
Ghai Essential Pediatrics8th
.pdf
Table 12.20: Algorithm for diagnosis of disseminated intravascular coagulation (DIC) using the DIC score
Risk assessment
Does the patient have an underlying disorder known to be associated with disseminated intravascular coagulopathy? (If yes, proceed. If no, do not use this algorithm).
Order global coagulation tests
Platelet count; prothrombin time, fibrinogen, soluble fibrin monomers or fibrin degradation products
Score test results |
Score |
(a) Platelet count >100,000/mm3 |
0 |
50,000-100,000/mm3 |
1 |
<50,000/mm3 |
2 |
(b) Elevated fibrin-related marker (soluble fibrin |
|
monomers or fibrin degradation products)* |
|
No increase |
0 |
Moderate increase |
2 |
Strong increase |
3 |
(c) Prothrombin time |
|
<3 sec |
0 |
>3 but <6 sec |
1 |
>6 sec |
2 |
(d) Fibrinogen level |
|
>1 g/1 |
0 |
<1 g/1 |
1 |
Calculate score |
|
Score .<'.5: Compatible with overt DIC; repeat daily
Score <5: Suggestive of non-overt DIC; repeat in 1-2 days
* Values of D-dimer above the upper limit of normal are moderately elevated; values above 5 times the upper limit of normal are strongly increased.
Hematological Disorders -
Specific indications for such therapy include the presence of arterial or large vessel venous thrombosis. These patients should continue to receive replacement therapy with heparin during continuous monitoring of platelet counts and fibrinogen levels and prothrombin, activated partial thromboplastin and thrombin time.
Therapy with activated protein C and tissue factor pathway inhibitor has not been shown to be beneficial in controlled trials in children.
Suggested Reading
Levi M. Disseminated intravascular coagulation: What's new? Crit Care Clin 2005;21:449-67
Taylor FB,Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scori11g system for disseminated i.ntravascular coagulation. Thromb Haemost 2001; 86:1327-30
Thrombotic Disorders
The incidence of thrombosis is lower in children than in adults, but is associated with significant morbidity and mortality. Till 6 months of age, children have lower levels of the vitamin-K dependent coagulation factors II, IX and X as compared to adults. Levels of thrombin inhibitors, such as antithrombin, heparin cofactor II, plasminogen and proteins C and S are low at birth. Protein S level approaches adult value by the age of 3-6 months, but protein C levels remains low during childhood. In newborns compared with adults, thrombin generation is delayed and decreased, probably due to low prothrombin level. The incidence of thrombosis is maximal in infancy and during adolescence.
Table 12.21: Types of blood component therapy, their constituents and guidelines for use
Component |
Constituents |
Indication |
Dose |
Precautions |
Fresh frozen |
All coagulation factors as |
Coagulation factor |
plasma (FFP) |
in normal plasma; contains |
deficiencies with pro- |
|
0.7-1.0 U/ml of |
longed prothrombin |
|
factors II, V, VII, VIII, IX, |
time; thrombotic |
|
X, XI, XII, XIII and |
thrombocytopenic |
|
2.5 mg/ml fibrinogen |
purpura |
Infuse soon after thawing; need ABO compatible units; may cause fluid overload
Cryoprecipitate |
Fibrinogen 150 mg/bag, |
|
factor VIII 80-120 units/ |
|
bag, factor XIII and vWD |
|
(does not contain factor |
|
IX) |
Random donor |
Platelets; 2':5.5 x 1010 |
platelets (RDP) |
platelets per bag |
Fibrinogen deficiency or consumption; factor VIII deficiency (hemo- philia A), vWD disease; factor XIII deficiency
Thrombocytopenia |
One unit raises platelet |
Infuse rapidly; do NOT |
|
counts by 5-10,000/ |
refrigerate priorto transfusion |
|
mm3; 1 unit every |
|
|
10 kg raises counts |
|
|
by 30,000-50,000/mm3 |
|
Single donor |
Platelets; contains at least |
Thrombocytopenia |
One collection is equi Precautions as above |
platelets (SDP) |
3 x 1011 platelets |
|
valent to approxi |
|
|
|
mately 6 units of |
|
|
|
random platelets |
Fresh blood |
All components of blood |
To replace acute and |
Only to be used in |
|
|
massive blood loss |
severe trauma |
Not a good source for platelets or coagulation factors

- Essential Pediatrics
Clinical Evaluation
Congenital heart disease and, recent cardiac catheteri zation are important causes of arterial thrombosis in children. Other predisposing factorsfor arterial or venous thrombosis include recent surgery, trauma, use of central venous catheter, nephrotic syndrome, dehydration, sepsis and collagen vascular disorders (Table 12.22).
Limb edema, erythema and tenderness on dorsiflexion of the foot (positiveHoman sign)suggestdeep veinthrom bosis. Signs of arterial thrombosis include diminished or absent peripheral pulses and cool extremities. Manifes tations of pulmonary embolism include anxiety, breathlessness, pleuritic chest pain, fever, tachypnea and cough, and a high index of suspicion is required to make the diagnosis. Symptoms of central nervous system thrombosis include vomiting, lethargy, seizures or weaknessinan extremity. Strokes may occurin utero; such newborns present with seizures and lethargy, while older children present with headaches or neurologic deficits such as hemiplegia. Patients with renal vein thrombosis may show flank pain and hematuria.
Laboratory Evaluation
Many clotting factors are consumed in acute thrombosis and factor levels may be fallaciously low in the acute phase. The child should be evaluated to rule out disseminated intravascular coagulopathy with complete blood count, peripheral blood smear, prothrombin time, activatedpartialthromboplastin time andfibrinogenlevel. Levels of D-dimer indicates activity of the coagulation cascade, and is a sensitive indicator of underlying DIC.
