
Ghai Essential Pediatrics8th
.pdf
is due to metabolic acidosis and respond well to fluid expansion/blood transfusion and not diuretic therapy
•Assessment of pulse rate, blood pressure, respiratory rate, core body temperature, pallor, level of conscious ness at least 4 hourly to enable early detection of complications. Monitoring of urine output by indwelling catheter and watch for hemoglobinuria. If facilities are available and clinical conditions warrant central venous pressure and arterial blood pressure should be monitored. Blood glucose in children withcoma should be ideally monitored initially every hour; then 2-4 hr, or if clinical deterioration occurs. Blood counts, electrolytes and creatinine should be monitored on a regular basis. Monitor parasite count 6 hourly for the first 48 hr
•Broad spectrum antibiotics (3rd generation cephalo sporins and arninoglycoside, beta lactam-beta lactamase inhibitor combinations) should be given in (i) any child with localized signs suggesting bacterial infection; (ii) any child in shock; and (iii) any child with severe respiratory distress in whom deep breathing does not
rapidly improve with correction of dehydration or anemia.
•Avoidance of harmful treatments such as corticosteroids, osmotic diuretics.
Treatment of complications is outlined in Table 10.12.
Response to Therapy, Treatment Failures, Recrude scence and Relapse
An optimalresponse to therapy is defined as a count which on day 1 is less than day 0, a count on day 3 which is less than 25% of count on day 0, no parasites in peripheral blood 72 hr after starting therapy and up to 28 days and no fever after 72 hr.
Patients with parasitologically confirmed malaria who continue to have fever 72 hr after starting antimalarials are occasionally encountered in clinical practice. If they are smear negative, causes could be IV thrombophlebitis, secondary bacterial infections, coinfections such as typhoid or rarely immune phenomena. If the smear is
Infections and Infestations -
positive then this is early treatment failure; causes are choice of wrong drug, substandard drug, wrong dose, poor compliance or drug resistance. If drug resistance is suspected than treatment should be changed to alternative artemisinin based combination or quinine.
Reappearance of asexual parasites within 28 days of treatment is defined as recrudescence/late treatment failure. Causes again are choice of wrong drug, wrong dose, poor compliance or drug resistance. Recrudescence is fairly common if artemisinin monotherapy is used. Treatment of recrudescence includes optimizing drug therapy or change to an alternative regime.
Control and Prevention of Malaria
Control and prevention of malaria is based on elimination of the vector by strategies like insecticide spraying, use of insecticide treated bed nets and elimination of breeding places. Vaccines against malaria have been under development for a long time but are yet not commercially available nor veryeffective. In holoendemic areas like Africa chemoprophylaxis with pyrimethamine sulfadoxine administered twice during pregnancy reduces the prevalence of maternal anemia and low birth weight.
Chemoprophylaxis against malaria is recommended for travelers from nonendemic areas to endemic areas. Drugs commonly used are chloroquine (for areas known to be fully chloroquine sensitive), mefloquine, chloroquine proguanil, doxycycline and atovaquone-proguanil (expensive but safest). Prophylaxis should be started at least 1-2 weeks before departure and continued for 4 weeks after return (except atovaquone-proguanil where it can be started on the day of departure).
National Vectorborne Disease Control Program
These strategies are two-fold: early case detection and prompt treatment and vector control. It has laid out guidelines for detection and management of malaria and are more or less similar to what has been discussed earlier. The program recommends treatment of uncomplicated vivax malaria with chloroquine and falciparum malaria
Table 10.11: Drug doses for oral antimalarial drugs
Drug |
Oral dose |
Chloroquine (tablet 250 mg contains 150 mg of base; |
10 mg/kg of base loading; then 5 mg/kg after 6 hr, 24 hr and 48 hr |
syrup 50 mg of base/5 ml) |
|
Primaquine (tablet 7.5 mg, 15 mg) |
0.25-0.75 mg/kg |
Artemether lumefantrine (tablet 20 mg artemether |
Administer one dose each at 0, 8, 24, 36, 48 and 60 hr as follows: |
and 120 mg lumefantrine; syrup 40 mg artemether |
5-14 kg: 1 tablet; 5-24 kg: 2 tablet; 25-34 kg: 3 tablet; >35 kg: 4 tablet |
per 5 ml) |
Alternatively, give 1.7 mg/kg of artemether component q 12 hr |
|
for 3 days |
Artemether |
4 mg/kg single dose |
Mefloquine (250 mg tablets) |
Two doses, 15 mg/kg and 10 mg/kg, given 8 hr apart |
Pyrimethamine sulfadoxine |
1.25 mg/kg of pyrimethamine (max 75 mg) single dose |
Doxycycline |
3.5 mg/kg/day |
Clindamycin |
10 mg/kg twice daily |
Quinine (tablet 300 mg of salt) |
10 mg/kg of salt thrice daily |

|
----- |
--E•s•s•e •n•t-ia•l•P•e•d-ia.t.ri•cs•----------------------------- |
|
|
Table 10.12: Treatment of complications of severe malaria |
Hyperpyrexia |
Use tepid sponging, fanning, paracetamol to induce defervescence |
|
Avoid aspirin and other NSAIDs due to risk of gastrointestinal bleeding |
Cerebral malaria |
Nurse in lateral position; turn every 2 hr; continuous NG aspiration; maintain airway; irrigate and |
|
patch eyes |
|
If convulsions occur: Exclude hypoglycemia, hyperthermia; manage seizure with slow IV push of diazepam, |
|
midazolam or lorazepam; if seizure recurs, load with phenytoin 15-20 mg/kg |
|
Consider CT scan for intracerebral bleed, cerebral edema or herniation if consciousness deteriorates or new |
|
neurological abnormalities appear |
|
For raised intracranial pressure, nurse with head propped up and in midline position; control seizures; |
|
hypoglycemia; use hyperventilation; use of mannitol is controversial; corticosteroids do not have a role |
Anemia |
Transfuse packed cells if hemoglobin is <5 g/dl or in presence of impaired consciousness, hyperparasitemia, |
|
respiratory distress or metabolic acidosis; use diuretics and slow transfusion if fluid overload present; |
|
perform exchange transfusion in patients with overt congestive cardiac failure |
Hypoglycemia |
1 ml/kg of 50% glucose given slowly IV; followed by infusion of 10% dextrose; frequent monitoring of blood |
|
sugar; may use octreotide and glucagon if IV fluids restricted |
Metabolic acidosis |
Correct hypovolemia, hypoglycemia and anemia; administer oxygen; administer sodium bicarbonate slowly |
|
if pH <7 |
DIC |
Vitamin K, fresh frozen plasma and/or cryoprecipitate; exchange transfusion in presence of fluid overload |
Renal failure |
Careful fluid challenge with 20 ml/kg of normal saline, followed by furosemide 1 mg/kg (max 5 mg/kg) if |
|
no response noted; restrict fluid intake; dialysisindicatedinpresence of refractoryhyperkalemia ormetabolic |
|
acidosis, fluid overload and rapid rise of serum creatinine |
Hemolysis |
May prefer artemisinin derivatives as antimalarial drug; transfusion with packed cells to maintain hematocrit; |
|
monitor urine output and renal function for need of dialytic support |
Acute respiratory |
Occurs due to pulmonary capillary leak and, less commonly, from fluid overload; administer oxygen; |
distress |
nurse in propped up position; restrict fluid intake; loop diuretics may help; severe cases need mechanical |
syndrome |
ventilation with high peak end expiratory pressure |
Shock |
Send blood culture andadministerbroad spectrumantibiotics (e.g.cefotaximeand arnikacin); monitorcentral |
|
venous pressure; replace fluids; administer vasopressors and respiratory support as required |
Hyperparasitemia Consider exchange transfusion
withartemisinincombinationtherapy. For severemalaria, artemisinin derivatives or quinine may be used. Strategies for vector control include source control, elimination of breeding places, biologic control with use of larvivorous fish in water bodies and finally chemical vector control by indoor residual spray, space fogging and use of chemical larvicides like abate in water bodies.
