
Photochemistry_of_Organic
.pdf
Flash Photolysis |
105 |
c |
|
3 |
* |
|
r |
= |
|||
r |
|
|||
A |
= |
C |
* |
3 |
c |
|
|
3 |
|
ε |
Aij = ∑ cik |
εkj |
k =1 |
|
|
|
|
Equation 3.11
In real life, we will have spectral data A such as those shown in Figure 3.19, but do not know the underlying matrices C and «. The goal of global analysis is to revert the process, that is, to recover the information about the number of components, their spectra, « and the rate laws with the associated rate constants defining the concentration profiles, C, by analysis of the complete set of spectral data, A. Global analysis consists of two steps, factor analysis and target factor transformation. For factor analysis we first form the covariance matrix from the data matrix A, M ¼ A A0, where A0 is the transpose of the data matrix A (Equation 3.12).d
r |
|
c |
|
r |
r |
= |
r |
* |
|
|
|
|
|
|
|
|
|
|
c |
M |
= |
A |
* |
A' |
Equation 3.12 Covariance matrix
The covariance matrix M is a symmetrical square matrix, Mij ¼ Mji, of size r r that can be diagonalized. Solving the secular determinant ||M dijl|| ¼ 0 yields r eigenvalues l with r associated orthonormal eeigenvectors ni, i ¼ 1, . . ., r of length r.f Collecting the eigenvectors in the columns of a matrix V we have found the matrix that diagonalizes M by the operation V0 M V (Equation 3.13). The eigenvalues appearing on the diagonal matrix S ¼ (dijli) are sorted by size.
r |
|
r |
|
r |
* |
r |
* |
V' |
* |
M |
* |
r |
r |
r |
= r |
V = S
Equation 3.13 Diagonalization of the covariance matrix
These matrix operations are easily programmed with mathematical software packages. In MATLAB, for example, only two statements, ‘M ¼ A A0;’ and ‘[V,S] ¼ eig(M);’, are required
d Another covariance matrix could have been constructed as A0 A, which would have had the much bigger size of c c. As the information content of A A0 and A0 A is the same, we chose the smaller one.
e The matrix product V V0 is a diagonal unit matrix (dij) of size r r.
f The mathematical procedure is the same as that used in quantum mechanics to optimize LCAO wavefunctions (Sections 1.4 and 4.2).

106 |
Techniques and Methods |
and with reasonably sized matrices such as c ¼ 1000 and r ¼ 20 the calculation is completed in seconds on a desktop computer. We are now in a position to determine the number of significant factors, that is, the number of independent components contributing to the spectral matrix A. Using the noise-free spectra (Figure 3.18), the diagonal matrix S contains only three non-zero diagonal elements on the bottom right. We have retrieved the information that A was generated using three components, n ¼ 3. Having decided on the number n of eigenvalues to be retained, we can reduce the matrices V and S to the shaded areas Vred of size r n and Sred of size n n (Equation 3.13) and reconstruct the transpose of the spectral matrix, Areconstr0, by forming the product Ured Sred Vred0 (Equation 3.14). To determine the matrix of the spectral eigenvectors, Ured, of size c n, we right-multiply Areconstr0 with Vred to get Areconstr0 Vred A0 Vred ¼ Ured Sred [the rows of Vred are orthonormal, Vred0 Vred ¼ (dij), hence Ured ¼ A0 Vred (Sred) 1]. The coefficients in the rows of Vred represent the weight of the three spectral eigenvectors Ured(c n), which is to be multiplied by the corresponding eigenvalue on the diagonal of Sred to reconstruct the spectra in the rows of A. The spectra reconstructed from the noise-free data are identical with Figure 3.18.
r |
c |
Areconst' |
n * n * n r
= c
n |
|
r |
|
|
|
c |
= |
c |
= Ured * Sred * Vred' |
where Ured = |
A' |
* n * n r
* Vred * (Sred)–1
Equation 3.14
Using the noisy spectra shown in Figure 3.19, the largest eigenvalues obtained by diagonalization of M are, in decreasing order, 135.1, 43.2, 0.19, 0.12, 0.11, 0.10, etc. The noise has produced 11 nonzero eigenvalues and it is difficult to decide whether the spectra were made up of two or more components. Note that the falloff after the second is 40% and very little thereafter. Using statistical criteria,201 one would conclude that the third eigenvalue is not significant at the 95% level. However, as chemists, we may have prior knowledge (or belief) that the reaction involves an intermediate and, hence, would want to choose three components. The spectra reconstructed retaining three eigenvalues are shown in Figure 3.21 (top).