Table 12.22: Factors which increase risk of thrombosis in children
Acquired conditions
Infections: Viral, bacterial sepsis Disseminated intravascular coagulation Dehydration
Central venous catheter Surgery, trauma
Cyanotic congenital heart disease Antiphospholipid antibody syndrome
Acute lymphoblastic leukemia; therapy (L-asparaginase and steroids)
Nephrotic syndrome
Inherited prothrombotic disorders
Resistance to activated protein C
Factor V Leiden
Protein C deficiency
Protein S deficiency
Antithrombin deficiency
Prothrombin gene G20210A mutation
Elevated lipoprotein (a) level
Hyperhomocysteinemia
Color Doppler shows absence of signals in thrombosed vessels and the lumen cannot be compressed with direct pressure. However, it may not be sufficiently sensitive to detect thrombosis in vessels such as subclavian veins, superior vena cava or brachiocephalic veins. Echocardiography is useful for vena caval and proximal subclavian vein thrombosis. An MRI in conjunction with magnetic resonance venography is more sensitive than CT scan for the diagnosis of cerebral venous thrombosis. Chest radiography may reveal findingsofpulmonaryembolismwhichincludesmallpleural effusions with wedge shaped pleural-based opacity of pulmonary infarction, but has poor sensitivity. Ventilation perfusion scanning is useful in suspected pulmonary embolism. Patients with elevated levels of D-dimer and intermediate probability of pulmonary thrombosis on perfusion scan should be screened by spiral CT.
Management
Screening tests for hypercoagulable state should ideally be sent prior to initiating anticoagulation. Patients with respiratory distress or neurological problems should be managed in an intensive care unit. Initial therapy requires heparin (unfractionated or low molecular weight) followedbyoralwarfarin. Unfractionated heparinexhibits antithrombin as well as anti-Xa activity, whereas the action of low molecular weight heparin (LMWH) has primarily anti-Xa function. Close monitoring is required to prevent overdosage and risk of bleeding. The international normalized ratio (INR), is useful for monitoring oral anticoagulation with heparin and should be maintained in the range of 2 to 3. The duration of therapy depends on the risk of recurrence, which can be assessed by testing for thrombophilic states. Theevaluationis best done after 3 months of the event and after stopping anticoagulants. Children with lower limb deep vein thrombosis should be fitted for compression stockings.
Recurrent Thrombosis
Recurrent thrombosis may occur due to inadequate anticoagulation therapy. The risk of recurrence is estimated at 4-5% in patients without adverseriskfactors, 17-20% for those with one predisposing condition and almost 50% with two or more risk factors.
Suggested Reading
Saxena R, Kannan M, Chaudhry VP. Laboratory studies in coagula tion disorders. Indian J Pediatr 2007;74:649-55
Tormene D, Gavasso S, Rossetto V, Simioni P. Thrombosis and thrombophilia in children: a systematic review. SerninThromb Hemost 2006;32:724-8.
WHITE BLOOD CELLS
Quantitative changes (more than ±2 SD) in white cell counts are the most frequently found abnormality on the

hemogram report. The differential count helps define the expanded population of cells. The percentage increase over normal range is important; very high counts are indicative of leukemoid reaction (a very high leukocyte responsetoinfectionthat may be confusedwith leukemia) or leukemia.
The examination of the peripheral smear is very useful. The morphology of cells may reveal abnormal size, immaturity, change in nuclear-cytoplasmic ratio, inclusions and abnormal granules. HowellJolly bodies are found with absent splenic function (asplenia, post splenec tomy) and toxic granulations and shift to the left suggest sepsis. Epstein-Barr virus infection results in large monocytoid cells which can be confused for blasts on peripheral smear.
Leukocytosis
The onset and duration of illness, history of intake of medications and prior hospitalization may provide a clue to the diagnosis.
Neutrophi/ia
Neutrophils increase in diverse conditions, like acute bacterial infections, acute blood loss, hemolysis and diabetic ketoacidosis (Table 12.23). Cytochemical staining for leukocyte alkaline phosphatase (LAP), an enzyme found in mature neutrophils, is useful; neutrophils granules containing LAP stain blue, resulting in a high LAPscore in infectionsand leukemoid reaction.In chronic myeloid leukemia, neutrophils are deficient inLAP so the score is low compared to normal neutrophils.
Table 12.23: Common causes of neutrophilia
Acute
Acute bacterial infections
Epinephrine, corticosteroids, granulocyte colony stimulating factor
Hemorrhage; hemolysis Hypoxia
Trauma, burns, exercise, heat stroke
Renal failure, diabetic ketoacidosis, hepatic failure Hodgkin lymphoma
Chronic
Chronic myeloid leukemia Rheumatological and inflammatory diseases Hemolytic anemias; sickle cell anemia Post-splenectomy
Chronic blood loss Thyrotoxicosis
Chronic idiopathic neutrophilia
Genetic causes or syndromes
Down syndrome Asplenia
Leukocyte adhesion defects
Hematological Disorders -
Monocytosis |
|
Monocytes, the circulating tissue macrophage precursors, |
|
are important for ingestion and killing of pathogenic |
|
bacteria, e.g.Mycobacterium tuberculosis, and parasites, e.g. |
I |
Leishmania. Monocytosis is noted in many infections |
|
(Table 12.24). Abnormality of macrophage activationmay |
|
cause disorders like familial hemophagocytic syndrome. |
|
Table 12.24: Causes of monocytosis |
|
Infections: Tuberculosis, typhoid, bacterial endocarditis, |
|
brucella, kala-azar, malaria |
|
Autoimmune diseases: Systemic lupus erythematosus, |
|
rheumatoid arthritis, ulcerative colitis, polyarteritis nodosa |
|
Post-splenectomy neutropenia; hemolytic anemia |
|
Malignancy: Lymphoma, chronic myeloid leukemia, juvenile |
|
myelomonocytic leukemia, myelodysplasia |
|
Myxedema |
|
Basophilia |
|
Basophilia is usually seen during acute hypersensitivity |
|
reactions, but may be found in chronic myeloid leukemia, |
|
Hodgkinlymphoma, varicella, hypothyroidismand while |
|
on antithyroid medications. |
|
Eosinophilia |
|
Eosinophilia is noted in many allergic disorders systemic |
|
inflammatory conditions and malignancies (Table 12.25). |
|
Parasites which invade tissue are more likely to cause |
|
eosinophilia (e.g. Toxocara which causes visceral larva |
|
migrans). Sustained elevation in eosinophil count is |
|
associatedwithcardiac toxicity. Moderate elevation refers |
|
to an absolute eosinophil count of 1500-5000 cells/µl and |
|
severe eosinophilia is >5000 cells/µl. |
|
Lymphocytosis |
|
Lymphocytosis is a common feature of many infections |
|
(Table 12.26). It is important to distinguish reactive from |
|
neoplastic lymphocytosis. |
|
Leukopenia
This is usually found in conjunction with pancytopenia, e.g. aplastic anemia, megaloblastic anemia, bone marrow replacement orinfiltration (malignancy, Gaucher disease, osteopetrosis) and hypersplenism.
