourished |
Double of above |
|
children |
|
|
Pregnant and lactating |
500 |
20-25 |
mothers
Officers under the RCH programme. The various health services include regular health check-ups, immunization, management of malnutrition, treatment of diarrhea, deworming and distribution of simple medicines.
e.Referral services. During health check-ups and growth monitoring, sick or malnourished children are referred to the Primary Health Centre or its subcenter.
f.Nutrition and health education.
National Programme of Mid-day Meals in Schools
With a view to enhancing enrolment, retention and attendance and simultaneously improving nutritional levels among children, the National Programme of Nutritional Support to Primary Education (rechristened National Programme ofMid-dayMeals in Schools in 2007) was launched as a centrally sponsored scheme on 15th August 1995, initially in 2408 blocks in the country. The National Programme of Mid-day Meals in Schools covers approximately 9.70 crore children studying at the primary stage of education in 9.50 lakh Government (including local bodies), Government aided schools and the centers run under Education Guarantee Scheme and Alternative and Innovative Education Scheme.
The program provides a mid-day meal of 450 kcal and 12 gofprotein tochildrenattheprimarystage.Forchildren at the upper primary stage, the nutritionalvalue is fixed at 700 kcal and20 g ofprotein. Adequate quantities of micro nutrients likeiron, folic acid and vitaminA arealso recom mended. The program has helped in protecting children from classroom hunger, increasing school enrolment and attendance, improved socialization among children belongingto all castes, addressing malnutrition andsocial empowerment through provision of employment to women.
National Nutrition Anemia Prophylaxis Programme
This program was launched in 1970 to prevent nutritional anemia in mothers and children. Under this program, the expected andnursingmothersaswellas acceptorsoffamily planning aregivenone tabletcontaining100 mg elementary iron and 0.5 mg of folic acid. Children in the age group of 1-5 yr are given one tablet containing 20 mg elementary iron (60 mg offerrous sulfate) and 0.1 mg of folic acid daily for a period of 100 days.
Suggested Reading
Dalwai S, Choudhury P, Bavdekar SB, Dalal R, et al. Indian Academy of Pediatrics. Consensus statement of the Indian Academy of Pediat rics on integrated management of severe acute malnutrition. Indian Pediatr 2013;50:399-404
WHO Child Growth Standards and the identification of severe acute malnutrition in infants and children. A joint statement by WHO and UNICEF. 2009. Accessed from http//who.int/nutrition/publications/ severemalnutrition/9789241598163-eng.pdf

Micronutrients in
Health and Disease
Ashima Gulati, Arvind Bagga
Vitamins are organic compounds, required in tiny amounts, that cannot be synthesized byan individual, and must be obtained from the diet. Vitamins perform diverse bio-chemical functions, including as hormones (e.g. vitaminD), antioxidants (e.g. vitamin E),mediators of cell signaling and regulators of tissue growth and differen tiation (e.g. vitamin A). Several vitamins (e.g. B complex vitamins) function as precursors for enzyme cofactor biomolecules (coenzymes) that help act as catalysts and substrates in metabolism. Fat soluble vitamins (A, D, E, K) control protein synthesis at either transcriptional or post-transcriptional level. Breast milk is deficient in vita mins D and K and exclusively breastfed infants must be supplemented with these vitamins.
Certain minerals are required in trace or small amounts tosupportbiochemicalprocessesinvolved incellstructure and function. Important mineralsincludecalcium,chloride, cobalt,copper,iodine,iron,magnesium,manganese,molyb denum, nickel, phosphorus, potassium, selenium, sodium, sulfur and zinc. Essential trace elements are required in amounts ranging from 50 µg to 18 mg per day, and act as catalyticorstructuralcomponentsoflargermolecules.Mar ginalorsevereimbalancesintraceelementsareconsidered risk factors for several diseases. In addition to deficiencies ofironandiodine,featuresofdeficiencyofcopper,zincand selenium are recognized.
Intakes of micronutrientsrecommendedby theNational Academy of Science 2006 are available at www.nap.edu. Intakes proposed by the Indian Council of Medical Research in 2010 are listed in Table 6.1 and are also available at icmr.nic.in/final RDA-2010.pdf.