At this point, we have achieved two goals. First, the amount of data stored (and subsequently handled) in the matrices Ured, Sred and Vred is reduced by at least an order of magnitude. Second, as an additional bonus, much of the random noise has been eliminated (compare Figures 3.19 and 3.21). One should realize, however, that by choosing the number of eigenvalues we introduce some bias in the reconstructed data. This is illustrated in Figure 3.21 (bottom), in which the same data were reconstructed with only two eigenvalues. The data are now forced through an isosbestic point.
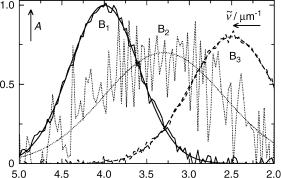
Figure 3.22 shows the three abstract eigenvectors (the columns of Ured Sred) that were used to reproduce the spectra. Clearly they do not correspond to the spectra of the species B1–B3. Rather, the first eigenvector (solid line) represents an average of the whole spectral series and is positive throughout. The second (dotted line) has one node and allows for a first correction in reconstructing the individual spectra and the last (dashed line,

Flash Photolysis |
107 |
Figure 3.21 Reconstructed spectra using three (top) or two (bottom) eigenvalues
multiplied by a factor of five) is a second correction . This is reminiscent of the molecular orbitals that are obtained by the same mathematical procedure (Section 4.2); the lowest MO is bonding throughout, the next has one node, and so on.
The row vectors in matrix Vred define points in the three-dimensional space of the orthogonal eigenvectors. They lie in (or near, in the presence of noise) a plane, consistent
Figure 3.22 The three eigenvectors of the spectral data. The smooth lines are the eigenvectors obtained from the noise-free spectra (Figure 3.18)

108 |
Techniques and Methods |
Figure 3.23 The reaction B1 ! B2 ! B3 proceeding in a plane of the eigenvector space
with a reaction involving three components, as shown in Figure 3.23. A one-step reaction B1 ! B2 would follow a straight line and a reaction involving more than three species would show systematic deviations from the regression plane.
Having completed factor analysis, we wish to determine the spectra of the chemical species that give rise to the observed spectral data. To achieve transformation of the eigenspectra to the component spectra (target factors), additional information is needed. We may know that the first spectrum corresponds to the starting material B1 and the final one to the product B3. The initial spectral changes are then attributed to the reaction B1 ! B2 and the final ones to B2 ! B3. We can therefore estimate the coordinates of the point in eigenvector space that represents the species B2 as the intersection of two straight lines in the regression plane (Figure 3.23).
Other, more powerful methods are available. For one, we know that the species spectra cannot have negative absorbances. This information may be sufficient to analyse spectra with little overlap, such as highly resolved IR spectra. If we know that the spectra are ordered in a meaningful sequence, for example because a chemical reaction proceeds in
time or the pH is changed continuously by titration, then self-modelling methods (evolving factor analysis) can be used.203,204 If we know, or have reason to assume, that the species
concentrations C follow a certain rate law (as in the present example) or the law of mass action (in a titration), then an initial guess of the C matrix can be made using trial parameters in the model function, for example the rate or equilibrium constants of
the assumed model. The model parameters are subsequently optimized by nonlinear least-squares fitting.201–203,205,206 All of the above methods aim at decomposing the
spectral data A to chemically meaningful concentration profiles C and species spectra «; (Equation 3.11). The best choice depends on the additional knowledge that is available about the system. It is recommended to test any chosen model by unbiased self-modelling methods.
To complete the global analysis of our example, we construct a trial matrix C (r 3) using the integrated rate laws for the species B1–B3 (Equation 3.8, Section 3.7.4) with trial parameters kAB and kBC. From this guess of C, a first guess for the species spectra « is
Time-Resolved IR and Raman Spectroscopy |
109 |
Figure 3.24 Recovered species spectra. The noisy traces were obtained from the spectral data shown in Figure 3.19
obtained without iteration as « ¼ (C0 C) 1 C0 A (MATLAB command: EPS ¼ C\A; ). The matrix « is then optimized by minimizing the sum of squares of the residuals, R ¼ A C «, upon variation of the nonlinear parameters kAB and kBC that determine C. The final species spectra contained in the rows of « are shown in Figure 3.24. The rate constants obtained from the noisy spectra are 0.49 and 2.2 s 1.