Neutropenia
Neutropenia may occur due to increased destruction or decreased production of neutrophils, and is usually acquired (Table 12.27) Rarely, neutropenia may be congenital, occurring as cyclic neutropenia, or as a part of inherited deficit. Transient neutropenia is common with viral infections. Severe neutropenia is present when the absolute neutrophil count is below 500 cells/µl.

|
i |
_ s |
_______________ |
_ E |
_s_s_ _en_t_a_i_P_ed_iat ric __________________ |
_ |
|
Table 12.25: Common causes of eosinophilia
Acute
Allergic disorders: Asthma, atopic dermatitis, urticaria, drug hypersensitivity, pemphigoid
Parasitic infestations: Toxocara, ascaris, amebiasis, strongy loidiasis, filaria, toxoplasmosis, trichinosis, schistosomiasis, malaria, scabies
Fungal infections: Bronchopulmonary aspergillosis, coccidio mycosis
Malignancy: Hodgkin lymphoma, T cell lymphoma, acute myelogenous leukemia, myeloproliferative syndrome
Hypereosinophilic syndrome
Chronic
Allergic disorders: Pemphigus, dermatitis herpetiformis Autoimmune disorders: Inflammatory bowel disease,
rheumatoid arthritis
Myeloproliferative syndrome, hypereosinophilic syndrome Loeffler syndrome
Immunodeficiency syndromes: Hyper IgE, Wiskott Aldrich syndrome; Omenn syndrome; graft versus host reaction Miscellaneous: Thrombocytopenia with absent radii; renal
allograft rejection; Addison disease
Lymphopenia
Lymphopenia is noted during severalinfections, including viral (hepatitis, influenza) and bacterial (typhoid, tuberculosis, sepsis) illnesses. The most common infectious cause is acquired immunodeficiency syndrome (AIDS). Lymphopenia is also found in inherited immuno deficiency syndromes due to decreased production of B or T lymphocytes (severe combined immunodeficiency, isolated CD4+ lymphocytopenia, ataxia-telangiectasia and Wiskott-Aldrich syndrome. It may also occur after anti thymocyte globulin treatment for aplastic anemia, use of corticosteroids, systemic lupus erythematosus and protein losing enteropathy.
QualitativeDefects
Qualititavedefects in leukocytes may give rise to immuno deficiencies. The work up of most immunodeficiencies needs specialized tests for estimate of immunoglobulins, T and B lymphocyte subsets and complement and functional assays.
Cl1ediak-Higaslzi syndrome is identified by its characteristic morphology showing giant lysosomes in the granulocytes and oculocutaneous albinism. Defects in CHSl or Lystgene impair lysosomal trafficking, resulting in ineffective granulopoiesis, delayed degranulation andaltered chemo taxis resulting in increased bacterial infections. In the
Table 12.26: Common causes of lymphocytosis
Infections: Infectious mononucleosis, infectious hepatitis, cytomegalovirus, tuberculosis, pertussis
Endocrine: Thyrotoxicosis, Addison disease Malignancy: Acute lymphoblastic leukemia, lymphoma
Table 12.27: Common causes of neutropenia
Acute
Infections: Severe sepsis; tuberculosis, Shigella, brucellosis; dengue, varicella, Epstein-Barrvirus, cytomegalovirus, HN; kala-azar, malaria; rickettsia
Drugs: Sulfonamides, phenytoin, phenobarbital, penicillin, phenothiazines
Bone marrow infiltration: Leukemia, lymphoma, neuroblastoma
Hypersplenism
Chemotherapy: Busulphan, cyclophosphamide, radiation
Chronic
Aplastic anemia: Acquired; inherited (Fanconi anemia) Autoimm.une diseases:Systemic lupus erythematosus, Crohn
disease, rheumatoid arthritis Vitamin B12 or folate deficiency
Bone marrow infiltration: Myelodysplasia, chronic myelogenous leukemia, chronic idiopathic neutropenia
Paroxysmal nocturnal hemoglobinuria
Inherited disorders: Cyclic neutropenia; severe congenital neutropenia; chronic benign neutropenia; Kostmann syndrome; Schwachrnan-Diamond syndrome; dyskeratosis congenita; Chediak-Higashi syndrome; glycogen storage disease type 1B
Associated with immunodeficiency: Hyper IgM syndrome; HN
accelerated phase, there is lymphohistiocytic infiltration of organs.
Leukocyte adhesion defect type 1 results from deficiency of CDll and CD18 on the neutrophils, lead to defects in adhesion, chemotaxis and C3bi mediated phagocytosis. This causes delayed umbilical cord separation in the newborn and leads to repeated, severe infections and periodontitis later in life.
Chronic granu/0111atous disease is an X-linked, (or rarely, autosomal recessive) inherited defect of the respiratory burst pathway in the granulocytes. It manifests as infections in the lungs, skin andgastrointestinaltract with S. aureus, aspergillus and Serratia marcescens, leading to formation of deep-seated granulomatous lesions. Many other primary immunodeficiencies have quantitative defects inT, B orboth lymphocyte subsetswithmaturation or functional defects, which lead to life threatening infections.

Otolaryngology
Sandeep Samant, Grant T. Rohman, Jerome W. Thompson
DISEASES OF THE EAR
Otitis Media
Otitis media is a common early childhood infection. Anatomic features that make young children particularly susceptible to ear infections include shorter, more horizontal and compliant eustachian tubes and bacterial carriage in the adenoids. Other risk factors include expo sure to cigarette smoke, overcrowding, bottlefeeding, cleft palate, Down syndrome, allergy and immune dysfunction. These risk factors contribute to the pathophysiology of the two common varieties of otitis media, acute otitis media and otitis media with effusion.
Acute Otitis Media
Acute otitis media (AOM) in children tends to have a bimodal age distribution, with children between ages 6 and 24 months and 5 to 6 yr at greatest risk.
Etiology. The most common organisms causing AOM are
Streptococcus pneumoniae and Haemophilus influenzae, accounting for approximately 65% cases; 15% are caused by Moraxella catarrhalis, Streptococcus pyogenes and
Staphylococcusaureus. Respiratoryvirusesplayan important role in initiating otitis media andmay bethe only pathogens in some cases, since 20% of middle ear aspirates are sterile.
