
Ghai Essential Pediatrics8th
.pdf
between the two circulations. In patients with intact ventricular septum, the mixing site is the atrial communi cation. Generally, the atrial communication is the patent foramenovale and this being small, the mixing is very poor (Fig. 15.28). The neonates become symptomatic due to severe hypoxemia and systemic acidosis soon after birth.
Presence of a VSD of adequate size results in good mixing. As the fetal pulmonary vasculature regresses, the pulmonaryblood flow increases and results in congestive failure around 4-10 weeks of age. The failing left ventricle as well as the large pulmonary blood flow increase the left atrial pressure. The patients, therefore, have pulmonary venous hypertension as well. The mixing with a large VSD can be so good that at times cyanosis can be missed. The presence of a large VSD equalizes pressures in the two ventricles as well as the great arteries. The pulmonary artery also carries a large flow. Patients with TGA and a large VSD develop pulmonary vascular obstructive disease (Eisenmenger physiology) early in life.
Clinical Features
Patients of complete TGA with intact ventricular septum are cyanotic at birth. Since the interatrial communication results in poor mixing, the neonates present with rapid breathing and congestive failure secondary to hypoxemia within the first week of life. The heart size can be normal in the first two weeks of life but enlarges rapidly. Physical examination shows severe cyanosis, congestive failure, normal first sound, single second sound and an insigni ficant grade one to two ejection systolic murmur. The electrocardiogram shows right axis deviation and right ventricular hypertrophy. The thoracic roentgenogram shows cardiomegaly with a narrow base and plethoric lung fields. The cardiac silhouette can have an "egg on side" appearance: The right upper lung fields appear more plethoric than other areas. The thymic shadow is often absent (Fig. 15.29).
RA |
LA |
)gi" |
LVI |
|
LI +-+ LI |
|
|||
+-+ |
Transposition |
RA |
|
|
( AO+-+ |
|
|
I |
|
PA |
....1 |
PA |
AO |
|
\ Br. Coll |
) |
|
|
|
Complete |
|
Corrected |
|
|
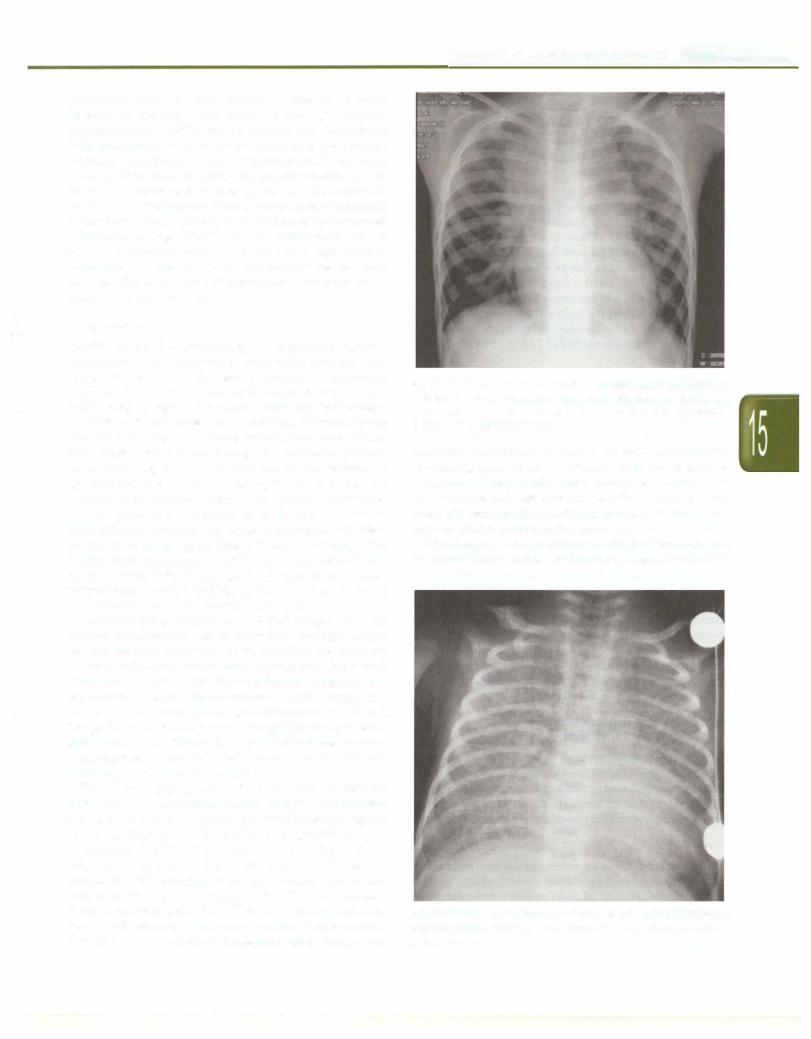
Fig. 15.28: The route of blood flow in complete TGA results in two separate circulations and survival depends on mixing. The mixing can occur at the atrial, ventricular or great vessel level. Bronchial collaterals (Br. Coll.) also increase pulmonary blood flow: In corrected TGA the route of blood flow is normal. Hemodynamicsdepend on associated anomalies
Disorders of Cardiovascular System -
Fig. 15.29: Egg on side appearance in transposition. This characteristic appearance is seen only in about one-third cases and results from a narrow pedicle of the heart because of malpostion of great vessels
Patients of TGA with VSD have increased pulmonary blood flow; mixing at the ventricular level determines the severity of cyanosis. They develop congestive failure around 4-10 weeks of age. Physical findings consist of cyanosis, cardiomegaly, congestive failure, normal first sound, single or normally split second sound and grade II-IV ejection systolic murmur. Apical third sound gallop or a mid-diastolic rumble may be present. Electrocardio gram shows right axis deviation with biventricular, right ventricular or left ventricular hypertrophy. Chest X-ray shows cardiomegaly, plethoric lung fields and features of pulmonary venous hypertension.