FAT SOLUBLE VITAMINS
Vitamin A
Vitamin A (retinol) refers to compounds structurally related to retinol thathave biological activity. Six isomers are known, including 5 cis-forms and trans-retinal. The active forms of vitamin A are the oxidation products of
retinol, all-trans-retinal and all-trans-retinoic acid. Carotenoids are provitamin A substances found in vegetables. All-trans -carotene is the most effective precursor and is widely distributed.
Absorption and metabolism Vitamin A is absorbed as an ester, as part of chylomicrons. Absorption is affected by impaired chylomicron formation and altered fat absorption. Retinol is absorbed asfreealcohol by an active transport system containing a cellular retinol binding protein (RBP) II. The yellow -carotene requires bile salts for absorption and is converted to vitamin A in the intestines. Once absorbed, vitamin A is stored in the liver as retinyl palmitate. The liver releases vitamin A to the circulation, bound to RBP and transthyretin.
Sources The richest sources of preformed vitamin A include oils extracted from shark and cod liver. Carrots, dark-green leafyvegetables,squash,orangesand tomatoes are also good sources. Many processed foods and infant formulas are fortified with preformed vitamin A.
Recommended daily allowance The recommended daily allowance of vitamin A is as follows: (i) infants 300400 µg; (ii) children 400-600 µg; (iii) adolescents 750 µg. 1 µg retinol = 3.3 international units (IU) of vit A;
= 12 µg -carotene. Hence, 30 mg retinol = 100,000 IU vit A.
Physiological functions The main functions of vitamin A are: (i) maintenance of vision, especially night vision; (ii) maintenance of epithelial tissues and (iii) differen tiationof various tissues, particularlyduringreproduction and gestation by regulating gene expression. The role of vitamin A in vision is related to the retinal form. Within the eye, 11-cis-retinal is bound to rhodopsin (rod cells) and iodopsin (cones). As light enters the eye, 11-cis-retinal is isomerized to the all-trans form. The all-trans-retinal dissociates from the opsin in a series of steps called bleaching. This isomerization induces a nervous signal
110

along the optic nerve to the visual center of the brain. Subsequently, the all-trans-retinal is recycled and converted to 11-cis-retinal form via a series of enzymatic reactions. Some of the all-trans-retinal is converted to all trans-retinol and transported with an interphotoreceptor RBP to pigment epithelial cells. Further esterification into all-trans-retinyl esters allows this form to be stored within the pigment epithelial cells to be reused when needed. Deficiency in vitamin A inhibits the reformation of rhodopsin and leads to night blindness.
Vitamin A Deficiency
In developing countries, a large number of preschool childrenbecomeblindowing tovitaminA deficiency, and many die because of increased vulnerability to infections especially measles. Defective dark adaptation is a characteristic early clinical feature, resulting in night blindness.The syndrome of vitamin Adeficiency in infants consists of Bitot spots, xerophthalmia, keratomalacia, cornealopacities (Fig. 7.1), hyperkeratosis,growthfailure and death. The deficiency disease in humans was called xerophthalmia (dry eyes) because of the prominence of the eye signs (Table 7.1). Other findings include infertility, metaplastic bones and keratinization of epithelial tissue particularly in theskin, genitourinary systemand thelung. Urinary calculi are common and fetal abnormalities are seen in pregnancy. Diets consisting of polished rice with little or no vegetables or fruits increase the risk of xerophthalmia.
Fig. 7.1: Bilateral keratomalacia in a child with protein energy malnutrition (PEM) severe vitamin A deficiency precipitated by an episode of pneumonia. Note the bilateral corneal opacification and corneal perforation in the left eye
Table 7.1: WHO classification of xerophthalmia
Primary signs |
Secondary signs |
||
XlA Conjunctiva! xerosis |
XN Night blindness |
||
XlB Bitot's spots |
XF |
Fundal changes |
|
X2 |
Corneal xerosis |
XS |
Corneal scarring |
X3A |
Corneal ulceration (<1/3 of cornea) |
|
|
X3B |
Corneal ulceration (>1/3 of cornea) |
|
|
Micronutrients in Health and Disease -
Laboratory tests show mild leukopenia and serum retinol level of 15 µg/dl or less (normal 20 to 80 µg/dl). Clouding ofthecorneain a childwithvitaminAdeficiency is an emergency and requires parenteral administration of 50,000 IU to 100,000 IU (15 to 30 mg retinol).