Suggested Reading ·
Malaria. Available at http://www.nvbdcp.gov.in
Severefalciparummalaria.WorldHealthOrganization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg 2000; 94 (Suppl) 1: Sl-90.
WHO. Guidelines for treatment of malaria. 2nd edition, 2010. Available at www.who.int/malaria/docs/TreatmentGuidelines 2006.pdf
Leishmaniasis
Leishmaniasis is caused by parasites of the genus Leishmania transmitted by the bites of female sandflies. Three major clinical forms are described: visceral leish maniasis (VL), cutaneous leishmaniasis, and muco cutaneous leishmaniasis (espundia). In India, the visceral and cutaneous forms are caused by Leishmania donovani. The term kala-azar (kala black; azar sickness), refers to the
hyperpigmented skin of patients with visceral leishmaniasis. About 100,000-300,000 cases are reported annually in India, chiefly from Bihar and Eastern UP.
Transmission and Etiopathogenesis
The parasite exists in two forms; as nonflagellated, round or oval shaped amastigotes (Leishman-Donovan bodies) in the human or animal reservoir and as flagellated promastigotes in the sandfly and culture media. In India, humans are the chief reservoir (anthroponotic cycle) and female sandfly of the genus Phlebotomus are the vectors of the parasite. When feeding on an infected animal or human, the sandfly may ingest an amastigote which developintoa promastigoteinthedigestive tract,migrates to the proboscis (salivary glands) and is injected into a susceptible host when the sandfly takes its next feed. Within the new host, promastigotes infect macrophages where they develop into amastigotes. These amastigotes multiply in the cells of themononuclearphagocytesystem.
Although all ages are affected, children aged 1-4 yr are more susceptible. The protective immune response in visceral leishmaniasisisprimarilycellmediated immunity which results in subclinical infection and spontaneous

cure in majority of cases. Failure of this immunity leads to leishmaniasis. Malnutrition and HIV predispose to clinical disease.
Clinical Features of Visceral Leishmaniasis
The incubation period is 3 to 8 months (range 10 days-34 months). Features include high grade fever, weight loss, hepatosplenomegaly, abdominal discomfort, lymph adenopathy and pallor. Fever may be high or low grade, remittent, intermittent or continuous; the 'double-rise' of temperature in a day (double quotidian), is uncommon. Splenohepatomegaly, with the spleen much larger than the liver, is usual. Spleen is usually huge, firm, smooth and nontender and is palpable by the end of first month of illness. Unlike African leishmaniasis, lympha denopathy is infrequent in Indian patients (<5%). Hyperpigmentation of skin is a characteristic feature and occurs in about two-thirds of patients in late stages of disease, affecting the face, hands and upper trunk. Progressive emaciation occurs inallcases, though appetite is preserved. Cough and diarrhea are common. Bleeding manifestations in the form of petechial hemorrhages, epistaxis and gum bleeding may be seen. Pedal edema mayoccurduetohypoalbuminemia.Jaundiceisuncommon. Diminished cell mediated immunity may account for the high incidence of secondary infections. Pancytopenia and hypergammaglobulinemia are characteristic.
Thediseasemay begininsidiouslyandbe asymptomatic initially, but usually runs a chronic course that may be fatal without or despite treatment. Death usually occurs within 2 yr in 75-95% cases, because of severe secondary bacterial infections or gastrointestinal bleeding.
Post Kala-azar Dermal Leishmaniasis
Postkala-azardermalleishmaniasis (PKDL) develops after resolution of visceral leishmaniasis and is seen in a small percentage of patients in Africa and India. This is usually due to infection by the L. donovani cluster. The interval to development of PKDL is variable; it usually occurs 1-10 yr after successful treatment of visceral leishmaniasis. Hypopigmentedmacular,maculopapular ornodularskin lesions are seen first in the perioral area, chin and lips and later appear over the neck, extensor surfaces of the arms, trunk and legs. Lepromatous leprosy is a close differential, but peripheralnervesarespared. Skin lesions may persist for up to 20 yr. These patients may act as chronic reservoir of infection.
Diagnosis
Visceral leishmaniasis should be suspected in a patient from an endemic area presenting with prolonged pyrexia and splenomegaly. Cases in nonendemic areas have been reported but are rare. Clinical differentials include chronic malaria, storage disorders, leukemia, lymphoma, chronic liverdiseasewithportalhypertensionandHIV.Preliminary tests show pancytopenia, mild elevation of liver enzymes
Infections and Infestations -
and hypergammaglobulinemia with reversal of albumin globulin ratio. The aldehyde test has poor sensitivity and specificity.
Diagnosis of visceral leishmaniasis is based on microscopic detection of amastigotes in smears of tissue aspirates or biopsy samples. Bone marrow aspirate or biopsy is frequently the tissue of choice with sensitivity between 55 and 97%. Lymph node aspirate smears (sensitivity 60%), liver biopsy (sensitivity 85%)andsplenic aspirates (sensitivity 97%) may be used for diagnosis. Though splenic aspirate has the highest sensitivity, the procedure may result in life-threatening hemorrhage; the procedure is contraindicated if the prothrombin time exceeds control value by 5 seconds or platelet count is below 40,000/mm3.