Treatment
Prostagladin El can help reduce cyanosis in selected cases by keeping the PDA open. Interim palliation can be accomplished through a balloon atrial septostomy (Fig. 15.30). This procedure can be accomplished in cathe terization laboratory or in the ICU under echocardio graphic guidance. Septostomy is successful only up to the age of 6-12 weeks andgives temporary relief by providing better mixing and reducing left atrial pressure.
The arterial switch operation is now established as the treatment of choice for TGA and most centers endeavor to offer this procedure for all infants with TGA. In this operation, the pulmonary artery and aorta are transected. The distal aorta is anastomosed to the proximal pulmonary stump (neo-aortic root) and the pulmonary artery to the proximal aortic stump (neopulmonary artery). The coronary arteries are moved along to the neo-aortic root along with a cuff of aortic tissue to allow suturing without

___E_ssen_ _ til aP_ed_ ia trics_________________________________
Fig. 15.30: Balloon atrial septostomy; this cartoon shows how a balloon atrial septostomy works. The figure on the left shows the physiology of transposition. The parallel circulation with poor inter circulatory mixing results in very low saturations in the aorta. Balloon atrial septostomy (right) creates an opening in the atrial septum and allows better inter-circulatory mixing with improved saturation that is often life saving
compromise of coronary blood flow. Most modern pediatric cardiac centers strive to achieve excellence with the neonatal arterial switch operation because the longterm results are very satisfying. There is a limited window of time for performing the arterial switch for TGA. Infants withTGA and intactseptumshould ideally undergo this procedure within the first 2-4 weeks of life. As pulmonary vascular resistance falls after birth the left ventricleregressesrapidly. In 1-2 months the leftventricle has the ability to adjust to the elevated systemic vascular resistance after the arterial switch through hyperplasia of the available muscle. After this it is difficult for the left ventricle to adapt to an arterial switch. Later in infancy, the atrial switch operation (Senning operation) is the only optionforTGA with intactventricularseptum.This is not an ideal longterm option because the right ventricle remains as the systemic ventricle for life. Overtime, right ventricle dysfunction and severe tricuspid regurgitation sets in. Additionally, extensive restructuring of atria predisposes to atrial rhythm disturbances.
In the presence of a sizable PDA or VSD there is no fear of early regression of LV because the PA pressures are elevated. Nevertheless, the window of time for operation ofTGA-VSD andTGA-PDA is also limited.This is because there is accelerated development of pulmonary vascular obstructive disease in these patients. Surgical correction involves the arterial switch operation with closure of the VSD or PDA.This should ideally be accomplished within 3 months of age. Beyond this age, anincreasing proportion of infants show irreversible changes in the pulmonary vasculature. Consequently postoperativerecoverymay be complicated by pulmonary hypertensive crisis and longterm results are unsatisfactory.
Many centers are able to perform arterial switch operations with operative mortality of <5%.Twenty year survival is >90%. Longterm concerns after surgery include development of aortic root dilation and aortic regur gitation, right ventricular outflow tract obstruction and coronary artery occlusion.
Corrected TGA
In correctedTGA the right atium is connected to the left ventricle and vice-versa. The left ventricle gives rise to the pulmonary artery andright ventricle to the aorta.The aorta lies anterior and to the left of the pulmonary artery (hence the term L-TGA). The ascending aorta forms the left upper border of the cardiac silhouette. Since the route of blood flow is normal, it is the associated anomalies that determine the clinical features.The associated anomalies are present in more than 98% cases. The commonest anomalies include (i) a VSD with or without pulmonic stenosis; (ii) left sided Ebstein anomaly of the tricuspid valve (clinically simulates mitral regurgitation); and (iii) atrioventricular conduction abnormalities including complete atrioventricular block, each in approximately 65% cases. The most useful clue for the diagnosis of correctedTGA is related toinversionof the ventricles.The precordial leads V4R, Vl, and V2 may show a 'Q' wave that is absent in the left precordial leads. Chest X-ray shows a smooth left upper border corresponding to the ascending aorta. The diagnosis depends on echocardio graphic identification of ventricular inversion as well as the additional anomalies. Management depends on the type of associated anomalies. The need to retain the morphologicleft ventricle as the systemic ventricle makes the surgical management of corrected transposition rather complex.
Total Anomalous Pulmonary
Venous Connecton (TAPVC) i
Here, all the pulmonary veins instead of joining the left atrium are connected anomalously to result in the total pulmonary venous blood reaching the right atrium. The anatomical classification ofTAPVC is into supracardiac, cardiac, infracardiac and mixed varieties. In the supracardiac TAPVC the pulmonary veins join together to form a common pulmonary vein that may drain into the left innominate vein or the right superior vena cava. In the cardiac TAPVC the veins join the coronary sinus or enter the right atrium directly. In the infracardiac variety the common pulmonary vein drains into the portal vein.
Hemodynamics
TAPVC results in the pulmonary venous blood reaching the right atrium, which also receives the systemic venous blood.This results in almost complete mixing of the two venous returns. The blood flow to the left atrium is the right toleft shunt through a patient foramen ovale or atrial septal defect. The oxygen saturation of the blood in the
pulmonary artery is often identical to that in the aorta because of mixing of the blood in the right atrium. Physiologically TAPVC can be divided into (a) patients with pulmonary venous obstruction, and (b) patients without pulmonary venous obstruction. Pulmonary venous obstruction results in pulmonary arterial hyper tension as well as restriction to pulmonary blood flow. In the absence of pulmonaryvenous obstruction, pulmonary blood flow is large and results in cardiac failure between 4-10 weeks of age. TAPVC of the infracardiac type is always obstructive whereas cardiac and supracardiac types may or may not have pulmonary venous obs truction. Generally the left atrium and ventricle are of normal size but can be small.