Treatment of vitamin A deficiency Specific treatment consists of oral vitamin A at a dose of 50,000 IU, 100,000 IU and 200,000 IU in children aged <6 months, 6-12 months and>1 yr, respectively. The same dose is repeated next day and 4 weeks later. Alternatively, parenteral water-soluble preparation are administered in children with persistent vomiting or severe malabsorption (parenteral dose is half the oral dose for children above 6-12 months and 75% in <6 months old). Local treatment with antibiotic drops and ointment and padding of the eye enhances healing.
Prevention Under the National Vitamin A Prophylaxis program, sponsored by the Ministry of Health and Family Welfare, children between 1 and 5 yr were previously given oral doses of 200,000 IU vitamin A every six months. Evaluation studies since then revealed inadequate coverage in most states. Currently, vitamin A is given only to children less than three years old since they are at greatest risk and the administration of the first two doses is linked with routine immunization to improvethecoverage. Hence, a dose of 100,000 IU is given with measles vaccine at 9 months and 200,000 IU with the DPT booster at 15-18months.Inendemicareas 3 more doses are administered at 24, 30 and 36 months. Dietary improvement is necessary to prevent vitamin A deficiency.Childrenwithmeaslesandseveremalnutrition should receive vitamin A at 100,000 IU if <1-yr-old and 200,000 IU if older.
Carotenemia
P-carotene is an important precursor of vitamin A in vegetable-based diets; 6 µg P-carotene has the biological potencyof 1 µgretinol.Excessivedietaryintakeof carotene containing foods, most commonly carrots and carrot containing products, does not produce symptoms other than yellow skin pigmentation. Carotene is normally present in keratin and subcutaneous fat. At high plasma levels, yellow pigmentation (carotenemia) shows in superficial skin (face, palms and soles), but not in sclerae. The color returns to normal within 2-6 weeks of discon tinuing intake of carrots. Carotenemia might be mistaken for jaundice.
HypeNitaminosis A and Teratogenicity
Toxicity has been observed in those ingesting more than 50,000 IU/day of vitamin A for several months in form of fish liver oil, therapeutic vitamin preparations or, in adole scents, as retinol or retinoic acid for acne. Acute manifestations include pseudotremor cerebri (vomiting, irritability, bulging fontanel, diplopia, headache). Patients

__E_s_s_e_n_ tia_l_P_e_di_atric_s_________________________________
with chronic hypervitaminosis may have dermatitis, alopecia, hepatosplenomegalyand/or hyperostosis.When taken by pregnant women in early gestation at daily levels ofmorethan7500 µg,fetal anomalies andpoorreproductive outcomes are reported. The WHO recommends that vitamin A intake during pregnancy should not exceed 3000 µg daily or 7500 µg every week.
Vitamin D
Vitamin Dis thegeneric term for secosteroids,which have an important role in maintaining calciumandphosphorus homeostasis. Secosteroids have three intact rings and one open ringwithconjugateddouble bonds. Various vitamin D metabolites differ in the side chains attached to the fourth ring. Vitamin D is a group of precursors of a hormone, 1,25-dihydroxycholecalciferol, synthesized and secreted by thekidneys under thecontrol of parathormone and tissue phosphate levels (Fig. 7.2). Dietary vitamin D is essential if the cutaneous synthesis of vitamin D3 is insufficient. When deficient, disease manifestations include rickets, with defective mineralization of growing bone, and osteomalacia with impaired mineralization of non-growing bones.
Absorption, metabolism and mechanism of action
Vitamin D refers to two prohormones, vitamin D2 (ergo calciferol; derived from plants) and D3 (cholecalciferol, available from animal sources) and their derivatives. Vitamin D is absorbed in the duodenum by an active transport system. In the enterocyte, vitamin D is incor porated into chylomicrons and transported to the liver,
where its hydroxylation take place to form 25-hydroxy vitaminD2 [250HD2]and25-hydroxyvitaminD3 [250HD3], known as ercalcidiol and calcidiol respectively. This hydroxylation is substrate dependent and without any negative feedback control. Calcidiol is released into the bloodstreamandhasa biological half-life ofapproximately 3 weeks. Subsequent hydroxylation by la-hydroxylase in the proximal renal tubule leads to formation of 1,25dihydroxyvitamin D2 [l,25(0H)iD2] or ercalcitriol and 1,25-dihydroxyvitamin D3 [l,25(0H)iD3] or calcitriol(Fig. 7.2). While circulating l,25(0H)iD almost exclusively results from renal production, extrarenal conversion also takes place in the skin, colon, macrophages, vascular smooth muscle cells, bone and parathyroid glands. Renal conversion is regulated by parathormone, calcitonin, calcium and phosphate; and inhibited by calcitriol and the phosphaturic hormone, fibroblast growth factor 23 (FGF-23). A negative relationship exists between serum 250HD and parathormone levels (Fig. 7.2). Vitamin D supplementation suppresses serum parathormone and increases bone mineral density.