In conclusion, the global analysis of spectral data is a very useful tool to validate a proposed model, when used with proper understanding and caution. However, statistical analysis in general is not able to eliminate any systematic errors that may be hidden in experimental data. On the contrary, it will emphasize any such deviations from a chosen model and might thereby insinuate false complexity of the system investigated. No mathematical treatment can ever make up for less than optimal methods of data collection.
3.8 Time-Resolved IR and Raman Spectroscopy
Before the advent of NMR, infrared (IR) absorption and the largely complementary Raman spectroscopy were the most important tools to identify functional groups by their characteristic vibrational frequencies and they still hold their place in analytical chemistry. Both IR and Raman spectroscopy provide fingerprints that permit an unambiguous identification of simple molecules and even of specific sites in biopolymers. Due to the low absorption coefficients of vibrational bands, time-resolved IR spectroscopy was hampered by a lack of sensitivity and was originally useful only for the detection of very strong vibrational bands such as those of metal carbonyls. This has changed dramatically over the last 20 years with the advent of Fourier transform instruments and amplified Ti:sapphire lasers that produce broadband femtosecond light pulses. Vibrational spectroscopic methods are now extensively used in structural studies of the dynamics of chemical and biological reactions.
In picosecond time-resolved Raman spectroscopy, the sample is pumped and probed by energetically well-defined optical pulses, producing a full vibrational spectrum over a 1000–2000 cm 1 window.207 One would expect vibrational spectroscopy to be restricted to the picosecond time domain and above by the Heisenberg uncertainty principle (Equation 2.1), because a 1 ps transform-limited pulse has an energy width of
110 |
Techniques and Methods |
15 cm 1 FWHM and a 10 fs pulse has an intrinsic energy bandwidth of 1500 cm 1. This impasse is elegantly circumvented by ultrafast femtosecond stimulated Raman spectroscopy, which can provide <100 fs temporal and <35 cm 1 spectral resolution by exploiting coherence.208,209 An additional femtosecond pump pulse (460–670 nm, <30 fs) that is significantly shorter than the period of the vibrationally active modes of the molecule is added to initiate a photochemical reaction. It produces a localized wave packet that evolves on the excited state surface. The spectral resolution is mainly limited by the bandwidth of the Raman pulse (<10 cm 1), while the time resolution is determined by the cross-correlation of the pump and Raman probe pulses (typically <50 fs). This technique has the additional advantages of an improved signal-to-noise ratio, short data acquisition times and insensitivity to background fluorescence. It was used to monitor the primary events of vision.210
To obtain IR spectra on a time scale of nanoseconds, the sample cell in conventional spectrometers is usually excited by an Nd:YAG laser. Flow cells with a pathlength of at least 0.1 mm must be used for photoreactive samples and the pulse repetition frequency is then limited to 1 Hz. In step–scan FTIR spectroscopy,211 the time evolution is collected at single points of the interferogram, which is then reconstructed point-by-point and subsequently transformed to time-resolved IR spectra. Alternatively, dispersive instruments equipped with a strong IR source can be used.212 The time resolution of both methods is about 50 ns. FTIR instruments provide a triggerable fast-scan mode to collect a complete spectrum within a few milliseconds.213
3.9 Quantum Yields
Quantum yields are fundamental quantities that define the photonic economics of processes induced by light absorption. They are required to determine rate constants of photophysical and photochemical processes (Section 3.9.7). Many different techniques are used to measure quantum yields depending on the process studied. In the following, we describe some procedures commonly used in the chemical laboratory. The measurement of quantum yields is an art that has a number of pitfalls. The experimenter has few options to double-check his or her own results other than reproducibility, which will not reveal any repeated systematic errors. Therefore, it is prudent to reproduce the quantum yield of a related, well-known process in the laboratory before determining an unknown one.