Immunochromatographicstrip (dipstick ELISA) testing of blood from a finger prick for leishmanial anti-K39 antibody has been usedsuccessfully infieldserodiagnosis with a sensitivity of 90-100% and high specificity (90%) insymptomaticpatients. Titers to rK39 decrease following successful therapy and tend to rise in cases of relapse, thus making it useful to recognize treatment failures. This test is useful in clinical management in resource-poor areas. Newer methods with high sensitivity and specificity include the detection of Leishmania antigen and antibody in the urine.
Treatment
Drug therapy Treatment of leishmaniasis is based on pentavalent antimonials; however, increasing resistance to antimonials is a major problem, most evident in North Bihar, where the failure rate for this treatment is more than 50%. Pentavalent antimony [Sb (V)] can be given in the form of sodium stibogluconate [Sb (V) 100 mg/ml] or meglumine antimonate [Sb (V) 85 mg/ml]; both may be givenintravenously orintramuscularly withequalefficacy (Table 10.13). The dose of Sb (V) is 20 mg/kg for 28 days. IM injections are painful and better avoided. IV injections should be diluted 1:10 with 5% dextrose and infused slowly over 20 min. Adverse effects include vomiting, fatigue, arthralgia, myalgia, abdominal pain, elevated serum transaminases, lipase and amylase levels, bone marrow depression, and ECG abnormalilties, including
Table 10.13: Treatment of visceral leishmaniasis
Drug |
Dose |
Duration |
Pentavalent antimony |
20 mg/kg/day IM or IV |
28 days |
Amphotericin B |
1 mg/kg IV alternate day |
28 days |
Liposomal |
2 mg/kg/day IV |
5 days |
amphotericin B |
|
|
Miltefosine |
2.5 mg/kg/day oral |
28 days |
Pentamidine |
4 mg/kg thrice |
8 weeks |
|
weekly IV/IM |
|
Paramomycin |
16-20 mg/kg/day |
21 days |
(Aminosidine) |
IV/IM |
|

- Essential Pediatrics
nonspecificST and T-wave changes and T-wave flattening or inversion. Weekly ECG monitoring is recommended during therapy. In some resistant cases, addition of IFNy to Sb (V) has been successful in inducing remission.
Amphotericin Bis an effective treatment used in Sb (V) resistant cases. It is toxic and requires admission to the hospital for a prolonged period. The main side effects include fever, chills, thrombophlebitis, hypokalemia and renal failure. The alternative is to use the liposomal form, which is highly effective and less toxic, but continues to be very expensive. Liposomal amphotericin Bis currently the drug of choice for resistant leishmaniasis. Compared to conventional amphotericin, its concentration in reticuloendothelial cells is ten-fold more and is ten times less toxic. Recent studies using lower doses of this agent show that it may be cost-effective even in resource poor areas with high antimonial resistance.
Miltefosineis the firsteffectiveorallyactivedrugagainst leishmaniasis. Studies of treatment with this drug for 3 or 4 weeks have shown a cure rate of 95-100%; cure rates at 6 months' followup are also comparable to amphotericin B.It has the added benefit of a very good safety profile; gastrontestinal side effects are frequent but mild.
Other drugs used in treating leishmaniasis include pentamidine and paromomycin. Pentamidine is used in treatment-resistant cases of visceral leishmaniasis. Its use islimitedbyitssubstantialtoxicity, including hypotension, hypoglycemia, renal damage, injection abscess, and diabetes, necessitatingcloseinpatient monitoring. Though paromomycin is inexpensive, nephrotoxicity and ototoxicity are concerns with its use. Sitamaquine, another oral agent, is associated with a 50-67% cure rate.
Supportive care Severe anemia and thrombocytopenia may necessitate packed cell and platelet transfusion. The child should receive a nutritious diet and coexisting nutritional deficiencies should be corrected. Concurrent infections should be treated using appropriate antimicrobial agents.
Response to treatment Fever, spleen size, hemoglobin, blood cell counts, serum albumin and body weight are monitored for response to therapy. In most patients, the feversubsideswithin7days,bloodcountsand hemoglobin levels rise, the patient feels better and spleen becomes smaller within 2 weeks. Parasitological cure should be documented at the end of therapy by splenic or bone marrowaspiration. Asrelapsesarecommoninthisdisease, patient should be followed for at least 6 months before a longtermdefinitecureispronounced. The spleenmaytake 6monthsto 1yrtoregresscompletely. Relapseissuggested byanincreaseofspleensize, afallin hemoglobinlevelsand is confirmed by the demonstration of parasites.
Treatment of PKDL Treatment is indicated only for those who have severe and prolonged disease. Pentavalent antimonials (2monthcourse) and liposomalamphotericin Bare both effective.
Leishmania-HIV Coinfection
Leishmaniasis may occur in HIV infected persons, either as an opportunistic infection or as a result of reactivation of subclinical infection. A high index of suspicion is required in patientswithHIVwiththetypicalpresentations of visceral leishmaniasis such as pyrexia, pancytopenia and hepatosplenomegaly. However, thepresentationmay be atypical with prominent gastrointestinal or upper respiratory tract involvement and absence of hepato splenomegaly. The Leishmania antibody test (direct agglutination test) is frequently negative. The main risk group is intravenous drug users where an anthroponotic cycle is involved, with leishmania organisms present in used syringes being inoculated intravenously.
In leishmania-HIV coinfection, leishmaniasis is often intractable and is associated with a high relapse rate. Visceral leishmaniasis in HIV infection is being proposed for inclusion in the Centers for Disease Control clinical category C for the definition of AIDS as an indicator disease. Although treatment of coinfection has not been adequatelystudied,pentavalent antimonials arecommonly used. Meglumine antimonite, liposomalamphotericinand oral miltefosine have been used in small studies. Secondary prophylaxis, using pentavalent antimonials administeredonceevery28daysor liposomalamphotericin Bevery 21 days, prevents relapse and improves survival . Secondaryprophylaxisshouldbecontinuedat CD4counts below 200/mm3•
Prevention and Control
Control of leishmaniasis involves controlling the source of infection and eradicating the vector and depends on local epidemiology. Where sand flies are mostly endophilic (rest mostly indoors after feeding), spraying houses with insecticide is effective, while use of treated and untreated bed nets is effective where sand flies are endophagic (feed mainlyindoors).Insecticidetreatmentofdogsanddogcollars is useful where canines are important reservoirs. In India, where anthroponotic transmission is important, effective treatment of patients, especiallythosewithPKDL (who may act as longterm reservoirs), has been found to be effective in controlling transmissionwhencombinedwithvectorcontrol.