Clinical Picture
TAPVC of the non-obstructive type is commoner than the obstructive type. Patients present with cyanosis and congestive failure as the fetal pulmonary vasculature regresses. The onset of congestive failure is around four to ten weeks of age. Occasionally, with large pulmonary blood flow, the cyanosis may be minimal or clinically not recognizable. The patients are irritable and have failure to thrive. Besides features of congestive failure the patients have cardiomegaly, hyperkinetic precordium normal or accentuated first sound, widely split and fixed second sound with accentuated pulmonic component, a grade two to four pulmonary ejection systolic murmur and a tricuspid flow murmur. The physical findings are identical to that of an atrial septal defect. Presence of congestive failure at this age suggests TAPVC since congestive failure in atrial septa! defect at this age is very rare. A continuous venous hum may be audible at the upper left or right sternal border or in the suprasternal notch.
Patients with obstructive type of TAPVC present with marked cyanosis and congestive failure typically within the first one to two weeks of life. In India, however, we do frequently come across infants presenting later with obstructed TAPVC. The physical findings consist of a normal sized heart with parasternal heave, normal first sound, accentuated pulmonic component of S2 and insignificant murmurs. Tricuspid regurgitation can occur and results in cardiomegaly. These infants are severely compromised and need admission in an intensive care unit and emergency corrective surgery.
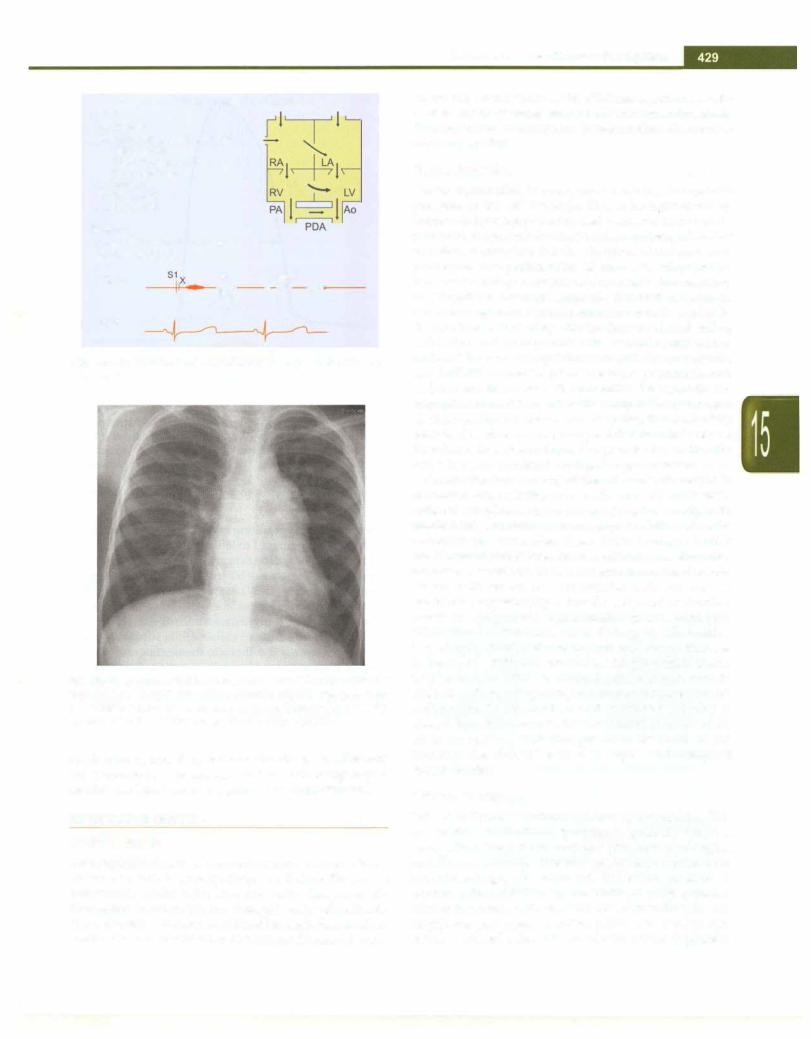
The electrocardiogram in TAPVC with or without pulmonary venous obstruction shows right axis deviation and right ventricular hypertrophy. Chest roentgenogram shows cardiomegaly with plethoric lung fields in non obstructive TAPVC. The characteristic pattern of the "snowman" or figure of '8' configuration in the supra cardiac TAPVC draining to left innominate vein is seen only after the age of 2 yr (Fig. 15.31). The characteristic X-ray of the obstructive TAPVC consists of a normal sized heart with severe pulmonary venous hypertension resulting in "ground glass" appearance of the lungs very
Disorders of Cardiovascular System -
Fig. 15.31: Chest X-ray in unobstructed supracardiac total anomalous pulmonary venous connection to the innominate vein via the left vertical vein in an 8-year-old child. This is the characteristic figure of ·s· sign or the snowman's sign
much like that of hyaline membrane disease (Fig. 15.32). Echocardiogram allows confirmation of the diagnosis, definition of the individual pulmonary veins and assess ment of the site of obstruction. In addition the pulmonary artery pressure can be quantified. In most situations echo alone is adequate for surgical planning.
The diagnosis of the obstructive TAPVC is made in a neonate with cyanosis and normal sized heart with
Fig. 15.32: Chest X-ray from a newborn with obstructed infracardiac total anomalouspulmonaryvenousconnection. Note the characteristic ground glass appearance

n i i aitrcs ______________
__E_s_s__aet__i_P_e_d___________________
"ground glass" lung fields. The diagnosis of non obstructive TAPVC should be suspected if the auscul tatory features of atrial septal defect are associated with either cyanosis with or without congestive failure in the first two to three months of life.
Management
Operation is indicated as early as possible since 80% of infants die within the first 3 months oflifewithout surgical help. Obstructed TAPVC needs surgery at short notice. The results of surgery for both forms of TAPVC are good in most modern centers but newborns and infants with obstructed TAPVC need a long time to recover after surgery. These patients are prone to develop pulmonary hypertensive crisis in the postoperative period. A small proportion of infants develop progressive pulmonary venousobstructionafterrepairofTAPVC that isoftennot easy to correct.
