
Ghai Essential Pediatrics8th
.pdf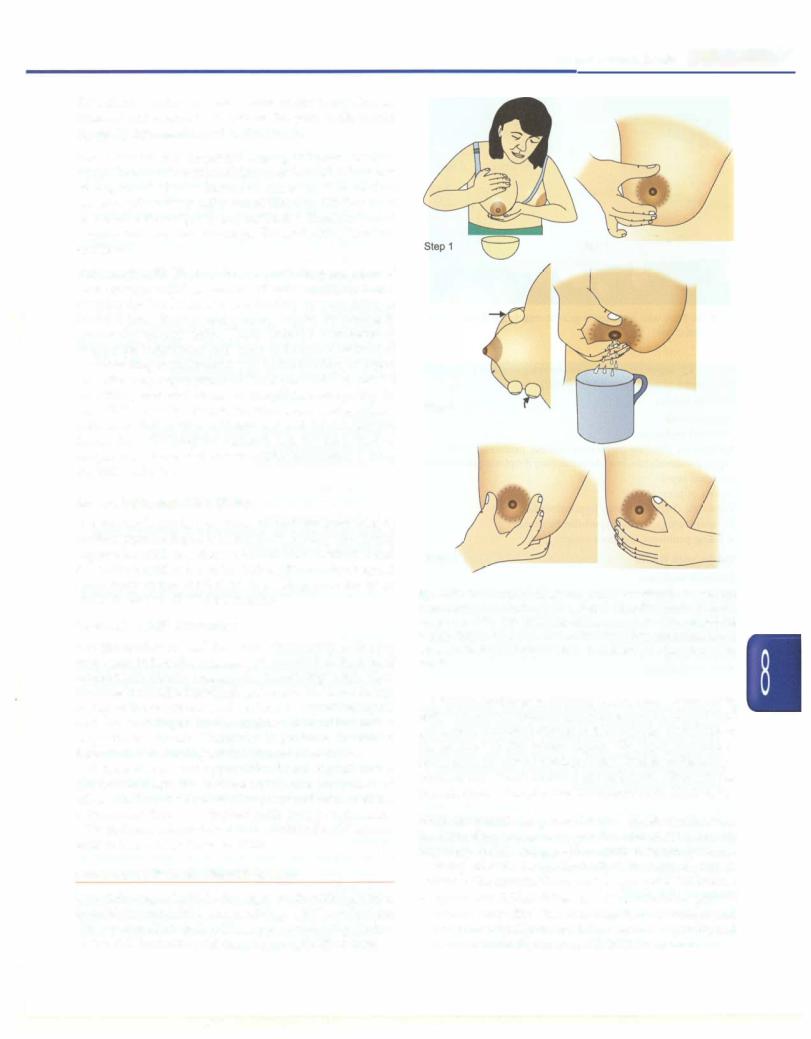



-.....E s s e n tial P e d iatrics_________________________________
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Term LBW infants started on IV fluids (because of their sickness) can be put on the breast once they are hemo dynamically stable.
|
Choice of Milk |
|
|
All LBW infants, irrespective of their initial feeding |
|
|
method should receive only breast milk. This can be |
|
|
ensured by giving expressed breast milk (mothers' own |
|
|
milk) for those infants fed by paladai or gastric tube. |
|
|
Expressed breast milk (EBM). All mothers should be |
|
|
counseled and supported in expressing their own milk |
|
|
for feeding their preterm infants. Expression should |
|
|
ideally be initiated within hours of delivery so that the |
|
|
infant gets the benefits of feeding colostrum. Thereafter, |
|
|
it should be done 2-3 hourly so that the infant is |
|
|
exclusively breastfed and lactation is maintained in the |
|
|
mother. Expressed breast milk can be stored for about 6 hr |
|
|
at room temperature and for 24 hr in refrigerator. |
|
|
The steps of breast milk expression are given in Fig. 8.35. |
|
|
Sick mothers/contraindication to breastfeeding. In these rare |
|
|
circumstances, the options available are |
|
|
i. Formula feeds: |
|
B |
a. Preterm formula in VLBW infants, and |
|
b. Term formula in infants weighing>1500 g at birth |