Active vitamin D affects calcium homeostasis through its action on the intestine, kidney and bones. In the intestine,thehormone induces calciumtransport proteins and an intracellular calcium-binding protein (calbindin) which aid in transport of calcium across the enterocyte. In the kidney, the hormone enhances calcium resorption in the tubule by a similar mechanism. It also inhibits the activity of la-hydroxylase and stimulates renal 24hydroxylaseactivitythatinactivates both thesubstrateand calcitrio1.
Dehydrocholesterol |
|
Dietary (vitamin 02, 03) |
|||||||
|
V mioD/ |
||||||||
24,25-dihydrox |
yvitamin D .__ |
24·hyd_rox_y1_as_e |
|
l |
; !: roxylase |
|
|||
|
|
|
|
|
|
||||
(inactive) |
- |
_ 25-hydroxyvitamin D |
|
||||||
|
|
|
- |
|
Kidney |
|
|||
|
|
|
|
|
1a·hydroxylase |
P |
|||
|
|
|
|
|
+l1 |
|
+ |
|
arathormone |
|
|
|
|
|
|
|
Low phosphorus |
||
|
|
|
|
|
I |
• ::. - - - - - - - Fibroblast growth factor |
|||
|
|
|
1,25-dihydroxyvitamin D ----• Decrease parathormone |
||||||
Bone |
|
|
Kidney |
|
|
|
Intestine |
||
|
|
Increased calcium and |
Increased calcium and |
||||||
Increased mineralization |
|
||||||||
|
phosphate reabsorption |
phosphate absorption |
|||||||
Fig. 7.2. Vitamin D metabolism. Serum levels of fibroblast growth factor (FGF) 23 are elevated in response to increased serum phosphate and also inhibit the production of parathormone

Sources The major source of vitamin D is its synthesis in the skin following exposure to ultraviolet B (UVB) solar irradiation(wavelength 290-315nm). The dermal concen tration of melanin regulates the amount of UV rays that reach the epidermal layers (stratum basale and spinosum) containting the highest concentrations of the substrate, 7-dehydrocholesterol. The time required for adequate sun exposuredepends on skinpigmentation,timing of theday, season and clothing. The exposure of the skin to UV-B is measuredas theminimumerythema dose (amountofUV B exposure that will cause minimal erythema). Exposure of40% ofthe bodytoone-fourthoftheminimum erythema dose results in generation of <1000 IU vitamin D per day. Excessive exposure to sunlight does not increase vitamin D production as previtamin D3 is degraded into inert products such as lumisterol-3 and tachysterol-3, and vitamin D3 photoisomerizes to suprasterol and inert products.
Dietary vitamin D usually accounts for 5-10% of the total vitamin D. Significant dietary sources are fish and fish oils, egg yolk, supplemented cereals and margarine. The amount in vegetable sourcesis negligible,and dietary intakes arelow in the absence of food fortification. Human milk contains only 30-40 IU/L. Exposure to sunlight and vitamin D supplementation to the nursing mother can increase the vitamin D content in breast milk.
Vitamin D requirements Since vitamin D3 is produced endogenously in the skin through the action of sunlight on7-dehydrocholesterol,thereisnonutritionalrequirement for vitamin D when sufficient sunlight is available. However, when shielded from sunlight, breastfed infants will develop rickets unless supplemented with vitamin D. The recommended daily allowance in infants is 5 µg (200IU)perdayandchildren 10µg(400IU)perday.Human milk is deficient in vitamin D and contains only 30-40 IU per liter, mostly from 25(0H) D3. Breastfed infants must therefore receive an additional source of vitamin D.
Hypervitaminosis D
An epidemic of 'idiopathichypercalcemia' ininfants,with anorexia, vomiting, hypertension, renal insufficiency and failure to thrive in England in the 1950s was traced to an intake of vitamin D between 2,000 and 3,000 IU/day. In adults, dosages of 10,000 IU/day of vitamin D for several months have resulted in marked disturbances in calcium metabolism with hypercalcemia, hyperphosphatemia, hypertension, anorexia, nausea, vomiting, weakness, polyuria, polydipsia, azotemia, nephrolithiasis, ectopic calcification and renal failure.