3.9.1Differential Quantum Yield
The definition of a quantum yield for a given process x, Fx(l) ¼ nx/np, was given in Equation 2.24, Section 2.1.7. Here nx is the amount of photochemical or photophysical events x that occurred during irradiation and np is the amount of photons at the irradiation wavelength l that were absorbed by the reactant. Both nx and np are measured in moles or einsteins (1 einstein ¼ 1 mol of photons) and the dimension of Fx is unity.
With photoreactive systems, the amount of light absorbed by the reactant changes as the reaction proceeds and the amount of product formed may change further when irradiation of the photoproducts leads to secondary photoreactions. To deal with this, the quantum yield Fx(l) ¼ nx/np must be defined differentially (Equation 3.15). As infinitely small conversions cannot be measured, one is forced to use finite doses of light to determine

Quantum Yields |
111 |
quantum yields. Exact integration of Equation 3.15 is possible in certain cases and will be dealt with below (Section 3.9.3). In any case, small conversions (<10%) are preferable to avoid corruption by secondary photoreactions. The best compromise depends on the sensitivity of the method of analysis used (GC, absorbance, luminescence, etc.).
FxðlÞ ¼ dnx=dnp
Equation 3.15 Differential quantum yield
For monochromatic light, the molar photon flux q0m;p ¼ np=t, the amount (in moles or einsteins) of photons incident on a sample cell per unit time, is proportional to the incident
spectral radiant power Pl0 (Equation 3.16). The unit of q0m;p (Equation 2.3) is mol s 1.
q0m;p ¼ P0l=NAhcn~
Equation 3.16 Molar photon flux
When only the reactant absorbs at the wavelength of irradiation l, the amount dnp of photons absorbed by the sample in a short time interval dt is equal to the absorbed molar photon flux qm,p (Equation 3.17).
dnp ¼ q0 ; ½1 TðlÞ&dt ¼ q0 ; ½1 10 Aðl;tÞ&dt
m p m p
Equation 3.17
If the fraction of light transmitted is substantial, a small correction should be applied to account for the light reflected back into the liquid from the rear face of the cell (about 4%). When other species that absorb at the wavelength of irradiation are present or when the photoreaction produces absorbing photoproducts, the right-hand side of Equation 3.17 must be multiplied by the fraction of light absorbed by the reactant A, AA(l,t)/A(l,t),
Equation 3.18. |
|
|
|
|
|
|
|
0 |
AA |
|
l; t |
|
|
||
dnp ¼ qm;ph1 10 Aðl;tÞi |
A |
|
ð |
|
Þ |
dt |
|
|
l; t |
Þ |
|||||
|
|
ð |
|
|
|||
Equation 3.18
For small absorbances, A(l,t) 0.1, the quotient ½1 10 Aðl;tÞ&=Aðl; tÞ approaches ln(10) ¼ 2.303, so that dnp/dt 2.303qm,pAA(l,t). For large absorbances, A(l,t) > 2, the term 10 A(l,t) may be neglected. If the reaction progress is measured by analytical methods such as GC, HPLC or NMR, then the quantum yield is usually calculated by Equation 3.15, simply replacing the differential terms dnx and dnp by small increments Dnx and Dnp measured after short time intervals Dt of irradiation. Average values for the absorbances A(l,t) and AA(l,t) during the irradiation interval should then be used to calculate the amount of light Dnp absorbed by component A (Equation 3.18) and the conversion per time interval Dt should be kept well below 10%. Quantum yields determined in this way for consecutive irradiation periods should remain constant; a decreasing trend indicates that secondary photoreactions are occurring.

112 |
Techniques and Methods |
3.9.2Actinometry
Recommended review.214
To determine a quantum yield using Equation 3.18, the radiant power Pl0 ¼ q0m;pNAhc n~ incident on the sample cell must be known. Absolute measurements of radiant power are difficult to perform with precision and are rarely done in the chemical laboratory. In practice, radiant power is measured by actinometry. An actinometer is a chemical system that undergoes a light-induced reaction, for which the quantum yield Fact(l) is known accurately. Equations 3.15 and 3.18 are then used in reverse, that is, the radiant power of the light source is determined by measuring the conversion of the actinometer per unit time, dnx/dt ¼ dnact/dt, and inserting the known value of Fact(l). Requirements for a good chemical actinometer are that its quantum yield is well established and preferably constant over a wide range of wavelengths, radiant power and total radiant energy, and also high sensitivity and precision coupled with simplicity of use and ready availability of the photosensitive material.