Diagnosis. AOM is characterized by the rapid onset of symptoms, which may be local, e.g. otalgia or ear tugging, and/or systemic, e.g. fever or crying.Older children may report impaired hearing. History of recent upper respira tory tract infection is common. Otoscopic examination reveals a red andbulgingtympanicmembranewith redu ced mobility as measured by either tympanometry or insufflation through the otoscope (pneumatic otoscopy). Rupture of the drum with ear discharge (suppuration) may have already occurred, in which case the ear canal contains an opaque yellow-green or reddish-brown fluid. Cleaning of this fluid usually reveals an intact drum, as theruptureissmallandclosespromptlyafterspontaneous perforation. The diagnosis of AOM is considered certain if all of the following criteria are met: (i) rapid onset; (ii) signs of middle ear effusion; and (iii) signs and symptoms of middle ear inflammation.
Treatment. Antimicrobial therapy is recommended. However, in some cases children mayqualify fora trial of observation (Table 13.1). Amoxicillin should be the first line therapy for AOM. Higher doses (80-90 mg/kg/day) may be considered where streptococcal resistance is endemic. Agents with -lactamase resistance (e.g. amoxicillin-clavulanic acid, cefaclor, cefuroxime or newer cephalosporins) are useful second-line drugs. Initial
Table 13.1: Criteria for choice of treatment or observation in children with acute otitis media
Age |
Diagnosis certain |
Diagnosis uncertain |
<6mo |
Antibacterial therapy |
Antibacterial therapy |
6-23mo |
Antibacterial therapy |
Antibacterial therapy if illness severe* |
.?.24mo |
Antibacterial therapy if illness severe* |
Observation is an option if illness not severe** |
|
Observation is an option if illness not severe** |
|
*Severe illness is defined as moderate to severe otalgia or fever ;?.39°C
*Observation is appropriate only if follow-up can be ensured; antibacterial therapy is started if symptoms persist or worsen
Adapted from guidelines of the American Academy of Pediatrics and American Academy of Family Physicians. Clinical Practice Guideline: Subcommittee on Management of Acute Otitis Media. Pediatrics 2004;113:1451-65
359
- E_s_s_ e tn ia_i_P_ed_ia_nct·s __________________________________
antibiotic therapy should last at least 7 days. Re examination is indicated after 3-4 days and at 3 weeks.
Adjuvant treatment with oral and topical decongestant drugs is not necessary. Antihistaminic agents, which contribute little to the resolution of otitis media and may precipitate sinus infections due to their drying effect on mucosal secretions, are also not recommended. Tympano centesis (aspiration of the middle ear fluid) with a bent 18-gauge spinalneedle on a syringe may provide specimen for culture in patients with complicated AOM who cannot tolerate tympanostomy tube insertion. Tympanocentesis improves otalgia but does not shorten the course of the illness.
Many children present with recurrent episodes of AOM. A child that has 4 episodes of AOM in 6 months or 6 episodes in 12 months should be considered for tym panostomy tube insertion. If a child requires a second set of tympanostomy tubes, concurrent adenoidectomy is considered. No benefit from concurrent tonsillectomy has been demonstrated in patients with recurrent AOM.
Otitis Media with Effusion (OME)
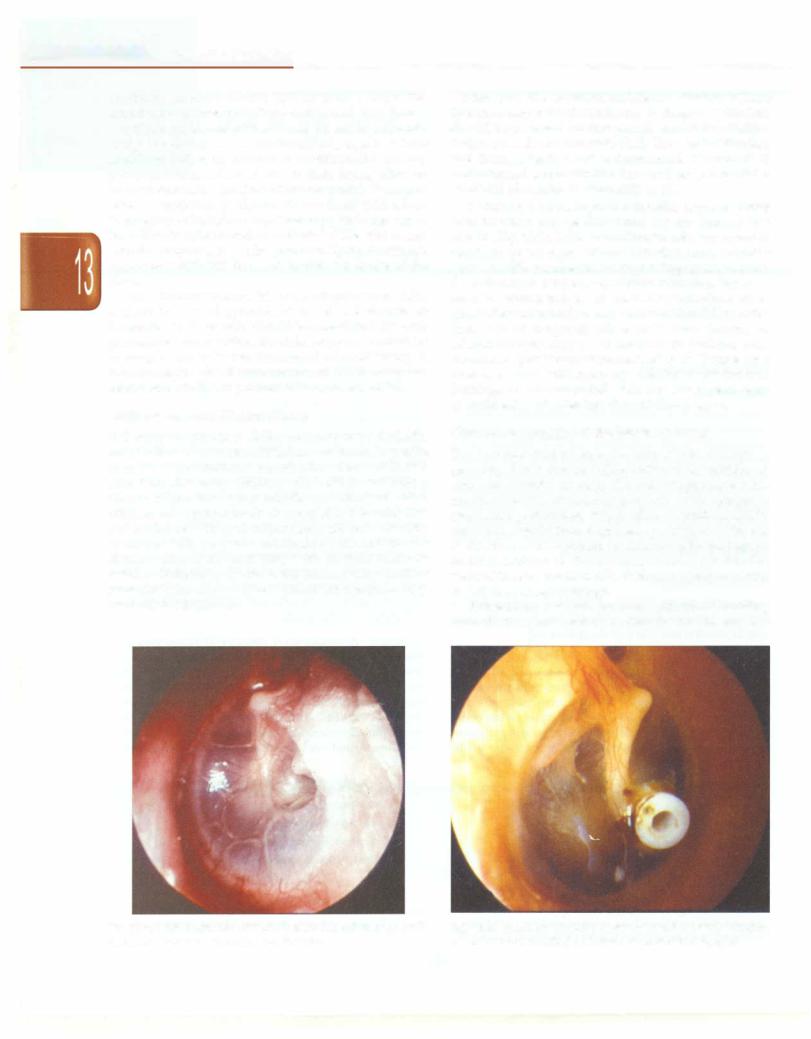
Following an episode of AOM, serous or mucoid middle ear effusions may be seen. Effusions are found to persist in up to 40% of children 1 month after AOM and in 10% after 3 months. Many children with OME do not have a history of previous acute middle ear infections. Most children are asymptomatic or complain of hearing loss and ear fullness. Otalgia is not normally present. Otoscopy reveals a dull tympanic membrane with middle ear effusion (Fig. 13.1), frequently with air fluid levels or bubbles. Reducedtympanic membrane mobility on either pneumatic otoscopy or type B pattern on tympanometry confirms the diagnosis.