Addi tional Cond itions with
Cyanosis and High Pulmonary Flow
Apart from transposition of great vessels and total anomalous pulmonaryvenous connection, singleventricle without obstruction to pulmonary blood flow, persistent truncus arteriosus, tricuspid atresia with absence of obstruction to pulmonary blood flow and double outlet right ventricle without pulmonic stenosis present with cyanosis and increased pulmonary blood flow. Clinically patients present with congestive failure in the neonatal period and are characterized by cyanosis, cardiomegaly and failure to thrive. Almost 80% die within 3 months of life due to congestive cardiac failure or pulmonary infection. Those who survive develop pulmonary arterial hypertension due to pulmonary vascular obstructive disease. Echocardiography is necessary to arrive at the specific diagnosis. Since the mortality of unoperated patients is high and patients develop Eisenmenger syndrome early in life, it is necessary that patients presenting with cyanosis and increased pulmonary blood flowbe referred to specialized centers as early as possible.
Cyano tic Heart Disease with
Pulmonary Arterial H ypertension
Patients with Eisenmenger syndrome have severe pulmonary arterial hypertension resulting ina rightto left
shunt at the atrial, ventricular or pulmonary arterial level. Eisenmenger complex consists of pulmonary arterial
hypertension with a VSD providing the right to left shunt.
Hemodynamics
The pulmonary arterial hypertension is due to pulmonary vascularobstructivedisease. If acommunicationispresent at the pulmonary arterial level or the ventricular level, the right ventricular pressure cannot go beyond the systemic pressure. The right to left shunt decompresses the right ventricle. The right ventricle has only concentric
hypertrophy without significant increase in the size. In patients who have a PDA or VSD, there is only a mild parasternal impulse without significant heave. In patients who do not have a VSD or PDA, the right ventriclebesides hypertrophyalsodilates.The rightto leftshunt at the atrial level is an indication of right ventricular failure to accommodate this volume and push into the pulmonary artery. Patients of Eisenmenger syndrome with commu nication at the atrial level only, exhibit a parastemal heave and cardiac enlargement. The right ventricular pressure may even be higher than the systemic pressure.
A right to left shunt at the atrial level or the ventricular level reaches the ascending aorta and is thus distributed to the whole systemic circulation. This results in equal cyanosis of fingers and toes. A right to left shunt through
a PDA is directed downwards into the descending aorta, which results in differential cyanosis affecting lower limbs,
with pink upper limbs.
Clinical Features
Patients present with history of cyanosis, fatigue, effort intolerance and dyspnea. There may also be history of repeated chest infections in childhood. On physical examination they havecyanosis andclubbing. Differential cyanosis separates patients who have a PDA from those who have a VSD or atrial septal defect. The features indicative of pulmonary arterial hypertension consist of parasternal impulse and palpable second sound. The pulmonarycomponent ofthesecondsoundisaccentuated and louder than the aortic component. The splitting of the secondsoundremainswideandfixedinatrialsepta!defect. Due to superimposition of A2 and P2 the second sound is single in patients who have a VSD. Patients who have a PDA continue to have a normally split second sound. A constant pulmonary ejection click, unlike in patients of valvar pulmonic stenosis, is well heard both during inspiration and expiration at the second left interspace. A functional pulmonary regurgitation murmur can be present along the left sternal border. Patients with atrial septaldefect, inwhom Eisenmengerphysiology isuncom mon, can develop tricuspid regurgitation (Fig. 15.33).
The electrocardiogram reveals right axis deviation and rightventricular hypertrophy, P pulmonale may bepresent.
The chest radiograph is characteristic, showing promi nence of the pulmonary arterial segment and large right
and left main pulmonaryarteries and their branches. The peripheral lung fields are oligemic. Thus the hilar area suggests pulmonary plethora whereas the peripheral lungfields suggest pulmonary oligemia (Fig. 15.34).
Treatment
Ideally pulmonaryvascularobstructive disease should be prevented. This means early diagnosis and correction of all CHD associatedwith increased pulmonary blood flow. Patients with cyanosis and increased pulmonary blood flow develop Eisenmenger physiology very early and
Eisenmenger Syndrome
Sounds
S1: Normal
S2:ASD: Widely split and fixed
VSD : Single
PDA: Normally split
P2accentuated
X: Constant
S3: RVinASD
S4: RAinASD
Murmurs
Pulmonary regurgitation (Graham Steell)
Ejection systolic ±
|
..... |
11 ....... |
Phono |
S2 |
ESM |
|
Early |
|
|
DM |
|
ECG |
|
|
Fig. 15.33: Summary of auscultatory findings in Eisenmenger syndrome
Fig. 15.34: Chest X-ray in Eisenmenger syndromefollowing ventricular septal defect. The proximal right pulmonary artery is enlarged. There is a relative paucity of vasculature in the periphery with a sudden tapering of caliber of the right pulmonary artery (pruning)
need to be operated by 2-3 months of age. Medications are available for the management of pulmonary hyper tension (see later section on pulmonary hypertension).
OBSTRUCTIVE LESIONS
Aortic Stenosis
Pathologically thesite of obstructionmaybeatvalvelevel, above the valve (supravalvar) or below the valve (subvalvar). At the valve level the aortic stenosis results fromeither an unicuspid or a bicuspid aortic valve. Rarely the aortic valve annulus may itself be small. Supravalvar aortic stenosis results from obstruction in root of aorta,
Disorders of Cardiovascular System
above the aortic valve, as in Williams syndrome. Sub valvar aortic stenosis may be discrete (membranous), fibromuscular or muscular (hypertrophic obstructive cardiomyopathy).