Suggested Reading
Davies CR, Kaye P, Croft SL, et al. Leishmaniasis: new approaches to disease control. BMJ 2003;326:377-82
Murray HW, Berman JD, Davies CR, et al. Advances in leishmaniasis. Lancet 2005;366:1561-77
Paredes R, Munoz J, Diaz I, et al. Symposium: Leishmaniasis in HIV infection. J Postgrad Med 2003;49:39-49
Piscopo TV, Azzopardi CM. Leishmaniasis. Postgrad Med J 2006; 82:649-57
Amebiasis
Amebiasis is defined as infection with Entamoeba histolytica. Clinical features of amebiasis range from

asymptomatic colonization to amebic dysentery and invasive extraintestinal amebiasis, occurring most commonly as liver abscess.
Epidemiology
E. histolytica is thought to infect 10% of the world's population and 2-55% Indians. However, these may be overestimates, because two morphologically identical, genetically distinct but apparently nonpathogenic Entamoeba species, namely E. dispar and E. moshkovskii, cause most asymptomatic cases.
Amebic dysentery and extraintestinal amebiasis is associated with a high rates of morbidity and mortality. It is less common in children, accounting for less than 3% of diarrhea in children below 5 yr.
Etiopathogenesis
An ingested cyst divides in the small intestine to form 8 trophozoites that colonize the mucosa of the large intestine. Trophozoites cause tissue invasion and destruction with little or no local inflammation, resulting in characteristic flask shaped ulcers in cecum, transverse colon and sigmoid colon. Extraintestinal complications occur when trophozoites invade the bloodstream and migrate through the portal circulation to lodge, most commonly, intheliver.Amebicliverabscessis usually single (95%) and more frequently involves the posterosuperior part of right lobe of the liver. The abscess may regress, rupture or disseminate; transdiaphragmatic rupture may cause amebic empyema and pulmonary amebiasis. Rare complications includeamebic involvementof peritoneum, pericardium, pleura, lungs, brain, genitourinary system and skin.
Clinical Features
Asymptomatic cyst passage is the most common mani festation of E. histolytica. In most cases the infection resolves spontaneously; uncommonly, amebic dysentery and other invasive manifestations may occur later.
After an incubation period varying from weeks to months,about10% individualscolonizedwithE. histolytica develop symptomatic disease. Amebic colitis presents as abdominal pain or tenderness (in 80%), with watery, bloody or mucous diarrhea. Some may have only intermittent diarrhea alternating with constipation. Fever is unusual. Occasionally, fulminant amebic colitis may occur, with profuse bloody diarrhea, fever, widespread abdominal pain, diffuse tenderness and pronounced leukocytosis. Toxic megacolon, ameboma, cutaneous amebiasis and rectovaginal fistulae can occur as compli cations of intestinal amebiasis.
Amebic liver abscess, seen in about 1% of infected individuals, mayoccurmonths to yrafterinfection. While some individuals may have concurrent amebic colitis, more commonly, thereare nobowel symptoms. The child usuallypresentswithfeverwithchillsandrigorsandright
Infections and Infestations -
upper quadrant pain of acute onset (<10 days). Exami nation reveals toxic appearance, right upper quadrant tenderness and hepatomegaly; jaundice is unusual (1015%). Cough along with dullness or crepitations in the right lung base may be present. Complications include rupture into the pleura pericardium and superinfection with bacteria.
Diagnosis
Diagnosis of amebic colitis is established by demons tration of motile trophozoites by direct microscopic examination of fresh fecal sample. At least 3 stool specimens takenonconsecutive days shouldbe examined because the test has poor sensitivity (<60%; -90% with 3 fresh samples). Stool contains plenty of erythrocytes but few leukocytes, unlike bacillary dysentery, where leukocytesare plentiful. Presenceofingested erythrocytes within trophozoites is pathognomonic for E. histolytica. Presence of cysts of E. histolytica in stool samples is of no clinical significance and should not be treated.
Serological tests are routinely employed for diagnosis of extraintestinal disease with E. histolytica especially for differentiating amebic from pyogenic liver abscess. They are positive in 70-80% patients with invasive disease at presentation and in >90% cases beyond first week of symptoms. IgG antibodies persist for yr after E. histolytica infection, whereas the IgM antibodies indicate present or current infection. Commonly used serologic tests in clinical practice are ELISA, IHA and IFA. Serological response as detected by ELISA becomes negative 6-12 months after infection.The IHA test is simple to perform and has been shown to be highly specific (-99%) and sensitive. The test may stay positive for as long as 10 yr following complete recovery, limiting itsutility inendemic areas. The IFA test is rapid, reliable, reproducible and helps to differentiate amebic liver abscess from other nonamebic etiologies.
In case of a liver abscess chest radiograph shows elevated diaphragmandpleural reaction ontheright side. Ultrasound, CT, MRI, or isotope scan can localize the abscess in mostcases. Leukocytosis without eosinophilia, mild anemia, raised alkaline phosphatase, and a high erythrocyte sedimentation rate (ESR) are common laboratory findings in these patients.
Treatment
The practice of giving antiamebic drugs for all children presenting with diarrhea should be strongly discouraged sinceamebiasisisrelativelyuncommonin young children.
Metronidazole is the drug of choice for treating amebic colitis (Table 10.14). Alternatives include tinidazole, orindazoleandsecnidazole. Since metronidazoledoesnot destroy the cysts, a luminal agent (paromomycin, iodoquinol, or diloxanide furoate) should be used to eradicate colonization. When possible, fulminant amebic

...e.s.s.e.n.t .ia•l•P•e•d•-at.r.ic•s |
|
---------------------- |
||||
- |
|
----------- |
|
|
|
|
|
|
|
|
|||
|
|
|
Table 10.14: Treatment of amebiasis |
|
||
Medication |
|
Pediatric dose |
|
Adult dose |
||
Invasive disease |
|
Colitis |
|
Liver abscess |
|
|
Tissue arnebicide |
Metronidazole |
35-50 mg/kg/day |
|
35-50 mg/kg/day for |
750 mg 8 hourly |
|
|
|
|
for 7 10days (in |
|
7-10 days (in 3 divided |
|
|
|
or |
3 divided doses) |
|
doses) |
|
|
|
Tinidazole |
50 mg/kg/day for |
|
50 mg/kg/day for |
2 g once a day |
|
|
|
3 days (once daily) |
|
3-5 days (once daily) |
|
Followed by |
|
|
|
|
|
|
Luminal |
Paromomycin |
25-35 mg/kg/day for |
|
25-35 mg/kg/day for |
25-35 mg/kg/day |
|
amebicide |
|
7 days (in 3 divided doses) |
7 days (in 3 divided |
(in 3 divided doses) |
||
|
|
or |
|
|
doses) |
|
|
|
Diloxanide |
20 mg/kg/day for |
|
20 mg/kg/day for 7 |
500 mg 8 hourly |
|
|
furoate or |
7 days (in 3 divided doses) |
days (in 3 divided doses) |
|
|
|
|
Iodoquinol |
30-40 mg/kg/day for |
|
30-40 mg/kg/day for 20 |
650 mg 8 hourly |
|
|
|
20 days (in 3 divided doses) |
days (in 3 divided doses) |
|
|
Asymptomatic intestinal colonization |
|
|
|
|
||
|
|
Paromomycin or |
As above |
|
|
|
|
|
Diloxanide |
As above |
|
|
|
|
|
furoate or |
|
|
|
|
|
|
Iodoquinone |
As above |
|
|
|
colitis, even with perforation, is managed conservatively, with the addition of antibiotics to deal with bowel flora.