Since over 65% of serous middle ear effusions resolve spontaneously within 3 months, newly diagnosed effusions should be observed for this period. Antibiotic adminis tration is not shown to resolve OME. Use of antihistamines and decongestants is not recommended. The benefit of corticosteroid administration has not been proven but a brief trial of steroids is commonly used.
If effusion persists beyond 3 months, tympanostomy tube insertion may be considered for any hearing loss >25 dB (Fig. 13.2). Other indications of tube placement in OME are speech delay, altered behavior, major sequelae such as otitic meningitis or impending cholesteatoma formation from tympanic membrane retraction. Improve ment in hearing and ear discomfort is immediate. Mean time before extrusion is usually between 12 and 18 months. Insertion of longterm tubes (of T-tube design) or adenoidectomy may be considered in patients with recurrent or persistent symptomatic effusion. T-tubes have been associated with tympanic membrane perforation. Earplugs are recommended while the tubes are in place to avoid entry of water into the middle ear space.
Chronic Suppurative Otitis Media (CSOM)
Ear drainage that persists for longer than 6 weeks is generally due to chronic inflammation of the middle ear space or mastoid air cells. Chronic suppurative otitis media (CSOM) invariably presents with tympanic membrane perforation, which allows otorrhea. CSOM most often results from neglected episodes of AOM and is therefore more common in children with inadequate access to health care. It most often occurs in the first five years of life as eustachian tube dysfunction plays a central
role in the pathophysiology.
Clwlesteatoma, a sac of squamous epithelium extending from the tympanic membrane into the middle ear, also
Fig. 13.1: Otitis media with effusion. Note the dull lustreless tympanic |
Fig. 13.2: Tympanostomy tube in situ in the anteroinferior quadrant |
membrane. (Courtesy: Textbook of ENT, Hazarika) |
of the tympanic membrane (Courtesy, Textbook of ENT, Hazarika) |
_________________________________o_t_o_,a_ryo,no gg_y
presents with a chronically draining ear. Most cholesteatoma is acquired. It remains unclearwhetheracholesteatomaarises from extension of a tympanic membrane retraction pocket, or from aberrant inward migration of normal epithelium. Rarely, it may be congenital, arising de nova through the eustachian tube by passage of neonatal epithelium. Though not malignant, cholesteatoma may cause serious compli cations by slow expansion and local destruction.
Etiology. The most commonly isolated organism is
Pseudomonas aeruginosa; other organisms include Staphylo coccus aureus, Proteus spp, E. coli and anerobes. Fungi, especiallyAspergillus and Candida spp., may be important.
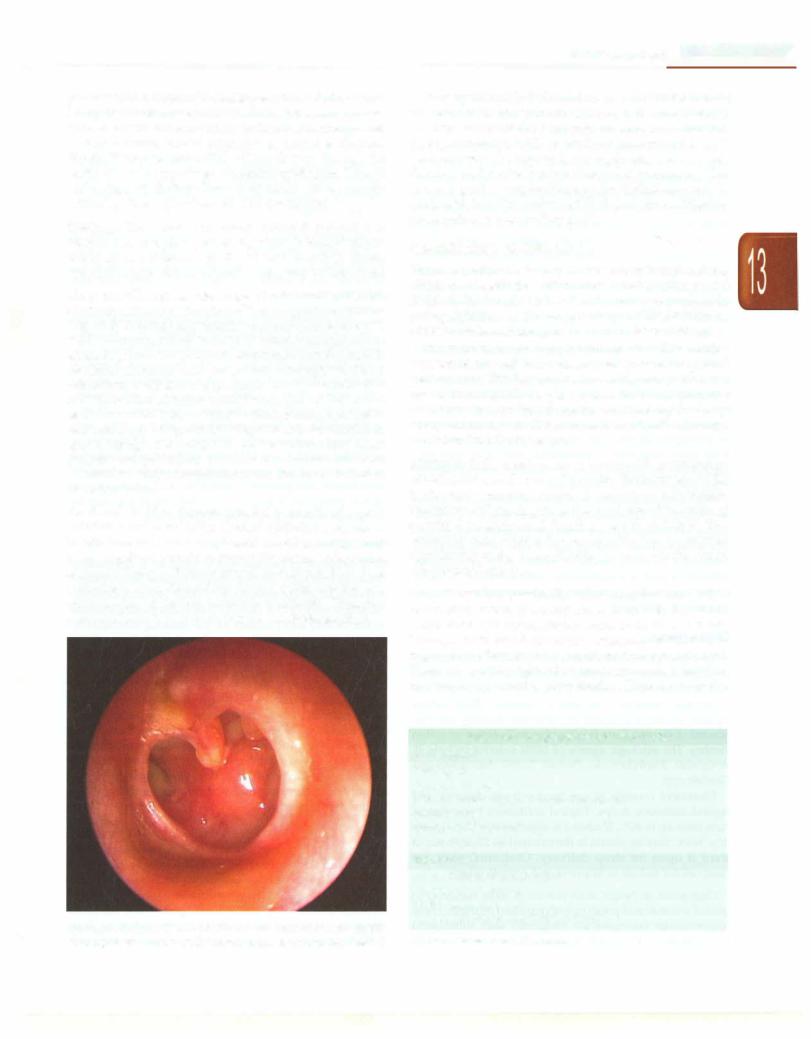
Diagnosis Chroniceardischargeisthehallmark ofCSOM. Otoscopy reveals perforation of the tympanic membrane (Fig. 13.3). A chronicallydraining earmayalsobeseenwith cholesteatoma, which is a sac of squamous epithelium extending from the tympanic membrane into the middle ear. Most cholesteatoma is acquired, although whether it arises from extension of a tympanic membrane retraction pocket, or from aberrant inward migration of the normal eardrum epithelium, remains unclear. Rarely, the choles teatomamaybecongenital,arisingdenovainthemiddleear space. Though not malignant, cholesteatoma may cause serious complications by slow expansion and local destruction. These complications are discussed further in the next section.