Hemodynamics
Valvar obstruction is overcome by raising the systolic pressure of the left ventricle. This is brought about by concentric hypertrophy of the left ventricle. Because of a powerful, muscular left ventricle, the emptying of the left ventricle is complete but the duration of the systole is prolonged. The prolongation of left ventricular ejection time causes delayed closure of the aortic valve resulting in delayed A2. The flow across the obstruction results in the aortic ejection systolic murmur that is typically diamond shaped, startingafterthefirst soundandending before the aortic component of the second sound with a mid-systolicpeak. Thesystolicmurmur is always palpable as athrillat thesecond right interspace, suprasternalnotch and the carotid vessels. The powerful left ventricle can maintain anormalforwardcardiac output. The prolonged ejection results in the characteristic pulse that can be best described as slowly rising to a peak that is sustained and then has a slow down-slope. The peak is low so that the pulse is of low amplitude and prolonged duration.
Concentric hypertrophy of the left ventricle results in decreased distensibility of the left ventricle in diastole reduced compliance. In severe aortic stenosis (AS), with marked left ventricular hypertrophy, the left ventricular diastolic pressure also rises. With increase in left ventricular diastolic pressure, theleft atrial pressure must increase to be able to fill the left ventricle during diastole. Hence, with severe AS accompanied with marked left ventricular hypertrophy, a forceful left atrial contraction results in a palpable as well as audible fourth sound (S4). When the left ventricle starts failing in AS, besides hypertrophy dilatation also appears and causes increase in heart size. With left ventricular failure a third sound (S3) becomes audible. In valvar AS there is post stenotic dilatationofascendingaorta,seenon posteroanteriorchest radiograph. In supravalvar and subvalvar AS, this is absent. In valvar stenosis, the first sound is followed by an aortic ejection click that precedes the starts of the murmur; the click is heard at the apex, and along left sternal border.
Clinical Features
Patientswithmild tomoderateASareasymptomatic.With severe stenosis, the initial symptom is generally dyspnea on exertion. The patients may also give history of angina on effort and syncope. Presence of any one of these three symptoms suggests severe AS. The blood pressure is normal with mild disease; the width of pulse pressure relates inversely with severity of AS resulting in low amplitude prolonged duration pulse. The cardiac size remains normal unless left ventricular failure is present.

__E_s_s_ _en_t_ial_P_ed_iat _rics _________________________________ _
The apical impulse is forcible or heaving. In severe AS thefourthsound may be palpable. If left ventricular failure is present the thirdsound may be palpable. A systolic thrill is palpable at the second right interspace, suprasternal notch and the carotid arteries. The first sound is normal and followed by an ejection click in valvar aortic stenosis. The aortic component of the second sound (A2) is delayed but not diminished in intensity in AS. The delay results in closely split, single or paradoxically split second sound according to the severity of obstruction. With severe AS, S4 isaudible, while in patients with leftventricularfailure, S3 is palpable and audible. The ejection systolic murmur starting after the ejection click reaches a peak in mid systole (Fig. 15.35). With increasing severity the peak gets delayed so that the maximum intensity of the murmur is closer to the end rather than being midsystolic. With immobile valves, either due to severe fibrosis or calcification the systolic click as well as the A2 diminish in intensity and may become inaudible (Fig. 15.36).
Subvalvar AS is differentiated from valvar lesions by absence of ejection click and the post stenotic dilatation of the ascending aorta in the thoracic roentgenogram. An aortic regurgitation murmur may be audible. The maximum intensityof the systolic murmur and thrill may be in the third or fourth left interspace.
Supravalvar AS (William syndrome) is associated with elfin facies, mental retardation, dental abnormalities, strabismus and peripheral pulmonary arterial stenosis. Since the obstructionis above theaorticvalve, the pressure in the segment of the aorta before the obstruction is elevated and results in loud A2. The jet through the supravalvar narrowing may be directed toward the
|
|
Aortic Stenosis |
|
|
J\ |
|||
Sounds |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
S1-Normal |
|
|
|
|
|
LA |
||
S2-A2 Delayed |
|
|
|
|
||||
P2 Normal |
|
|
|
II |
|
/! |
||
Normal splitting single |
|
|
||||||
Paradoxical splitting |
|
|
LV |
|||||
S3: With LV failure |
|
|
|
|||||
S4: Severe stenosis |
|
|
|
|
||||
|
|
|
|
|||||
X: Constant Valvar |
|
|
PA |
|
|
|||
Murmurs |
|
|
|
|
|
|
|
|
Ejection systolic (Diamond shaped) |
|
|
|
|||||
(INSP) |
|
X |
ESM |
A2 2 |
Mild |
|
|
|
(INSP and E |
P) |
s111 |
•• I t |
|
|
|
||
|
|
S2 |
Moderate |
|
|
|||
|
X |
|
|
|
|
|||
|
|
11• I |
|
|
|
|||
(EXP) |
|
S4 X |
P2 |
|
Severe |
|
|
|
|
11 |
•1 A2 |
|
|
||||
|
|
I |
|
|
1 |
|
|
|
ECG
Fig. 15.35: Summary of auscultatory findings in aortic stenosis, S4 fourth sound; X aortic click
LV
|
---- |
'' |
-- |
Ao |
|
- |
LA |
f------- |
|
|
' |
-::.;::..----- |
|
-, ·+!.....
S1 X
ESM
-7,, |
|
|
i |
|
|
• |
I |
- ==--- |
|
.......... |
|
A2 |
|
|
Fig. 15.36: Aortic stenosis: Diagrammatic portrayal of the hemodynamic basis for aortic stenosis murmur. The first sound (51) occurs as the left ventricular (LV)pressure increases above left atrial (LA) pressure. This is followed by the ejection click (X) occurring after the aortic valve opens. The shape of the gradient between LV and aorta(Ao)correspondsto the shape of the aortic ejection systolic murmur (ESM).The murmur ends before the aortic components of the second sound (A2)
innominate artery resulting in higher systolic pressure in the right arm compared to left arm.