Most amebic liver-abscesses, even large ones, can be cured without drainage. Most patients show a response to treatment (reduced fever and abdominal pain) within 72-96 hr. Individuals with amebic liver abscess should also receive a luminal agent to eliminate intestinal colonization. Therapeutic aspiration guided by CT in the treatment of uncomplicated amebic liver abscess is controversial. Large amebic liver abscesses (>300 ml)may benefit from aspiration with decrease in duration of hospital stay and faster clinical improvement recovery when compared to those managed medically alone. Abscess cavity resolves slowly over a period of several months.
Aspiration is reserved for individuals in whom diagnosisis uncertain (where pyogenic abscessorbacterial superinfection is a concern), those who have not responded to metronidazole therapy (persistent fever or abdominal pain after 4 days of treatment), individuals withlargeleftlobeabscesses(becauseof the riskof rupture into the pericardium), size more than 8-10 cm (suggesting impending rupture) and severely ill patients with an acceleratedclinicalcourse andlargeabscesses. Aspiration, percutaneous catheter drainage, or both, improve out comes in the treatment of amebic empyema after liver abscess rupture, and in treatment of amebic pericarditis or peritonitis.
suggested Reading
Fotedar R, Stark D, Beebe N, et al. Laboratory diagnostic techniques for Entamoeba species. Clin Microbial Rev 2007;20:511-32
Stanley SL. Amoebiasis. The Lancet 2003;361:1025-34
Giardiasis
Giardiasis, caused by Giardia lamblia (also known as G. intestinalis or G. duodena/is), is a majorcauseof diarrhea in children and in travelers.
Epidemiology
Theinfectionis endemic indevelopingcountries with poor sanitation. Individuals with malnutrition, humoral immunodeficiencies and cystic fibrosis are particularly susceptible. Children appear to be more severely affected than adults.
Etiopathogenesis
Giardia exists in two stages, cysts and trophozoites. Outsidethehuman body it exists intheform of cysts. Cysts are hardy, capable of surviving in cool, moist environ ments for up to 2 months and in water that has been routinely chlorinated, but are destroyed by boiling for 10 min. Transmission of infection isthroughcysts,which may be ingested in contaminated water or food or spread by direct person-to-person contact. Ingestion of 10-100 cysts is sufficient for causing infection. Low pH of the duodenum facilitates excystation and release of tropho zoites.Trophozoitescolonizetheduodenumand proximal jejunum of the host, where they attach to the intestinal brush border. It is believed that the infection causes diarrhea via a combination of intestinal malabsorption and hypersecretion. These effects cause malabsorption and maldigestion and in addition, may facilitate the develop ment of chronic enteric disorders, including inflammatory bowel disease and irritable bowel syndrome.

Clinical Features
Theincubationperiod after ingestion of cysts is1-2 weeks. Most infectionsinbothchildrenandadultsare asymptomatic. Symptomatic infections are more common in children than in adults and usually take the form of acute diarrhea with sudden onset of explosive, watery, foul smelling stools, along with nausea and anorexia; others may also have abdominal distension, flatulence, epigastric cramps and mild fever. Thereis no blood or mucus in stools. The illness may last 3--4 days and is usually self limiting in normal immunocompetent children. Variable degree of mal absorption may occur. Some patients may have a protracted course, with persistent or recurrent mild to moderate symptoms such as brief episodes of loose foul smelling stools alternating with constipation. Persistent diarrheamay be seen in 30-50% cases. A few children may develop chronic diarrhea, lactose and fat malabsorption and failure to thrive.
Diagnosis
Diagnosis of giardiasis is established by microscopic examination of at least 3fresh specimens of stools collected on alternate days. Thereis noblood or leukocytes in stools. Enzyme immunoassay (EIA) and direct fluorescent antibody test for Giardia antigens in stools have been reported to have better sensitivity and require less expertise than traditional microscopy. Where diagnosis is strongly suspected, duodenal aspirate or biopsy may yield high concentration of Giardia when fresh wet mount is examined for trophozoites. Where duodenal aspirate is negative, intestinal biopsy may be considered in presence of features like lactose malabsorption or abnormal radiographic findings (edema or segmentation in small intestine), or a suggestivesetting like absentsecretoryIgA or hypogammaglobulinemia.
Treatment
All symptomatic cases-acute and persistent diarrhea, failure to thrive and malabsorption syndrome-require drugtreatment. Asymptomatic cystcarriersare not treated except in specific situations like for outbreak control or for prevention of spread from toddlers to immunocompro mised family members. Treatment options are listed in Table 10.15.
Suggested Reading
Buret AG. Pathophysiology of enteric infections with Giardia duodenalis. Parasite 2008;15:261-5
Escobedo AA, Cimerman S. Giardiasis: a pharmacotherapy review. Expert Opin Pharmacother 2007;8:1885-902
Kiser JD, Paulson CP, Brown C. Clinical inquiries. What's the most effective treatment for Giardiasis? J Fam Pract 2008;57:270-2
Amebic Meningoencephalitis
The term amebic meningoencephalitis refers to infection of the central nervous system by free living amebae. The
Infections and Infestations -
Table 10.15: Agents for treatment of giardiasis
Drug |
Dosage |
Drugs of choice |
|
Tinidazole |
50 mg/kg (maximum 2 g) orally, single dose |
Metronidazole |
5-10 mg/kg (maximum 250 mg) orally |
|
q 8 hr for 7 days |
Nitazoxanide |
Age 1-3 yr: 100 mg orally q 12 hr for 3 days |
|
Age 4-11 yr: 200 mg orally q 12 hr for 3 days |
|
Age >11 yr: 500 mg orally q 12 hr for 3 days |
Alternative agents |
|
Albendazole |
10-15 mg/kg (maximum 400 mg) orally |
|
once daily for 5 days |
Mebendazole |
200 mg orally q 8 hr for 5 days |
Paromomycin* |
10 mg/kg orally q 8 hr for 5-10 days |
Furazolidone' |
1.5 mg/kg (maximum 100 mg) orally q 6 hr |
|
for 7-10 days |
Quinacrine |
2 mg/kg (maximum 100 mg) orally q 8 hr |
|
for 5 days |
*Has poor intestinal absorption; useful in treating giardiasis during pregnancy
"Monoamineoxidase inhibitor;significantfood and drug interactions if used for >5 days
disease occurs in two clinical forms: primary amebic meningoencephalitis caused by Naegleria fowleri and granulomatous amebic encephalitis induced by amebae of Acanthamoeba and Balamuthia genera.