Treatment. Medical therapy consists primarily of topical antibiotics and aural toilet. Topical quinolones appear to be effective and safe. Complicated infections and/or any signs of systemic involvement require the use of systemic antibiotic therapy. Parents should be instructed to avoid water exposure. Secondary fungal otitis externa is a complication of topical antibiotic treatment. Otolaryn gology referral is necessary to rule out cholesteatoma.
Surgery is usually indicated for cases of CSOM that do not respond to conservative treatment. Surgical therapy involves repair of the tympanic membrane perforation (tympanoplasty) with or without mastoidectomy. If cholesteatoma is suspected, ear exploration via mastoi dectomy and cholesteatoma removal is mandatory. The primary goal of surgical therapy for cholesteatoma is to create a 'safe ear' by removal of all cholesteatoma. Hearing preservation is a secondary goal.
Complications of Otitis Media
Untreated otitis media may cause serious complications, which are classified as either intracranial or extracranial (Table 13.2). Complications of AOM are more common in young children, while complications of CSOM with or without cholesteatoma are common in older children.
The most common complication of CSOM is hearing loss, which may affect language development and school performance. The hearing loss is usually conductive and results from middle ear edema and fluid and tympanic membrane perforation. Sensorineural hearing loss may rarely occur due to direct extension of inflammatory mediators into the inner ear.
Meningitis is the most common intracranial complication of both acute and chronic otitis media. Furthermore, AOM is the most common cause of secondary meningitis. Pneumococcal meningitis is the most common cause of acquired sensorineural hearing loss in children. The mortality rate from otitic meningitis has decreased significantly in the postantibiotic era and with the use of streptococcal vaccines.
Brain abscess is a potentially lethal complication. Unlike meningitis, which is caused more frequently by AOM, brain abscesses result almost exclusively from CSOM. Therapy with broad spectrum parenteral antibiotics is begun immediately and surgical drainage considered. Thrombosis of the sigmoid or transverse sinus is another important intracranial complication. Patients typically
Fig. 13.3: Otoscopy in a child with chronic suppurative otitis media showing subtotal central perforation (Courtesy, Textbook of ENT, Hazarika)
Table 13.2: Complications of otitis media• lntracranial
Meningitis Epidural abscess
Dural venous (sigmoid sinus) thrombosis Brain abscess
Otitic hydrocephalus Subdural abscess
Extracranial
Acute coalescent mastoiditis Subperiosteal abscess Facial nerve paralysis
Labyrinthinitis or labyrinthine fistula
*Listed from most to least common

.....E_s_s_en_ t_ia_i_P_e_d_ ia trics_________________________________
-
present with headache, malaise and high spiking fever in a 'picket fence' pattern. Treatment involves parenteral antibiotics and surgical drainage of the mastoid.
Acute coalescent mastoiditis. This results from the spread of
infection into the mastoid bone. The entity should be differentiated from fluid effusion within mastoid air cells, which is sometimes mistakenly reported radiologically as 'mastoiditis'. Such opacification is commonly seen with AOM or OME, is readily apparent on CT and is of little clinical significance. Coalescent mastoiditis, on the other hand, presents with postauricular erythema, tenderness, and edema. The auricle is displaced inferiorly and laterally. The CT scan shows fluid and breakdown of the wall separatingthe mastoid air cells. Untreated, coalescent mastoiditis may spread externally, leading to the formation of subperiosteal or deep neck abscesses.
Acute coalescent mastoiditis should initially be treated with parenteral antibiotics directed against the afore mentionedpathogensassociated with AOM. If mastoiditis is superimposed on a chronically draining ear, coverage should be added for gram-negative and anerobic organisms. Surgery in the form of cortical mastoidectomy with tympanostomy tube insertion is indicated for cases with poor response to parenteral antibiotic therapy, presence of an abscess or an intracranial complication or acute mastoiditis in a chronic ear.
Othercomplicationsinclude labyrinthinefistula andfacial nerve paralysis. Labyrinthine fistula, in which a choleste atoma has eroded into the inner ear, presents with vertigo and sensorineural hearing loss. Facial nerve paralysis secondary to otitis media is treated with appropriate antibiotics and tympanostomy tube insertion. If facial nerve paralysis is secondary to cholesteatoma, mastoi dectomy is indicated.
Otitis Externa
Acute otitis externa (swimmer's ear) presents with itching, pain and fullness. Erythema and edema of the ear canal and tenderness on moving the pinnae or tragus are diagnostic features. Otorrhea is common. Risk factors include swimming, impacted cerumen, hearing aid use, eczema or trauma from foreign objects (hairpins or cotton swabs). The etiologic agents of otitis externa include P.
aeruginosa, Staphylococcus, Proteus, E. coli, Aspergillus and Candida spp.
Treatment consists of ear canal culture, cleaning and topical antibiotic drops. Topical antibiotics have clinical cure rates up to 80%. If edema is significant, ribbon gauze or a 'wick' may be placed in the externalauditorycanal to stent it open for drop delivery. Oral antibiotics are reserved for failure to improve and complications.
Otomycosis or fungal otitis externa is most common in
humid weather andpresentswithpain andpruritus.These opportunistic infections are frequently seen subsequent to treatment of a bacterial infection. Examination reveals
fungal spores and filaments. Aspergillus and Candida are the most common pathogens. Aural toilet and a topical antifungal (e.g. clotrimazole) are curative.
Otic furunculosis is an exquisitely painful, superficial abscess intheouter portion oftheearcanal, typically from S. aureus. Oral antistaphylococcal antibiotics and anal gesics bring about prompt relief. Incision and drainage may be necessary.
Eczematous or psoriatic otitis externa describes a group of
inflammatory conditions in which there is drainage, pruritis and/or scaling of the ear canal skin. Underlying causes include contact dermatitis, atopic dermatitis and seborrheic dermatitis.
Malignant otitis externa is a rare invasive infection of the external auditory canal cartilage and bone. Immunocom promised children (acquired immunodeficiency syn drome, leukemia, diabetes mellitus, immunosuppression after organ transplant) are at risk. Pseudomonas aeruginosa is the most common etiology. Invasive fungal species, especially Aspergillus, are also seen. The external auditory canal is tender and facial or scalp necrosis may arise, with or without cranial nerve abnormalities. Diagnosis is confirmed with CT and MRI scan and/or scintigraphy for osteomyelitis of the temporal bone. Aggressive surgical debridementandparenteralantibioticsand/or antifungals for 4-6 weeks are required. Treatment response may be monitored with serial Gallium67 bone scans.