The electrocardiogram reveals left ventricular hypertrophy. Presence ofST and T wave changes suggest severe disease (Fig. 15.37). It should be remembered that a normal electrocardiogram does not exclude severe aortic stenosis. The chest X-ray shows a normal sized heart with dilated ascending aorta in valvar AS. In supravalvar and subvalvar stenosis the thoracic roentgenogram may be normal. Presence of cardiac enlargement indicates severe AS. Echocardiogram can not only identify the site of stenosis, butusing Doppler assess the gradient across the obstruction fairly accurately.
Assessment of Severity
The severity of AS should be determined, based on the following:
i.Symptomatic patients have severe AS; lack of symptoms does not exclude severe disease
ii.Narrower the pulse pressure, the more severe the AS
iii.Systolic thrill at second right interspace suggests at least moderately severe AS
iv.The later thepeak of the ejectionsystolicmurmur, the more severe the narrowing
v.The delay in A2 correlates well with severity. With mild AS, the S2 is normally split, with moderate AS it is closely split, with severe or critical AS it is single or paradoxically split

Fig. 15.37: Electrocardiogram from a patient with severe aortic stenosis depression and T wave inversion in lateral leads (strain pattern)
vi.Presence of S4 is indirect evidence for severe AS
vii.Presence of S3 indicates severe AS and congestive cardiac failure
viii.ST and T changes in the electrocardiogram suggest severe stenosis
ix.Cardiac enlargement on chest radiograph indicates severe AS with left ventricular failure
x.Doppler can quantitate the gradient across the aortic valve accurately. Two-dimensional echo reveals concentric left ventricular hypertrophy; ventricular dysfunction is associated with heart failure.
Treatment
Patients with AS should be followed closely, with 6-12 monthly electrocardiogram. Symptoms should be care fully evaluated. Doppler echo can be used to quantitate the gradient at each visit and ventricular function should be monitored. Severe AS is risk for sudden death. Patients should be discouraged from outdoor games, athletics, competitive sports and strenuous exercises if AS is significant (gradient of 50 mm Hg or more). Balloon aortic valvuloplasty is the procedure of choice for valvar AS. A balloon introduced through the femoral artery can be placed at the aortic valve and inflated to tear the valve along the commissure. It is indicated if the gradient is above 75 mm Hg. Supravalvar and subvalvar AS do not respond to balloon dilation; the procedure should also be avoided in patients with significant aortic regurgitation.Surgical options include aortic valve repair and replacement with a prosthetic valve. Patients need to
Disorders of Cardiovascular
I - |
I\r ,. |
|
I |
' , |
l |
l |
1 |
..... |
- |
|
|
1;,. Is• |
||
'" |
·· y |
•. |
|
> |
1 |
|
· |
|
|
|
, |
System
.,.l ,f\.,..i.-,.. D I• -
,.,. '" I· ti ,-f
'!·
showing prominent left ventricular voltages together with
-
[I
I ...
ST segment
be administered anticoagulants if they have a prosthetic valve replacement and careful followup to prevent/detect complications, such as restenosis, thrombus or pannus formation and infective endocarditis.
Coarctation of the Aorta
Coarctation of the aorta is located at the junction of the arch with the descending aorta. It is a sharp indentation involving the anterior, lateral and posterior wall of the aorta; the medial wall is spared. It may be distal or proximal to the ductusor ligamentum arteriosus and also the left subclavian artery. Forty to 80% patients have a
bicuspid aortic valve. Hemodynamics
In fetal life, the right ventricular output passes down the descendingaortathroughawideductusarteriosus. Theleft ventricularoutputemptiesintotheinnominate,leftcarotid and left subclavian arteries and little output reaches the descending aorta.Theportion of the aorta distal to the left subclavian and before the portion where the ductus arteriosusjoins is calledtheisthmus. At birth,theisthmus is the narrowest part of the aorta.Following closure of the ductus arteriosus, the descending aorta must receive its total supplyfrom the left ventriclevia the ascending aorta. Neonates withsevere coarctation therefore become symp tomatic immediately as the duct starts to close.However, a significant proportion present late. The exact mechanism for the production of systemic hypertension in coarctation is not known. The aortic

___E_s_s_ _en_t_ _ial_P_ed_iat _r_ics _________________________________ _
obstruction is certainly partly responsible for it. The narrow pulse pressure in the descending aorta distal to thecoarctation hasbeenimplicated intherenal mechanism for the causation of hypertension in coarctation. The obstruction stimulates growth of collateral vessels bet ween the proximal and distal segments. The intercostal vessels also participate in decompressing the hypertensive upper segment. They enlarge and become palpable at the lower borders of the ribs often later in childhood or adolescence. Palpable collaterals are also felt at the medial andinferiorangleof scapula. Becauseofthedecompression of the upper segment by the collaterals, the resting blood pressure in upper extremities may even be normal, but rises on exercise.
Clinical Features
Coarctation has a continuum of severity and the age at presentation is linked to severity. Newborns with severe coarctation presents as soon as the duct start to close (see duct dependent circulation). Infants with coarctation occasionallypresent withleft ventricular dysfunction and heart failure. It is important to examine femoral pulses in newborns and infants with heart failure. Later in life, coarctation is often not associated with symptoms.
The only symptoms in uncomplicated coarctation may be intermittent claudication, pain and weakness of legs and dyspnea onrunning. Examinationshowsdelayed and weak femorals compared to strong brachial arteries. The
heart size remains normal with a left ventricular forcible or heaving apex. A systolic thrill may be palpable in the
suprasternal notch. There are prominent arterial pul sations in the suprasternal notch and the carotid vessels. The first sound is accentuated and sometimes followed by a constant ejection click. The second sound is normally split with a loud aortic component. A variable intensity ejection systolic murmur is heard with the point of maximumintensityover the backin theinterscapulararea. The murmur starts late in systole after a considerable gap fromthe first sound and click. It mayappear to go through the second sound suggesting a continuous murmur. This is because of delay in the transmission of pulse from the heart to the site of coarctation. Continuous murmurs may be audible over collaterals in the chest wall but are uncommon. An aortic ejection systolic murmur and/or an aortic regurgitation murmur may also be present because ofthe commonly associated bicuspid aortic valve (Fig. 15.38).