Vitamin D Deficiency and Rickets
Alack of adequate mineralization ofgrowingbones results in rickets and that of trabecular bone in osteomalacia. Osteoporosis is due to proportionateloss of bone volume and mineral,which in childrenis often caused byexcessive administration of corticosteroids.
Mlcronutrlents in Health and Disease
Etiology In most developed countries nutritional rickets (vitamin D deficiency rickets) was virtually eradicated by fortification of milk or direct administration of vitamin D. In India and many other developing countries, how- ever, nutritional rickets is still widely prevalent. Recent reports suggest that nutritional rickets is reappearing in thedevelopedcountries,particularlyamongdark-skinned infants who are exclusively breastfed for prolonged periods without vitamin supplements.
Rickets results from deficiency of either calcium or phosphorus, since both are needed for bone minerali zation. The former results from insufficient amount of vitamin D, resulting in secondary hyperparathyroidism; bloodparathormonelevels areraised. Besides poordietary intake and insufficient exposure to sunlight, vitamin D deficiency may result from various malabsorption syn dromes and chronic liver disease. Anticonvulsant drugs induce hepatic cytochrome P450 oxidase that leads to conversion of 25(0H)D3 into its inactive metabolites. Rickets may also occur secondary to severe dietary defi ciency of calcium; such patients show normal serum concentrations of 25(0H)D3 but elevated levels of 1,25(0H)zD3. Calcium supplements alone lead to healing of rickets in such cases.
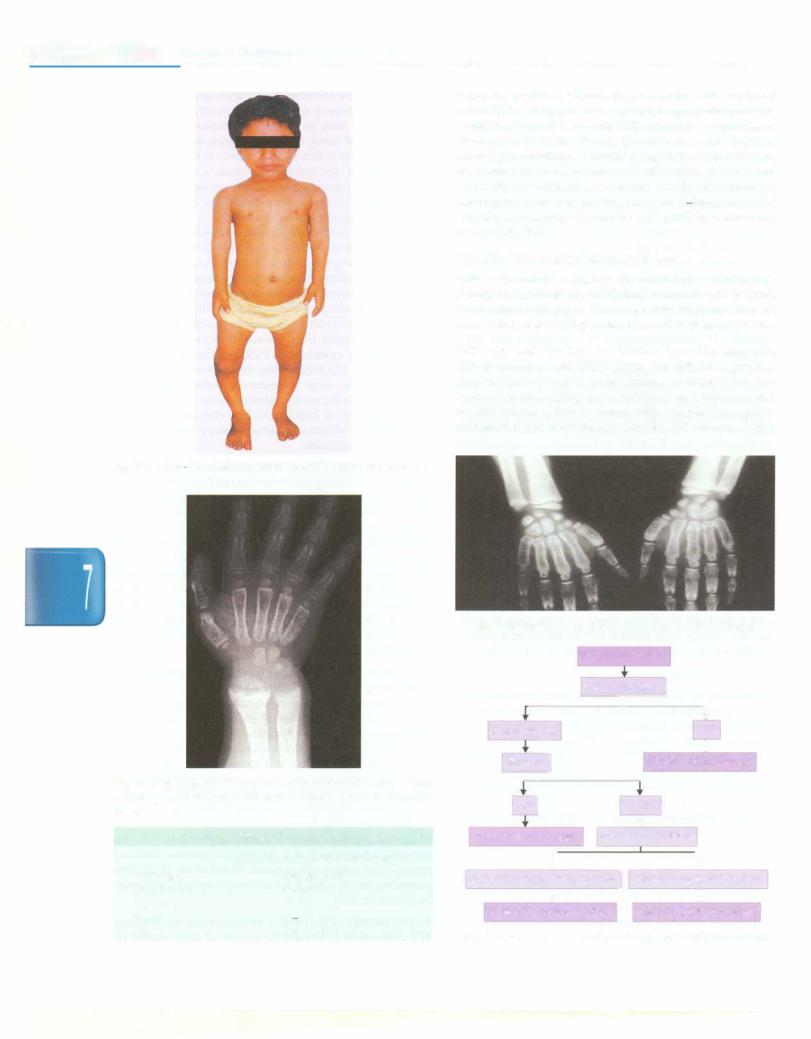
Clinical features These include skeletal deformities, includingbowlegs(genu varum) intoddlers,knock-knees (genu valgum) in older children, craniotabes (soft skull), spinal and pelvic deformities,growth disturbances, costo chondral swelling (rickety rosary), Harrison groove, double malleolidueto metaphysealhyperplasia,increased tendency for fractures, especially greenstick fractures, bone pain or tenderness, muscle weakness and dental problems (Fig. 7.3). Nutritional rickets usually presents in infancy or preschool age, usually as widened wrists or bowing of legs. Presentation inearlyinfancy and findings of seizures or tetany suggest a defect in vitamin D metabolism.