Hearing Loss
Early detection of hearing loss in children is imperative. Unrecognized earlyhearingloss can impede development ofspeech, language andcognitive skills. Separate differen tial diagnoses exist for deficits of both the conductive and sensorineural components of the hearing mechanism. Hearing loss in children can be classified as either
congenital or acquired.
Conductive Hearing Loss
Anyprocessthatinterferes withtheconductive mechanism of the ear canal, tympanic membrane, or ossicles may cause a conductive hearingloss.Themostcommonpedia tric cause of conductive lost is otitis media with effusion and is typically of mild to moderate severity. Several congenital syndromesmayalsobe associated with middle ear abnormalities, such as Apert, Crouzon and Treacher Collins syndromes.
Sensorineural Hearing Loss
Sensorineural hearing loss is caused by a lesion of the cochlea, auditory nerve or central auditory pathway. SNHL can be acquired or congenital, both being equally common. The most common postnatal cause of acquired sensorineural hearing loss is meningitis, while the most common prenatal cause is intrauterine infection (e.g. TORCH infections, syphilis). Other causes of acquired

_________________________________o_t_ o_ ,a_ry_no__g,o_ g_y __
hearing loss include prematurity, hyperbilirubinemia, perinatal hypoxia, acquired immunodeficiency syndrome, head trauma and ototoxic medications (aminoglycosides, loop diuretics).
Congenital causes of sensorineural hearing loss are of syndromic and nonsyndromic types. Although 70% of congenital hearing loss is nonsyndromic, over300 genetic syndromesareassociatedwithSNHL.Commonsyndromes include Pendred syndrome (euthyroid goiter), Jervell and Lange-Nielsen syndrome (prolonged QT waves, syncope), Usher syndrome (retinitis pigmentosa and blindness), Alport's syndrome, branchio-oto-renal syndrome, neuro fibromatosis and Waardenburg syndrome. Multiple chromosome loci and at least 65 genes associated with genetic hearing loss have been identified. Mutations in a single gene, GJB2, may be responsible for up to 50% of nonsyndromic congenital hearing loss. GJB2 encodes the protein connexin 26, which is widely expressed in cells of the inner ear. Screening tests for this mutation are available.
Neonatal Screening
All neonates with risk factors for hearing loss should be screened with an oto-acoustic emission test or an auditory brainstem response. The use of clinical indicators to focus hearing screens will miss as many as 50% of all cases of impairment. Hence universal newborn hearing screen programs are now commonplace in the United States and Europe. The importance of neonatal screening cannot be overemphasized. Infants in whom treatment for hearing loss is initiated by 6 months of age are able to maintain language and social development in line with their phys ical development. This is in contrast to those whose hearinglossisidentified after 6 months ofage.A limitation of newborn screening is that some forms of early-onset hearing loss are notapparentat birth. A UnitedStatesJoint Committee on infant hearing has identified 11 risk indicators that should prompt continued monitoring of hearing status even in theface of normal neonatal screens (Table 13.3).
Screening in Older Children
Clinical evaluation of hearing at routine well child assess ments iscritical for early detection of hearing impairment. Examination should include otoscopy with attention to middle ear pathology. Doubtful cases are referred for detailedaudiologicevaluationso that timely intervention may begin.
Multiple techniques exist to assess hearing sensitivity and are selected based on the age and the abilities of the child. For younger children unable to understand instruc tions, visual-reinforcement audiometryis performed. Pure tone audiometry is possible in children older than 5 yr. Tympanometry may be performed in nearly all children to assess ear drum mobility.
Table 13.3: Indications for continued hearing monitoring in children with normal hearing on neonatal screening
Caregiver concern regarding hearing, speech, or develop mental delay
Family history of childhood hearing loss
Neonatal intensive care for >5 days or use of any of the following, regardless of duration: Extracorporeal membrane oxygenation, assisted ventilation, exposure to ototoxic antibiotic (gentamycin, tobramycin) or loop diuretics (furosemide) and hyperbilirubinemia requiring exchange transfusion
In utero infections (CMV, rubella, syphilis, herpes, toxo plasmosis)
Findings of a syndrome associated with hearing loss
Postnatal infection known to cause hearing loss (e.g. meningitis)
Syndromes associated with progressive hearing loss (e.g. neurofibromatosis)
Neurodegenerative disorders (e.g. Hunter syndrome, Friedreich ataxia)
Head trauma
Recurrent or persistent ( 3 mo) otitis media with effusion
Chemotherapy or head radiation
Treatment of Hearing Loss
Once diagnosed, treatment of hearing loss is based on the extent of deficit and the underlying pathology. For very mild hearing loss, treatment may consist simply of preferential seating in school. For mild to moderate conductive hearing loss, treatment options include tympanostomy tubes or, if a perforation is present, tympanoplasty.
Treatment of significantsensorineuralhearing loss may require the use of hearing aids from as early as 3 months of age. The development of cochlear implants has rapidly reshaped the management of childhood hearing loss. Bilateral cochlear implantation may be considered for infantsas young as12 monthsof age who have a profound bilateral hearing loss and may be considered even earlier if the hearing loss is due to meningitis. If a child has never had auditory stimulus (secondary to profound congenital deafness), cochlear implantation before 6 yr of age is crucial todevelop the auditory cortex for sound awareness and speech development. Sign language and deaf education programs should be considered for children who are not candidates for cochlear implantation.
DISEASES OF THE NOSE AND SINUSES
Rhinitis
Allergic Rhinitis
Allergicrhinitisis aninflammatorydisordercharacterized
by sneezing, itching, nasal obstruction and clear rhino-
|
i |
i |
E_s_s_e_n_ tia_i_P_e_d_ |
a _trc-_s_________________________________ |
|
|
|
|
rrhea. The pathophysiology involves an IgE-mediated reaction to a specific allergen. Symptoms may be seasonal ('hay fever') or perennial. Examination reveals a pale nasal mucosa, congested nasal turbinates and mucoid rhinor rhea. Conjunctiva! itching and redness may be present. Inhaled allergens (e.g. pollen, spores and dust mites) are common causes. Accurate diagnosis may require demonstration of eosinophilia in a nasal smear, or the use of skin/serologic tests to show specific IgE response to allergens. These tests establish the atopic etiology and help differentiate from other conditions withsimilar symptoms.