Primary Amebic Meningoencephalitis
N. Jowleri causes fulminating meningoencephalitis, infecting mostly children and healthy young adults. History of swimming in fresh water lakes, pools and ponds, usually during hot summer months, is common. The amebae enter the nose through contaminated water (rarely, air), penetrate the nasalmucosa and the cribriform plate and travel along the olfactory nerves to the brain leading to a diffuse hemorrhagic necrotizing meningo encephalitis. Microscopic demonstration of motile amebae in fresh cerebrospinal fluid is required for diagnosis. CSP evaluation shows ahigh WBCcount (usuallyin thousands per mm3) with a polymorphonuclear predominance and elevated protein.
Disease progression is usually rapid, and might lead to death within 5-10 days. A combination of high dose amphotericin B, both intravenous and intrathecal, along with rifampicin and chloramphenicol, has been employed successfully.
Granulomatous Amebic Encephalitis (GAE)
This is an infection with Acanthamoeba species and, rarely, Balamuthia species, that is acquired through lung or skin andspreadshematogenously. Theinfectionisusuallyseen in immunocompromised children, e.g. those with AIDS, SLE or postrenal transplantation. Clinically, the disease has a subacute or chronic course similar to tubercular meningitis. Untreated, the condition is fatal. CSP

....e.s.s.e.n.t.ia•l•P-•d•iae.t.ri•c•s ---------------- |
----- |
- |
|
- |
------------ |
|
|
|
|
|
|
examination reveals motile trophozoites or cysts of Acanthamoeba in addition to elevated proteins and lymphocytic leukocytosis. A triplex real time PCR assay for simultaneous identification of Acanthamoeba spp., B. mandriallaris and N. fowleri has been developed. CT scan ofbrain may revealgranulomatous lesions and ventricular dilatation. Treatmenthasbeen attemptedwithfluconazole, ketoconazole, sulfonamides and cotrimoxazole, but prognosis is poor.
Suggested Reading
Kaushal V, Chhina DK, Ram S, et al. Primary amebic meningoen cephalitis due to Naegleria fowleri. J Assoc Phys India 2008:56:459-62.
Ma P. Naegleria and acanthamoeba infection: Review. Rev Infect Dis 1990;12:490-504
CONGENITAL AND PERINATAL INFECTIONS
Congenital and perinatal infections are often referred to by the acronym TORCH. This refers to toxoplasmosis (T), rubella (R),cytomegalovirus (C) andherpessimplex virus (H or HSV). The 'Others' group (0) is ever expanding and includes several infections like syphilis, malaria, tuberculosis, HIV, HCV, HBV, varicella, enterovirus and parvovirus infections. This section will focus on toxo plasmosis, rubella, cytomegalovirus, herpes simplex and syphilis as they are not discussed elsewhere and share common features.
General Principles
Fetal and neonatal infections occur only with primary infection in the mother. Latent infection or reactivation affects the baby very infrequently, with the exception of syphilis. Not all infections in mother are transmitted to the baby due to the placental barrier and not all infected babies are affected. The transmissibility and severity of fetal affection depends on the timing of gestation. Gene rally, infection during the first trimester has the most devas tating consequences. Congenitalandperinatalinfectionscan manifest during pregnancy as ultrasonographic findings, soon afterbirthor later in life. The commonmanifestations of intrauterine infections are abortions (recurrent only with syphilis), intrauterine growth retardation, intrauterine death, prematurity, deafness, chorioretinitis, aseptic meningitis, microcephaly and mental retardation, lymphadenopathy, hepatosplenomegaly, neonatal hepatitis, anemia, thrombocytopenia and skeletal abnormalities.
Tests that are useful in diagnosis include a complete blood count, liver and renal functions, skeletal survey, fundus examination, hearing evaluation and imaging of thecentralnervous system. Specific diagnosis is generally by serology and the TORCH screen is often ordered for. However, it must beremembered that serologic diagnosis by IgM and IgG estimation is tricky, should be done in both baby and mother and is interpreted with caution. Treatment is possible and rewarding for toxoplasmosis,
syphilis and herpes simplex, only partly successful for CMV and not available for rubella.
Congenital Toxoplasmosis
The maternal primary infection is generally subclinical. In HIVinfectedorimmunocomprornised women, reactivation of latent infection may also affect the fetus. The transmissi bility increases but the risk of fetal disease decreases with advancing pregnancy. The clinical manifestations are similar to those mentioned earlier. The classical triad of toxoplasmosis includes intracranial calcification, hydro cephalus and chorioretinitis. Infantsasymptomaticat birth may later present with mental retardation and deafness. Diagnosis is confirmed by demonstrating a positive toxoplasma IgM in serum of the affected child. Treatment is recommended for all affected babies even if they are asymptomatic. Therapy is with pyrimethamine, sulfa diazine and folinicacidfor a period of 1 yr. Sincematernal infection results from ingestion of food or water conta minated with oocysts or tachyzoites in infected meat, prevention centers around advising pregnant women to wash fruits and vegetables carefully, limit contact with soil and refrain from eating undercooked meat.
Congenital Rubella
Transmissibility is highest in the first trimester and so is the rate of fetal disease (90% at <11 weeks). The fetus is completely spared if infection occurs beyond 16 weeks. Clinical manifestations are as mentioned earlier; the classical triad of congenital rubella syndrome consists of deafness, cataract and congenital heart disease. Delayed manifestations such as diabetes mellitus and renal disease have also been described. Diagnosis is by demonstration of positive rubella IgM in cord or neonatal blood. No treatment exists. A unequivocal diagnosis of rubella in the first trimester of pregnancy is an indication for maternal termination of pregnancy. Vaccinating all children and particularly all adolescent girls against rubella is strongly recommended to reduce the burden of congenital rubella.