The electrocardiogram shows left ventricular hyper trophy; ST and T wave changes below the age of 15 yr suggests additional aortic stenosis or endocardial fibro elastosis. Chest X-ray shows a normal sized heart with prominent ascending aorta and the aortic knuckle. In an overpenetrated film, the site of coarctation can be well localized as the proximal segment is dilated and there is post stenotic dilatation of the distal segment. Barium swallow shows the characteristic 'E' sign and confirms
Coarctation of Aorta
Sounds
S1:Accentuated-loud M1
S2: Normal splitting
DelayedA2
AccentuatedA2
X: Constant
S3:With LV failure
S4: With severe hypertension
Murmurs
(a)Late ejection systolic
(b)Continuous
S1 X S2
Phono II •I•
Fig. 15.38: Summary of auscultatory findings in coarctation of the aorta. S3 third heart sound, S4 fourth heart sound
the site of coarctation. The characteristic notching of the lower borders of ribs tends to appear beyond the age of 10yr. Usingsuprasternal approach coarctation can be seen on echocardiogram and the gradient estimated.
Course and Compllcatlons
Coarctation may result in congestive failure in infancy. If congestivefailure does not occur in infancy, it is unlikely to occur throughout the pediatric age group unless com
plicated by infective endocarditis or anemia. The com plications of coarctation include rupture of berry intra cranial aneurysm and dissection of aorta. These complications are rare in children. Infective endarteritis mayin occur the wall of aorta distal to coarctation or there could be endocarditis involving the bicuspid aortic valve.
Treatment
Relief of coarctation is recommended as soon as diagnosis is made when coarctation is severe. In newborns and infants surgery is preferred. In older children, adolescents and adults, balloon dilation is often undertaken. The recurrence rates of balloon dilation in newborns is over 90% and this procedure should only be done as interim palliation in the face of heart failure and severeventricular dysfunction. Prostaglandin El is used to maintain ductal patency prior to surgery in the first few weeks of life.
It is likely that coarctation is not a localized disease at the junction of arch and descending aorta and there is perhaps generalized weakness of the arterial media. Resection of coarctationdoesnot guarantee freedom from complications like dissection of aorta. Systemic hyper tension can persist following operation and recoarctation of aorta can also occur, requiring repeat balloon angioplasty.

Pulmonic Stenosis (Pure Pulmonic Stenosis or Pulmonic Stenosis with Intact Ventricular Septum)
Pulmonic stenosis (PS) is usually valvar or subvalvar (iniundibular PS). Uncommonly pulmonic stenosis may be in the pulmonary artery above the valve or in the main right or left branches or the peripheral branches.
Hemodynamics and Clinical Features
Flow across the narrow pulmonary valve results in a pulmonary ejection systolic murmur and a thrill in the left second interspace. To keep the flow normal the right ventricle increases its systolic pressure and develops concentric right ventricular hypertrophy. The pulmonary artery beyond the obstruction shows poststenotic dilatation visible on the thoracic roentgenogram as a dilated pulmonary arterial segment. Because of the obstruction, the right ventricular systole is prolonged resulting in delayed closure of the pulmonic component {P2) of second sound. The delay in P2 results in a wide and variably split second sound. In valvar PS, a pulmonary ejection click is audible, soon after Sl and just before the onset of murmur, during expiration but disappears or becomes softer during inspiration. With increasing severity of stenosis the duration and intensity of the murmur increaseandthepeakgets delayed; theclick disappears and P2 becomes softer. With moderate PS, the murmur ends just short of the aorticcomponent of the second sound. The concentric right ventricular hypertrophy results in main taining a normal heart size, but reduces its distensibility. In severe PS with marked right ventricular hypertrophy, the ventricular diastolic pressure also increases. The right atrial pressure increases to be able to fill the right ventricle and results in a right atrial fourth sound (S4) as well as prominent 'a' waves in the JVP (Fig. 15.39).
Patients with mild to moderate PS are asymptomatic; with severe stenosis, dyspnea on effort appears. If foramen ovale is patent, a right to left shunt at the atrial level may occur in severe PS and result in cyanosis. Palpitation, easy fatigability and rarely chest pain may occur. Features of Noonan syndrome should be looked for. The cardiac size is normal and the hypertrophied right ventricle results in left parasternal heave. If the right ventricle fails, a right ventricular third sound may be audible. Rarely with right ventricular failure, tricuspid regurgitation may appear. Since the right atrium offers less resistance to flow of blood than obstruction at the pulmonary valve, the flow through the pulmonary valve diminishes reducing the intensity as well as the duration of ejection systolic murmur.
lnfundibular stenosis is distinguished from valvar PS by: (i) absence of click; (ii) absence of post stenotic dilatation; and (iii) a relatively lower point of maximum intensity of systolic murmur in 3-4th left interspace. Isolated pure iniundibular PS is rare.
The electrocardiogram shows right axis deviation and right ventricular hypertrophy. PS results in systolic
Disorders of Cardiovascular System -
Pulmonic Stenosis
Sounds
S1: Normal
S2: P2 Delayed and softer Widely split with normal movement
S3: With RV failure
S4: With severe stenosis
X : Inconstant (valvar)
Murmurs
Ejection systolic (diamond shaped)
(I |
|
S |
M |
A2 |
|
Mild |
) |
1X |
ES |
P2 |
|||
|
nsp.!--- -tlt...l ll411i.....--tl-r!.:. |
|||||
(lnsp and Exp) |
Ii |
|
|
2 |
Moderate |
||
|
ert1 |
||||||
|
|
||||||
(Exp) |
S4 |
|
A2 |
Severe |
|||
|
|
, ,, |
111 |
I |
|
||
ECG
Fig. 15.39: Summary of auscultatory findings in pulmonic stenosis
overload for the right ventricle, suggested by pure 'R' or 'qR' complex in V4R and Vl leads. P pulmonale suggests
severe PS. Chest X-ray shows a normal sized heart with normal pulmonary vasculature in mild, moderate as well as severe PS. Pulmonary oligemia occurs if the patients develop a right to left shunt at the atrial level in severe or critical PS. The main pulmonary artery exhibits poststenotic dilatation. Echocardiography can identify the site and severity of obstruction and helps in planning catheter intervention.