||
|
||
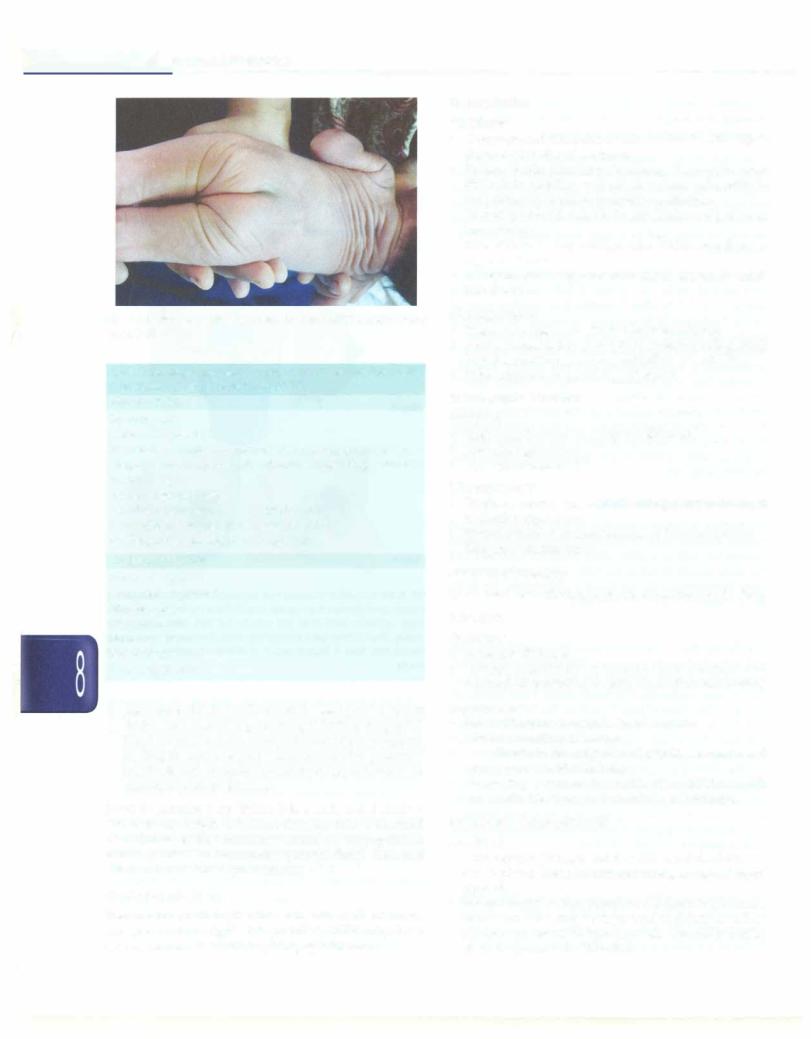
Figs 8.38A and B: (A) Paladai feeding; (B) Gavage feeding |
ii. Animal milk, e.g. undiluted cow milk |
Infant on!IV fluids
11 hemodynamically stable
[ Start trophic feeds by orogastric tube |
|
|
|
|
|
|
|
and monitor for feed intolerance |
|
|
|
|
|
|
|
! Ifaccepting well |
|
|
|
|
|
|
|
Gradually increase the feed volume, |
|
|
|
|
|
|
|
taper and stop IV fluids |
Infant on orogastric tube feeds |
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
Al 30-32 weeks' gestational age |
|
|
||
|
|
Try spoon feeds once or twice a day |
|
|
|
||
[ |
Also put on mother's breast and allow non nutritive suckling |
|
|
||||
|
|
If accepting spoon feeds well |
|
|
|||
|
|
Gradually increase the frequency |
|
|
|||
|
|
|
and amount of spoon feeds |
|
|||
|
|
Reduce orogastric feeds accordingly |
• |
• |
|
||
|
|
|
|
|
|
Infant on spoon or paladai feeds |
|
|
|
|
|
|
Put them on mother's breast before each feed |
J |
|
|
|
|
|
|
Observe for good attachment and effective sucking |
||
|
|
|
|
|
|
! If able to breastfeed effectively |
|
Taper and stop spoon feeds once the mother is confident
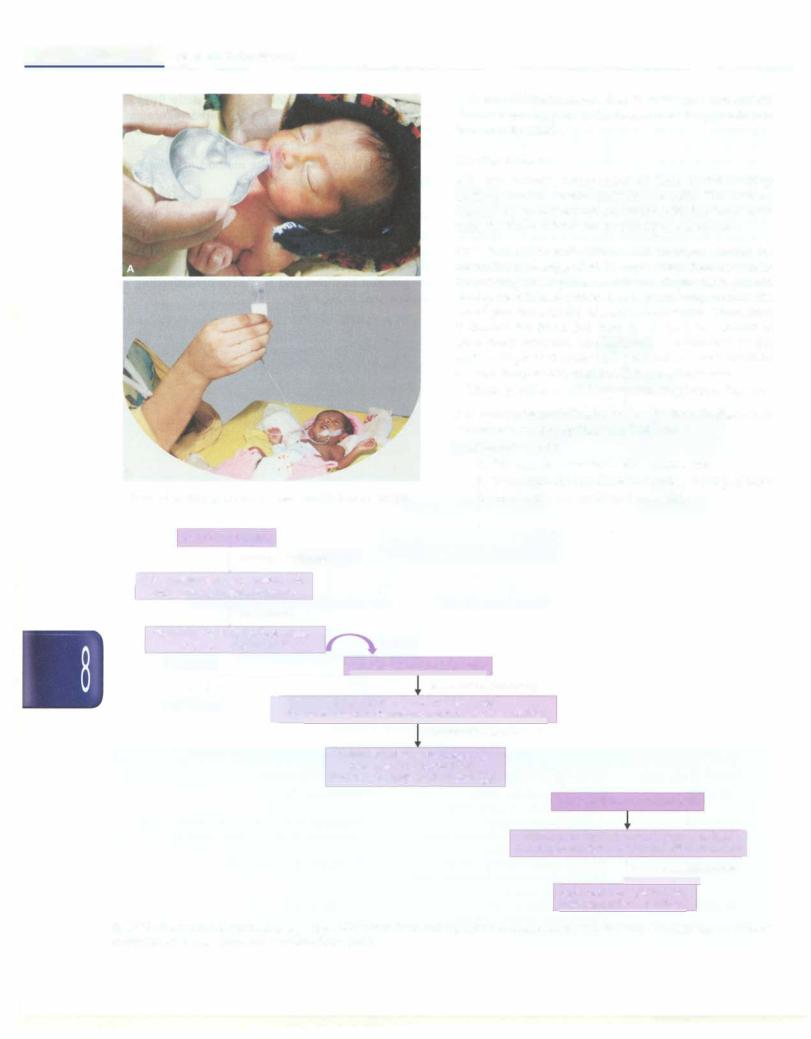
Fig. 8.39: Progression of oral feeding in preterm LBW infants. Term and near-term sick infants started on intravenous (IV) fluids can be initiated on breastfeeding once they are hemodynamically stable