Spectrophotometric monitoring of the reaction progress in both the sample and the actinometer solution (Section 3.9.3) is convenient and gives accurate results. Due to fluctuations of most light sources in time, the irradiation of sample and actinometer solution should be done simultaneously (Figure 3.29). Alternatively, standard analytical techniques following irradiation on a merry-go-round apparatus (Figure 3.30) are frequently used in the organic laboratory. A comprehensive list of established chemical
actinometers and recommended standard procedures is available.214 The ferrioxalate actinometer is probably the most widely used.214–216 It has been calibrated repeatedly by
comparison with a thermopile and also against other actinometers. It is prepared from readily available chemicals (Case Study 3.1) and can be used for a wide range of irradiation wavelengths (205–509 nm, Table 3.2). Its disadvantages are that it must be
Table 3.2 Quantum yields of the ferrioxalate actinometer at different wavelengths.
Wavelength/nm |
Quantum yield32 |
Quantum yield216 |
|
509 |
|
0.86 |
|
480 |
|
0.94 |
|
468 |
|
0.93 |
|
436 |
(0.15 M) |
1.01 |
|
436 |
(0.006 M) |
1.11 |
|
405 |
|
1.14 |
|
365/366 (0.15 M) |
1.18 |
|
|
365/366 (0.006 M) |
1.22 |
1.27 |
|
340 |
|
|
1.23 |
334 |
|
1.23 |
|
320 |
|
|
1.27 |
313 |
|
1.24 |
|
297/302 |
1.24 |
1.25 |
|
260–300 |
|
1.25 |
|
253.7 |
1.25 |
1.40 |
|
240 |
|
|
1.45 |
225 |
|
|
1.46 |
220 |
|
|
1.47 |
214 |
|
|
1.50 |
205 |
|
|
1.49 |
|
|
|
|

Quantum Yields |
113 |
handled in a darkroom under red light and that its quantum yield is wavelength dependent. For details on the convenient, reversible actinometer azobenzene, see Section 3.9.4. Aberchrome 540, another reversible and widely used actinometer, is no longer commercially available and exhibits fatigue and also side-reactions.217 Its use is not recommended. The self-sensitized photo-oxidation of meso-diphenylhelianthrene can be used for irradiation wavelengths in the range 475–610 nm (Scheme 6.259).218 Several compounds giving standard transient absorbance have been recommended for the actinometry of laser pulses.214
Case Study 3.1: Actinometry – Ferrioxalate
Pure potassium ferrioxalate is prepared by mixing three volumes of 1.5 M potassium oxalate with one volume of 1.5 M ferric chloride, both of analytical quality. The precipitated green potassium ferrioxalate [K3Fe(C2O4)3 3H2O] should be recrystallized three times from warm water and the crystals dried in a current of warm air (45 C). The whole procedure must be carried out in a darkroom lit with a red safelight. The crystals can be stored indefinitely in the dark.
The 0.006 M actinometer solution is prepared by dissolving 2.947 g of the ferrioxalate crystals in 800 ml of water. Sulfuric acid (1.0 N = 0.5 M, 100 ml) is added, the solution diluted to 1 l and mixed. The 0.15 M solution is made in a similar manner using 73.68 g of ferrioxalate. At least 99% of the light is absorbed by the 0.006 M solution up to 390 nm and by the 0.15 M solution up to 445 nm in a cell of pathlength 1 cm. Above these wavelengths, the fraction absorbed at the irradiation wavelength should be measured.
On exposure to light, the following reactions occur:
½ |
Fe |
C2O4 |
Þ& |
þ |
hn |
Fe2þ |
þ |
C2O . |
|
|
ð |
. |
|
|
! |
|
4 |
2 |
|||
C2O4 |
þ ½FeðC2O4Þ&þ ! 2 CO2 þ Fe2þ þ C2O4 |
|||||||||
After photolysis, the ferrous ion formed is converted to its 1,10-phenanthroline complex and the latter determined by spectrophotometry. The minimal detectable amount of light is about 2 10 10 einstein ml 1 and the maximal light dose that can
be measured accurately ( 2%) is about 5 10 6 einstein ml 1.