Treatment
Valvar PS generally does not increase in severity with time unless it is severe or diagnosed in the newborn period. Patients with mild PS (gradients of 50 mm Hg or less) need annual review. Balloon pulmonary valvuloplasty is the treatment of choice for isolated valvar PS. The procedure is sometimes technically challenging in newborn with critical PS. Surgical treatment is indicated only if balloon valvotomy is unsuccessful, as in patients with dysplastic valves or small pulmonary valve annulus. lnfundibular PS requires surgical resection.
Suggested Reading
Allen HD, Shaddy RE, Driscoll DJ, Feltes TF, Moss Adams' Heart disease in infants, children and adolescents, 7th Edition, Kluwer/ Lippincott William and Wilkins, Philadelphia, USA, 2008
RHEUMATIC FEVER AND RHEUMATIC HEART DISEASE
Rheumatic Fever
Rheumatic fever is an immunological disorder initiated by group A beta hemolytic streptococci. Antibodies

- E_s_s__ent_ia1 P_ed_iat rics
1.- _ _________________________________
produced against selected streptococcal cell wall proteins and sugars react with the connective tissues of the body as well as the heart and result in rheumatic fever. There is no single test for the confirmation of diagnosis. There is a strong relationship with streptococcal infection and it is possible to prevent rheumatic fever by prompt treatment of streptococcal infections with penicillin.
Epidemiology
Rheumatic heart disease (RHD) constitutes from 5 to 50% of the cardiac patients in Indian hospitals. A survey conducted by the Indian council of medical research (ICMR) involving 133,000 children 6-16 yr old showed the incidence to be 5.3/1000. Selected parts of India that have experienced improved human development are now reporting a significant decline in incidence of rheumatic fever. Surveys conducted at Chandigarh, Indore, Cochin and Vellore involving more than 100,000 children (2004 to 2007) indicate that the prevalence has dropped to between 0.5 and 1/1000. Surveys using echocardiography alone have suggested a higher prevalence of RHD in selected regions (as high as 20/1000).
Age and sex. The incidence of rheumatic fever following streptococcal throat infection is 0.3% in the general popu lation and 1 to 3% in presence of epidemics of strepto coccal sore throat. The commonest age group involved is 5-15 yr, and first episodes of RF are rare before 3 yr or after30 yrage.Althoughthesexesare nearlyequallyaffec ted, mitral valve disease and chorea is more common in girls whereas aortic valve involvement is more often seen in boys.
Predisposing factors. Poor socioeconomic conditions, unhygienic living conditions and overcrowded house holdspredisposeto streptococcal infections.However, an epidemic in United States occurred in upper middle class families in the absence of overcrowding and with good medical facilities in the mid 1980s.
Etiopathogenesis
The etiology of rheumatic fever is unknown. A strong association with beta hemolytic streptococci of group A is indicated by a number of observations:
i.History ofprecedingsore throat isavailablein less than 50% patients
ii.Epidemics of streptococcal infection are followed by higher incidence of rheumatic fever
iii.The seasonal variation of rheumatic fever and streptococcal infection are identical
iv.In patients with established RHD streptococcal infection is followed by recurrence of acute rheumatic fever
v.Penicillin prophylaxis for streptococcal infection prevents recurrences of rheumatic fever in those patients who have had it earlier
vi.More than 85% of the patients with acute rheumatic fever show elevated levels of anti-streptococcal antibody titer
Streptococci have never been isolated from rheumatic lesions in joints, heart or the blood stream. Considerable evidence suggests that rheumatic fever is an antigen antibody reaction. Following streptococcal sore throat, there is a latent period of 10 days to several weeks before the onset of rheumatic fever. This latent period is similar to other antigen-antibody diseases like serum sickness. Streptococcal cell wall proteins as well as carbohydrates have thecapacitytoproduceantibodiescapableof reacting with human connective tissue, resulting in rheumatic fever. Rheumatic fever appears tobe theresult of the host's unusual response at both the cellular and humoral level to Streptococcus. There is no marker that identifies genetic susceptibility to rheumatic fever.
Only heart valves are permanently damaged during an episode of rheumatic fever. All other affected tissues typically heal without residua: pericarditis, chorea and arthritis resolve completelywithout constriction, longterm neurologic consequences or joint disability, respectively. Concepts about the pathogenesis of rheumatic fever and RHD are summarized in Fig. 15.40.
Clinical Features
The clinical features of rheumatic fever consists of streptococcal sore throat with fever followed 10 days to a few weeks later by recurrence of fever and the various manifestations of acute rheumatic fever. The history of sore throat is available in less than 50% of the patients. Guidelines for the clinical diagnosis of acute rheumatic fever, originally suggested by T. Duckett Jones have been revised by the American heart associationand WHO. The guidelines consist of major, minor and essential criteria (Table 15.16). Two major or one major and two minor criteria are required in the presence of essential criteria to diagnose acute rheumatic fever. It is important to emphasize that these guidelines are meant to help a physician in making a firm diagnosis of rheumatic fever and do not mean that a physician should not use his clinical judgment in making a diagnosis in the absence of these criteria.
Major criteria These include:
(i) Carditis. It is an early manifestation of rheumatic fever. Studies utilizing echocardiography indicates that carditis occurs in almost 90% patients. In 60-70% it is clinically obvious whereas in the remaining, the diagnosis is based on echocardiographic findings labeled as subclinical carditis. Rheumatic carditis is designated as a pancarditis involving the pericardium, myocardium and endocar dium, although studies indicate limited myocardial component. Almost 80% of those patients who develop carditis do so within the first two weeks of onset of rheumatic fever.