Treatment includes allergen avoidance, use of topical nasal steroidsprays for prevention andoral antihistamines for symptom relief. The use of oral decongestants is controversial.Topical decongestants should also generally be discouraged as they cause rebound congestion (short term) and chemical rhinitis or rhinitis medicamentosa (longterm).
Viral Rhinitis
Viral rhinitis or common cold is the most common cause of both nasal obstruction and rhinorrhea in children. Children normallyaveragebetween six andeight of these upper respiratory infections per year. Malaise, low to moderate grade fever, nasal congestion and rhinorrhea are the presenting symptoms. A number of different virusescanbe responsible, includingrhinovirus, influenza and adenovirus. Treatment is symptomatic and involves antipyretics, saline nasal spray. Use of oral decongestants and antihistamines are controversial. A number of deve loped countries recommend annual influenza vaccination in children older than 6 months. Otitis media and sinusitis are frequent complications.
Sinusitis
Sinusitis can be classified as either acute or chronic. The ethmoid and maxillary sinuses are the earliest to develop and are the ones most commonly infected in pediatric sinusitis. The frontal sinuses may become involved only after 5-6 yr of life; isolated sphenoid disease is rare. Risk factors associated with sinusitis include recurrent upper respiratory infections, allergic rhinitis, cystic fibrosis, immunodeficiency, ciliary dyskinesia, daycare attendance and exposure to tobacco smoke. Ten-fifteen percent of upper respiratory tract infections are complicated by sinusitis. A sinus infection should be considered in any child whose cold symptoms have not resolved by 7-10 days.
Etiology. The most common isolates in acute sinus infec tions are 5. pneumoniae, H. influenzae and M. catarrhalis.
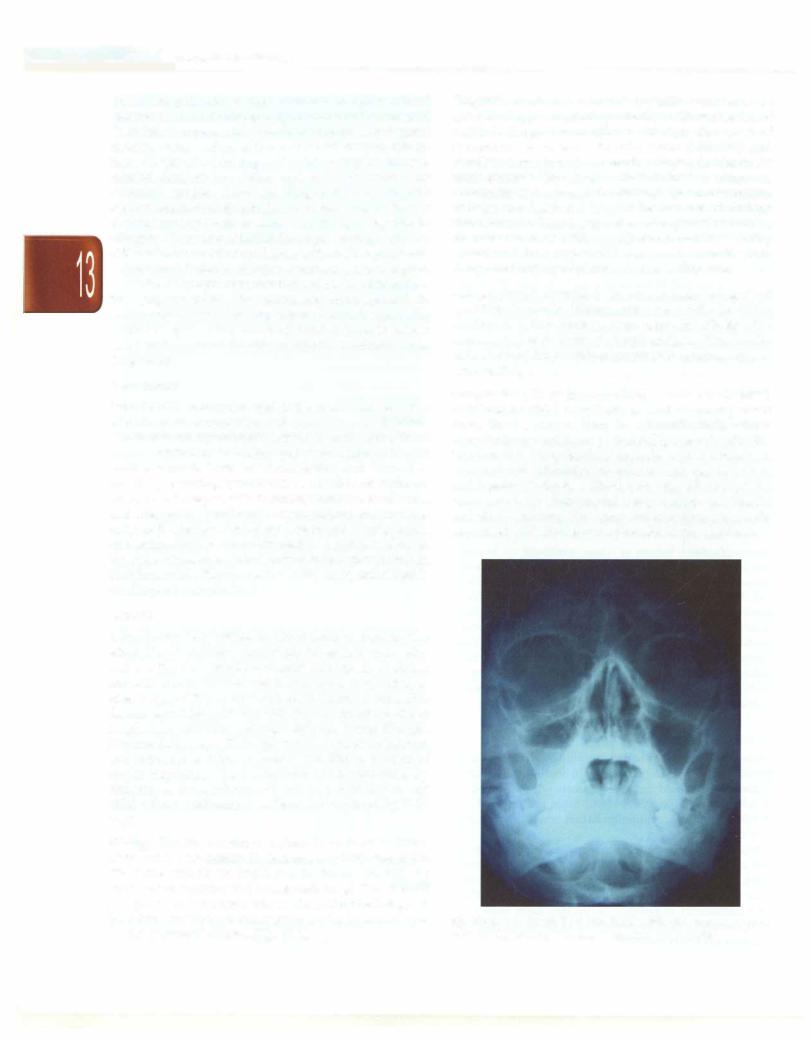
The same bacteria are implicated in chronic sinusitis, as are 5. aureus, anerobes and occasionally fungi.Theadenoid pad plays an important role in the pathophysiology of pediatric sinusitissinceit may serve as a bacterial reservoir for the paranasal sinuses (Fig. 13.4).
Diagnosis. Acute rhinosinusitis typically presents as an episode of upper respiratory infection with worsening of nasal discharge and cough 7 to 10 days after onset of symptoms. A severe URI with fever (>38.5°C) and purulent rhinorrhea also meets the diagnostic criteria for acute sinusitis. Chronic sinusitis is defined as symptoms of sinusitis lasting longer than 30 days. Nasal obstruction, malaise and headache may all be features of chronic rhinosinusitis. Imaging is not necessary and should be reserved for cases with complications and those being considered for surgery. CT scan is superior to plain X-rays for imaging of paranasal sinuses (Fig. 13.4).
Allergic fungal sinusitis is an increasingly recognised condition in atopic, immunocompetent patients. Older children and adolescents aremostcommonlyaffected.The cause is hypersensitivity to fungal antigens. This results in the form of chronic rhinosinusitis that requires surgical intervention.
Complications. These include orbital or intracranial spread of infection. Orbital complications most commonly result from direct extension from the ethmoids. Early orbital complicationsmanifestas periorbital(preseptal)cellulitis. More severe complications include orbital abscess or cavernous sinus thrombosis. Ophthalmoplegia, vision loss, and toxemia indicate a life-threatening infection of the cavernous sinus. Intracranial complications (meningitis and abscesses) may also occur and are more commonly associated with frontal and sphenoid sinus infections.
Fig. 13.4: Note the air fluid level in right maxillary sinus in a patient with maxillary sinusitis (Courtesy: Textbook of ENT, Hazarika)