Congenital Cytomegalovirus (CMV) Infection
CongenitalCMVisreportedtobe thecommonestcongenital infection in developed countries. Data from developing countriesislacking.Mostfetal infectionsoccurwith primary infection in the mother; transmission and fetal disease occur throughout pregnancy. The overall transmission rate with primary infection is 30% and 10% of all infected babies are symptomatic. CMV remains latent in the body and can reactivateanytime;similarlyreinfectionscanalsooccur. The transmission riskwith reactivation or reinfection is only 1% and only 5-10% of infected babies are symptomatic.
Congenital infection can affect all organ systems; periventricular calcification is characteristic of CMV (Fig. 10.25). CMV transmission can also occur during delivery and breastfeeding and is of consequence only in the preterm and very low birthweight babies. In this
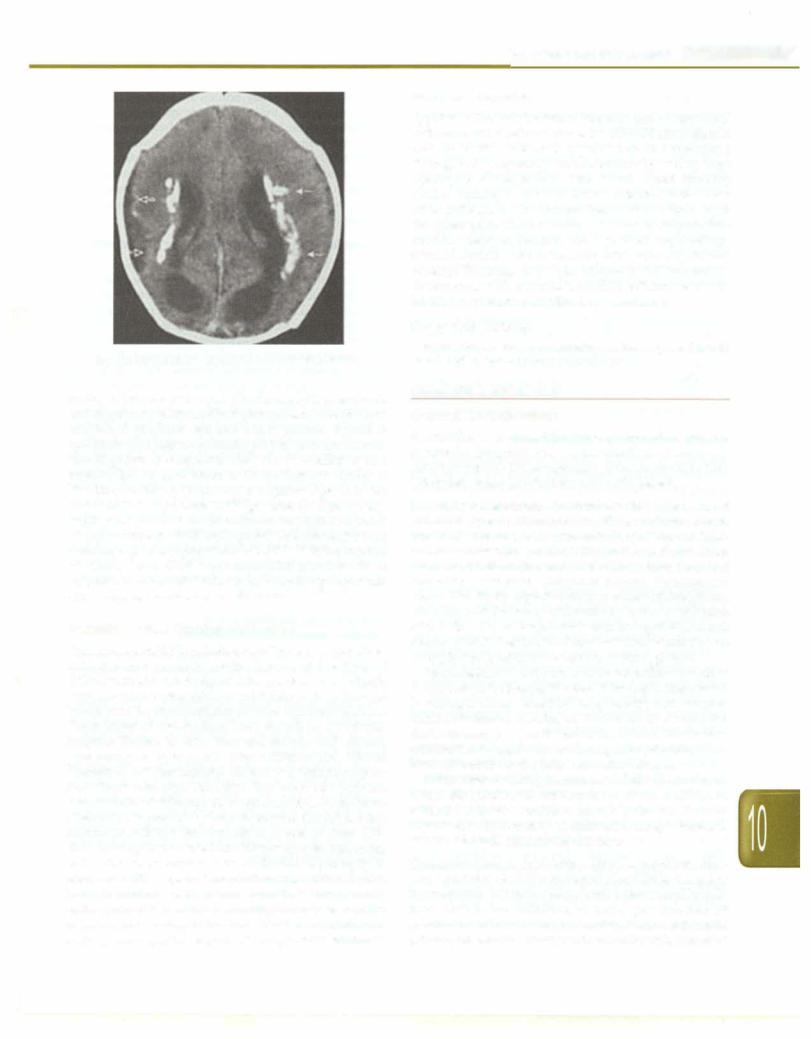
Infections and Infestations -
Congenital Syphilis
Syphilis is the only maternal infection that is associated withrecurrentabortions;hence, the TORCH screenshould include VDRL. Maternal syphilis can be transmitted throughout pregnancy, more commonly during later pregnancy as the placenta thins down. Apart from the clinical features mentioned earlier, infected babies have other pathognomonic features like skeletal lesions, snuf fles, pneumonia alba andbullous skin lesions.Some babies manifest delayed features like depressed nasal bridge, notched central incisors, keratitis, saber shins and frontal bossing. Diagnosis is by quantitative VDRL estimation. Treatment is with procaine penicillin; ceftriaxone should be used if procaine penicillin is not available.
|
Suggested Reading |
|
Shet A. Congenital and perinatal infections: throwing new light with |
Fig. 10.25: Classical periventricular calcification of CMV |
an old TORCH. Indian J Pediatr 2011;78:88-95 |
setting it presents as a sepsis like illness with pneumonia and respiratory distress. CMV transmission due to blood and blood products can also cause anemia, thrombo cytopenia and hepatosplenomegaly in preterm infants. The diagnosis of congenital CMV can be confirmed by a positive IgM in cord blood or in the first two weeks of life. The sensitivity is low and a negative IgM does not rule out CMV. A positive CMV IgM after the first 2 weeks of life can also occur due to postnatal transmission and is not an accurate method for diagnosis. In babies older than 2 weeks, a positive quantitative CMV PCR is the method of choice. Antiviral treatment with ganciclovir or valgancicloviris available but is indicated only in patients with progressive disease and deafness.
Perinatal Herpes Simplex Virus (HSV)
Transmission of HSV to babies usually occurs in mothers who develop primary genital herpes at the time of delivery. Reactivation of genital herpes is associated with very low rates of transmission and fetal affection.Infected babies may be asymptomatic or have fulminant disease. Three forms of disease have been described; a vesicular eruption limited to skin, eyes and mouth, CNS disease presenting as meningitis with seizures and altered sensoriumanddisseminated disease presenting as sepsis like illness with high mortality. The latter two may not have associated skin eruptions, whichfurther complicates diagnosis. Diagnosis is by Tzanck smear of the skinlesions, culture or PCR for the virus from lesions or from CSF. HSV serology has no role in making diagnosis. Treatment with intravenous acyclovir should be started promptly in neonates with suspected or confirmed infection. Babies born to mothers with active herpetic lesions during delivery should be watched carefully for disease. Elective cesarean section should be considered in mothers with activeprimarygenitalherpesand unruptured membranes.
HELMINTHIC INFESTATIONS
General Considerations
Helminthiasis is caused by three groups of worms, i.e. nematodes (roundworms),trematodes (flukes), andcesto des (tapeworms).These organisms differmarkedlyin their life cycles, mode of infections and pathogenesis.
Nematodes (roundworms). Intestinal nematodes are the most common type of helminthiasis. These include Ascaris lumbricoides, Strongyloides stercoralis, Ancylostoma duodenale and Necator americanus (all residing in the small intestine). Trichuris trichiura (located in the large intestine) and Enterobius vermicularis (lodged in cecum). Transmission occurs directly by ingestion of eggs in case of Enterobius, trichuris, and Ascaris or indirectly by larval penetration of the skin, i.e. in Ancylostoma and Strongyloides. Adult stages inhabit the human intestinal lumen but do not multiply there, with the exception of Strongyloides.