Evaluation Radiologic changes are characteristically seen at the metaphysis. The first change is loss of normal zone ofprovisional calcificationadjacentto themetaphysis.This begins as an indistinctness of the metaphyseal margin, progressing to a frayed appearance with widened growth plate due to lack of calcification of metaphyseal bone (Fig. 7.4). Weight bearing and stress on uncalcified bone gives rise to splaying and cupping of metaphysis. Even tually, a generalized reduction in bone density is seen.
Laboratory diagnosis of vitamin D deficiency is based on low circulating levels of 25(0H)D3. Values below 10 µg/ml are indicative of deficiency (Table 7.2). An increasedplasmalevelof 1, 25(0H)zD3 indicates deficient intake of calcium or phosphorus. Blood levels of alkaline phosphate areelevated;calciumand phosphatelevels may be normal or low.
Management Vitamin D is administered orally either in a single dose of 600,000 IU or over 10 days (60,000 IU
|
s ------- |
---- |
e_s_se_n_1tiPaed_1at_cri ---------- |
||
__ |
---------- |
|
daily for 10 days) followed by a maintenance dose of 400-800 IU/day and oral calcium supplements (3075mg/kg/day)for2 months. Followingadequatetherapy, most patients with vitamin D deficiency rickets show radiological evidence of healing (Fig. 7.5)within 4 weeks. Reduction in blood levels of alkaline phosphatase and resolution of clinical signs occur slowly. If radiologic healing cannotbedemonstrated, despite 1 2- largedosesof vitamin D, patients should be evaluated for refractory rickets (Fig. 7.6).
Familial Hypophosphatemic Rickets
This is the most commonly inherited form of refractory rickets, being inheritedas X-linkeddominantwithvariable penetrance. Sporadic instances are frequent and an autosomal recessive inheritance has also been reported.
Pathogenesis The gene for X-linkedhypophosphatemic rickets is termed the PHEX gene (phosphate regulating gene with homology to endopeptidases on the X chromo some). Theunderlying defect involves impairedproximal tubular reabsorption of phosphate. Despite hypophos phatemia the blood levels of 1,25(0H)z03 are low, which
Fig. 7.3: A 5-yr-old child with rickets with wide wrists and bow legs
Fig. 7.4: Radiograph of wrist in 4-yr-old boy with rickets. Note widening, cupping and fraying at the metaphyseal ends of forearm bones
Table 7.2: Vitamin D levels in serum
|
25-hydroxyvitamin D level (ng/ml) |
Deficient |
Less than 10 |
Insufficient |
10-20 |
Optimal |
20-60 |
High |
60-90 |
Toxic |
Greater than90 |
Fig. 7,5: Healing of the growth plate after vitamin D therapy
|
|
|
Refractory rickets |
|
|
||||
|
|
|
Serum phosphate] |
|
|
||||
|
|
|
|
I |
|
|
|
||
Low or Normal |
|
|
|
|
|
+ |
|||
|
|
|
|
|
High |
||||
Blood pH] |
|
|
|
|
|
+ |
|||
|
|
|
Chronic kidney disease |
||||||
|
|
I |
|
|
|
|
|
|
|
Lowl |
|
|
Normal |
|
|
||||
Renal tubular acidosis |
|
|
... |
|
|
|
|||
Serum PTH, calcium |
i |
||||||||
|
|
i |
|
|
|
|
|
||
High PTH, low or normal calcium] |
Normal PTH, normal calcium |
||||||||
+ |
|
|
|
|
|
+ |
|
||
Vitamin D dependent rickets |
Hypophosphatemic rickets |
||||||||
Fig. 7.6: Biochemical evaluation of a child with refractory rickets