The following procedure is taken from the literature.32,219 It is recommended to prepare a calibration graph by mixing solutions of (a) 0.4 10 3 M of Fe2+ in 0.1 N sulfuric acid (freshly prepared by dilution from a standardized solution of 0.1 M FeSO4 in 0.1 N sulfuric acid), (b) a buffer solution of 600 ml of 1 M sodium acetate and 360 ml of 1 N sulfuric acid diluted to 1 l and (c) 0.1% (w/v) of 1,10-phenanthroline monohydrate in water. Solution (c) must be kept in the dark and stored for no longer than 3 months. Into a series of 20 ml calibrated flasks, sequentially add the following volumes of solution and mix: x ¼ 0.0, 0.5, 1.0, . . ., 4.5, 5.0 ml of solution (a). Add 5 ml of solution (b) and (10 x) ml of 0.1 N sulfuric acid. Add 2 ml of solution (c), make up to mark, mix and allow to stand for at least 0.5 h. Measure each absorbance at 510 nm in a 1 cm cell and correct each reading for the value obtained with the solution, to which no ferrous ion was added (should be 0.01). The solutions may be kept for several hours in the dark before measurement. The resulting plot of absorbance against the
114 |
Techniques and Methods |
|
|
molarity of ferrous |
ion should be linear with a slope corresponding to |
« = 11 050 M 1 cm 1. |
|
During irradiation of the actinometer, the solutions should be stirred with a magnetic |
|
bar. After irradiation, pipette an aliquot (2 ml) of the solution into a 20 ml calibrated |
|
flask. Add a volume of buffer (b) equal to half the volume of photolyte taken (1 ml) and |
|
2 ml of the phenanthroline solution (c). Make up to the mark with water, mix and allow |
|
to stand for at least 0.5 h. Measure the absorbance at 510 nm and repeat with the same |
|
volume of unexposed actinometer solution. Convert the absorbance difference of |
|
ferrous iron using the calibrated slope. Convert the quantity of ferrous ion formed in the |
|
total volume of the irradiated solution to a radiation dose (see Equation 2.24) using the |
|
recommended quantum yield given in Table 3.2. If necessary, allow for the fraction of |
|
light absorbed (Equation 3.17). |
|
|
|
3.9.3Spectrophotometric Determination of the Reaction Progress
A convenient, sensitive and highly accurate method of monitoring the progress of photoreactions is to record absorption spectra intermittently between successive periods of irradiation. It works best for uniform reactions; this does not imply that a single photoproduct must be formed, but that the product distribution remains unchanged throughout. Nonuniform reactions arise when transient intermediates have sufficient lifetime (>1 min) to interferewith spectrophotometric monitoring or when some photoproducts are unstable either thermally or under the irradiation conditions. When a photoproduct is light sensitive and undergoes secondary photolysis upon prolonged irradiation, then the quantum yield of the initial photoreaction may be estimated at low conversion, provided that the absorption spectrum of the light-sensitive photoproduct is known.
Sensitive tests for the uniformity of a reaction can be done by global analysis of the complete set of spectra recorded during photolysis. These methods, described in Section 3.7.5, provide the best evaluation of the minimum number of spectral components required to reproduce a sequence of spectra within experimental accuracy and the time-dependent species concentrations thus obtained accurately define the reaction progress. Simpler versions use absorbance differences observed at a few selected wavelengths where the changes are largest. Uniform reactions give linear plots of DA(l1,t) versus DA(l2,t). For two sequential photoreactions, absorbance difference
plots are curved, but plots of absorbance difference quotients, DA(l1,t)/DA(l2,t) versus DA(l1,t)/DA(l3,t), are linear.198,220,221 Isosbestic points provide the simplest criterion
for the uniformity of a reaction: An isosbestic point is a wavelength, wavenumber or frequency at which the total absorbance of a sample does not change, because reactant and product(s) accidentally have the same absorbance (Figure 3.25). A reaction is nonuniform if the intermediate spectra do not cross at the same point, as in Figure 3.18.
We now derive several equations for the spectrophotometric quantum yield determination of unidirectional photoreactions A ! B. Reversible photoreactions will be treated in Section 3.9.3. The reader should not be deterred by the complex appearance of some of these equations. They are easy to use and give highly reproducible results, because absorbance measurements are precise. The photoreaction is induced by continuous irradiation with a monochromatic light source that exposes the sample