Nonintestinal nematodes include microfilariae such as
Brugiamalayi, B. timorior Wuchereria bancrofti. Theseresult in filariasis characterized by lymphangitis and lympha denitis. Onchocerca volvulus and Loa loa are other nonintes tinal nematodes or public health importance. Filariasis is transmitted to man by larvae during mosquito bite, while black fly transmits the larvae in onchocerciasis.
Othertissue dwellingnematodesinclude Toxocara canis, Trichinella spiralis and Dracunculus medinensis. All tissue nematodes have a complex life cycle that involves an intermediatehost, mostly an arthropod, except Trichinella spiralis, which is transmitted directly.
Trematodes (flukes). Schistosoma (spp. haematobium, Man soni, japonicum, etc.) also known as blood fluke is a major flatworminfestationin Africa,South AmericaandMiddle East. Man is the definitive host and gets infected by penetrationofintact skin by cercariae. Snails are the main primaryintermediatehost. Clinicalmanifestations maybe

__E_s_s_..ent.1a_1_P_ e_d_1a.tc.ris_______________________________
acute (dermatitis, Katayama disease) or chronic (fibro obstructive sequelae due to egg embolism in liver, lungs and genitourinary tract).
Flukes of liver, e.g. Clonorchis sinensis, Opisthorchis, Fascia/a hepatica, lung, e.g. Paragonimus westermani, and intestine, e.g.Fascia/apsisbuski, Heterophyes are alsoknown. These flukes have two intermediate hosts. Snail is the common primary intermediate host to all of them while fish, crabs and aquatic plants serve as secondary intermediate hosts to liver, lung and intestinal flukes, respectively. Transmission to the definitive host, i.e. man occurs through ingestion of metacercariae lodged on aquatic plants and animals, including fish.
Cestodes (tapeworms). These include giant tapeworms
(Taenia saginata, T. solium and Diphyllobothrium latum), dwarf tapeworms (Hymenolepsis nana) and zoonotic cestodes (Echinococcus granulosus and E. multilocularis).
Man acquires Echinococcusthroughingestionofeggs, while T. saginata and T. solium infect humans through ingestion of cysticerca in contaminated food. D. latum infection is carried to man by ingestion of cysts in fresh water fish.
|
|
Ascaris tumbricoides (Roundworm) |
|
|
Ascariasis is the most common worm infestation of the |
|
|
humans, infecting nearly one-fourth of the world's |
|
|
population. Preschool children are vulnerable to infection |
|
|
due to their hand to mouth behavior. Infection may also |
|
|
be acquired through ingestion of contaminated fruits and |
|
|
vegetables. Most infected individuals are asymptomatic |
|
|
due to low worm load. Clinical manifestations occur due |
|
|
topulmonary hypersensitivityandintestinal complications. |
|
|
Clinical features Symptoms may be produced by |
|
|
migration of larvae through lungs (pulmonary ascariasis) |
|
|
or the presence of adult worms in theintestine.Pulmonary |
|
|
ascariasis presents as Loeffler syndrome, characterized by |
|
|
fever, cough, dyspnea, wheeze, urticaria, eosinophiliaand |
|
|
lung infiltrates. Pulmonary ascariasis should be differen |
|
|
tiated from other causes of Loeffler syndrome, namely, |
|
|
visceral larva migrans caused by Toxocara canis or |
|
|
hookworm, schistosomiasis, pulmonary aspergillosis and |
|
|
tropical pulmonary eosinophilia. |
|
|
Intestinalmanifestations of ascariasis include abdominal |
|
|
distension, vomiting, vague abdominal discomfort and |
|
|
irritability.Thechildmaypassadultwormsinthevomitus |
I |
-1[ |
or feces. In heavy worm infestation, small bowel obs |
truction can occur due to a mass of entangled worms. |
||
Occasionally, worms migrate to aberrant sites such as |
||
biliary and pancreatic ducts, where they can cause |
||
cholecystitis, cholgangitis, pancretitis and rarely intra |
||
|
|
hepatic abscess. As parasites compete with host for |
nutrients, heavyworm infestation maybe associated with poorgrowthandnutritional deficienciesinyoungchildren.
Management The diagnosis is established by identi fication of characteristic eggs in stool samples by
microscopy. Occasionally, adult wormin fecesorvomitus can be recognized by its large size and smooth cream colored surface. Ultrasound can identify worms in pancreaticobiliary ducts. Occasionally, worms can be incidentally diagnosed on contrast studies of the gastrointestinal tract, appearing as string shadows due to barium in the worms' alimentary canal, or as linear filling defects outlined by contrast media. Therapy of ascariasis is described in Table 10.16.
Enterobius vermicutaris (Pinworm or Threadworm)
Enterobius vermicularisisa small (1 cm long), white, thread like worm that lives in the cecum, appendix, ileum and ascending colon. Eggs are not usually liberated in the gut. Gravid females migrate at night into the perianal region and release eggs there. The egg become infective within 6 hr. Perianal scratching causes transfer of eggs 10 finger nails. Infection occurs when eggs are ingested. The larvae hatch and mature within the intestine. Perianal itching, especially in night is the most common complaint.
Management Stool microscopy is not useful as eggs are generally not passed in the stools. Eggs can be demon strated by examining the perianal swab obtained early in the morning before the child hasdefecated.Alternatively, a strip oftransparentcellulose acetate tape is applied with sticky side down on the perianal region. The tape is lifted and pressed on a glass slide with the sticky side down. All the members of the family should be treated simultaneouslytoprevent cross-infection and reinfection. The nails of the child should be cut short and scrubbed. Single dose mebendazole or albendazole or pyrantel pamoate are highly effective. The course may be repeated after 2 weeks (Table 10.16).
Ancytosfoma duodenate and
Necafor americanus (Hookworm)
Hookworm infestation is an important cause of iron deficiency anemia. Most infected persons are asympto matic.
Clinical features Chronic blood loss due to hookworm infestation causes iron deficiency anemia and, occa sionally, hypoproteinemia.Onlyheavily infected children become symptomatic.
Infective larvae may produce a pruritic maculopapular eruption known as ground itch; at the site of skin penetration. Larvae migrating through lungs may also cause transient lung infiltration, but this is less common than with Ascaris. Nonspecific complaints like abdominal pain, anorexia, and diarrhea have also been attributed to the hookworm infection.
Diagnosis The diagnosis is established by identifying the characteristic oval hookworm eggs in the feces. Eggs of the two species are indistinguishable. Blood examination reveals microcytic, hypochromic anemia, occasionally eosinophilia.
